Abstract
The NDI1 gene encoding rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria was cotransfected into the complex I-deficient Chinese hamster CCL16-B2 cells. Stable NDI1-transfected cells were obtained by screening with antibiotic G418. The NDI1 gene was shown to be expressed in the transfected cells. The expressed Ndi1 enzyme was recognized to be localized to mitochondria by immunoblotting and confocal immunofluorescence microscopic analyses. Using digitonin-permeabilized cells, it was shown that the transfected cells, but not nontransfected control cells, exhibited the electron transfer activities with glutamate/malate as the respiratory substrate. The activities were inhibited by flavone, antimycin A, and KCN but not by rotenone. Added NADH did not serve as the substrate, suggesting that the expressed Ndi1 enzyme was located on the matrix side of the inner mitochondrial membranes. Furthermore, although nontransfected cells could not survive in a medium low in glucose (0.6 mM), which is a substrate of glycolysis, the NDI1-transfected cells were able to grow in the absence of added glucose. When glycolysis is slow, either at low glucose concentrations or in the presence of galactose, respiration is required for cells to survive. The mutant cells do not survive at low glucose or in galactose, but they can be rescued by Ndi1. These results indicated that the S. cerevisiae Ndi1 was expressed functionally in CCL16-B2 cells and catalyzed electron transfer from NADH in the matrix to ubiquinone-10 in the inner mitochondrial membranes. It is concluded that the NDI1 gene provides a potentially useful tool for gene therapy of mitochondrial diseases caused by complex I deficiency.
Mammalian NADH-quinone (Q) oxidoreductase (complex I) is composed of at least 43 distinct subunits and has the most intricate structure of the membrane-bound mitochondrial enzyme complexes (1). Of these subunits, seven are encoded by mitochondrial DNA and others are encoded by nuclear DNA (2, 3). Complex I contains noncovalently bound FMN and at least five EPR-detectable iron–sulfur clusters as prosthetic groups (4–7). It has been shown in recent years that structural and functional defects of complex I are involved in many human mitochondrial diseases (8–10). At present, mutations and deletions of the seven mtDNA-encoded subunits are not correctable and mutations of multiple subunits encoded by nuclear DNA are difficult to repair. Various chemotherapies have been reported to be ineffective at the present time (11). Dysfunction of complex I presents three problems (12): (i) impairment of the ability of the respiratory chain to oxidize NADH to NAD; (ii) impairment of the ability of this enzyme to pump protons, which results in a decrease in the rate of ATP synthesis; and (iii) production of superoxide radicals, causing mitochondrial DNA mutations, lipid peroxidation, and protein denaturation. Of the three problems, the impairment of proton pumping by one of the three proton translocation sites does not appear to be a severe health hazard as compared with the inability of mitochondria to oxidize NADH and damage by superoxide production.
There is another type of NADH-Q oxidoreductases that are distinct from complex I in that they do not contain a proton translocation site and are rotenone-insensitive. In contrast to mammalian mitochondria that are believed to contain only complex I, mitochondria of Saccharomyces cerevisiae lack complex I but instead have rotenone-insensitive NADH-Q oxidoreductases (13–15). In S. cerevisiae mitochondria, at least two distinct rotenone-insensitive NADH-Q oxidoreductases are considered to be present, because in contrast to mammalian mitochondria, a malate/aspartate shuttle that allows redox equilibration of NADH between the mitochondrial matrix and the cytoplasm is absent from this organism (15). Therefore, one NADH-Q oxidoreductase faces the intermembrane space (referred to as external, rotenone-insensitive NADH-Q oxidoreductase), and the other faces the matrix (designated internal, rotenone-insensitive NADH-Q oxidoreductase) (14).
The internal, rotenone-insensitive NADH-Q oxidoreductase of S. cerevisiae mitochondria is a single polypeptide enzyme with noncovalently bound FAD as a cofactor and no iron–sulfur clusters (13). The enzyme is reported to be a two-electron reaction enzyme, whereas complex I is believed to be a one-electron reaction enzyme (12, 13). If so, the yeast enzyme should not cause complications resulting from free radicals. The NDI1 gene encoding the enzyme has been cloned and sequenced by de Vries et al. (15). The DNA sequence indicates the presence of an ORF of 1,539 bp predicted to encode a precursor protein of 513 aa residues. Of these amino acid residues, 26 residues at the NH2 terminus serve as the signal sequence for import into mitochondria. The Ndi1 enzyme is believed to be attached to the inner membranes on the matrix side. It is the main entry point into the respiratory chain in this organism, just as complex I is in mammalian mitochondria (16, 17). Should the Ndi1 enzyme replace the functionality of complex I in the mammalian systems, it would solve problems (i) and (iii) described above. As described previously (12), Ndi1 is a versatile enzyme because the Ndi1 enzyme expressed in Escherichia coli acts as a member of the respiratory chain in the prokaryotic host cells. In addition, on the basis of the observation that complex I-type enzymes and Ndi1-type enzymes coexist in bacteria, plant, and fungal mitochondria (16, 17), it was anticipated that complex I in mammalian mitochondria may not hamper the association of the Ndi1 type enzyme with the inner mitochondrial membranes. Therefore, a possible approach for coping with complex I defects is to introduce into mammalian mitochondria an Ndi1-type enzyme. It was of interest to attempt the functional expression of Ndi1 in complex I-deficient mammalian cells in the hope that this might provide an assessment of the capacity of the yeast NDI1 gene to be useful for repair of complex I defects in mammalian cells.
In this paper, we demonstrate that the S. cerevisiae NDI1 gene has been transcribed and translated in Chinese hamster cells. The expressed Ndi1 has been incorporated predominantly into mitochondria by the leader sequence of Ndi1, and it restored the NADH oxidase activity of the complex I-deficient Chinese hamster cell mutant (CCL16-B2), which was isolated from lung fibroblasts by Scheffler and coworkers (18–21). The restored NADH oxidase is insensitive to rotenone, but is sensitive to flavone, a specific inhibitor for the yeast Ndi1.
MATERIALS AND METHODS
Two oligonucleotide primers were employed. One was to generate a KpnI recognition site 60 bp upstream from the initiation codon of the NDI1 gene: 5′-TCAGGTAGGGTACCAGTT-3′ (the underlined bases were changed from S. cerevisiae DNA, and italic bases indicate the KpnI site). The other was to construct a BglII site 213 bp downstream from the termination codon of the NDI1 gene: 5′-AGTGATCAACAGATCTTG-3′ (the underlined bases were mutated from S. cerevisiae DNA, and italic bases show the BglII site). Using pRVS2.3‖ as the template, site-specific mutagenesis was carried out (22). The resulting plasmid was designated pRVS(KpnI, BglII). The pRVS(KpnI, BglII) construct was cut with KpnI and BglII, and the 1.9-kbp KpnI/BglII fragment containing the full-length NDI1 gene (1,539 bp) was ligated into the KpnI/BamHI site in the mammalian expression vector pHook-2** (Invitrogen). The resulting expression plasmid was designated pHook(NDI1). The construct was verified by DNA sequencing of both strands as described previously (23, 24).
The complex I-deficient CCL16-B2 mutant of Chinese hamster male lung fibroblasts was grown in DMEM supplemented with 10% fetal calf serum/25 mM glucose/50 μg/ml gentamycin. Cells were maintained at 37°C in a 5% CO2 atmosphere (19–21).
Complex I-deficient CCL16-B2 mutant cells (1 × 105) in 1 ml of DMEM containing 25 mM glucose and 10% fetal calf serum were cotransfected with 8–10 μg of pHook(NDI1) and 2 μg of pHook(LacZ) by a calcium-phosphate precipitation method (25). The transfected cell lines were isolated by screening with 0.5 mg/ml of antibiotic G-418. The NDI1-transfected CCL16-B2 cells can be grown in the DMEM + 10% fetal calf serum + 0.5 mg/ml G-418 medium in the presence of 0.6 mM glucose.
Preparation of crude mitochondrial fractions from the transfected and nontransfected cells (26), measurement of respiratory chain activities by digitonin-permeabilized cells (27), protein determination (12), SDS/PAGE (28), DNA sequence analyses (29), determination of DNA sequence (23), immunoblotting (5, 6), and immunofluorescence (30) were performed according to the references cited. Any variations from the procedures and other details are described in the figure legends. Prototype antibody specific to human mitochondria was a generous gift from Eng M. Tan (The Scripps Research Institute). This serum from the patient AMA with primary biliary cirrhosis reacted with the pyruvate dehydrogenase complex E2 subunit (70 kDa) and the 55-kDa polypeptide in human mitochondria (31).
RESULTS
Transfection of Complex I-Deficient Cells with the NDI1 Gene.
To investigate whether the yeast NDI1 gene can be functionally expressed in mammalian cells, we used complex I-deficient cells. Such mammalian cells have been reported by Scheffler and coworkers (Chinese hamster cells) (18–21) and Attardi and coworkers (human cells) (32, 33). We attempted to transfect the Chinese hamster complex I-deficient cell line CCL16-B2 with a full-length NDI1 gene (1,539 bp) inserted into pHook-2 vector. An initial experiment with transient transfection was only partially successful. However, the results were unclear because of a mixed population of transfected and nontransfected cells. Therefore, establishing stable cell lines was essential for performing reliable enzymatic activity assays and also for our ultimate goal of gene therapies. Several stable NDI1-transfected cell lines were established from single colonies after selection with the antibiotic G418. At present all isolated cell lines have exhibited the same properties as described below.
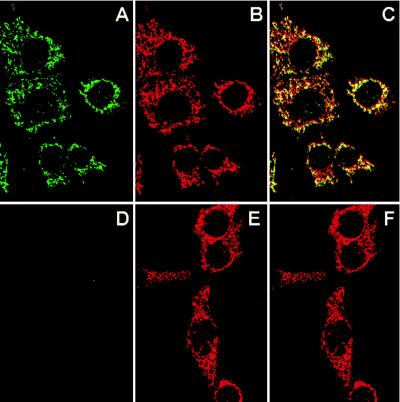
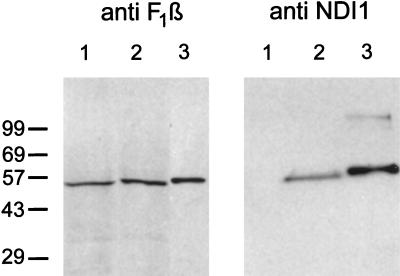
The NDI1-transfected CCL16-B2 cells were examined by confocal immunofluorescence microscopy by using anti-Ndi1 antibody and anti-human mitochondria antibody (Fig. 1). It is clear that the expressed Ndi1 is predominantly localized to mitochondria. In addition, the anti-human mitochondria antibody was recognized at the same location in the cells as the antibody to the β-subunit of bovine heart ATP synthase (data not shown). To provide additional evidence for the mitochondrial localization of the Ndi1 enzyme, we performed Western analyses (immunoblotting) of crude mitochondrial fractions from nontransfected and NDI1-transfected CCL16-B2 cells, using the affinity-purified antibodies to the S. cerevisiae Ndi1, and the β-subunit of bovine heart mitochondrial ATP synthase (Fig. 2). The antibodies to the β-subunit reacted with a single band (Mr = 55,000) of crude mitochondrial fractions of both CCL16-B2 cells. The antibody to the S. cerevisiae Ndi1 reacted with one band (Mr = 56,000) in the mitochondrial fraction of the NDI1-transfected cells, but this band was absent in the nontransfected cells. These results strongly suggest that the S. cerevisiae NDI1 gene was transcribed and translated in Chinese hamster CCL16-B2 cells. In addition, the precursor Ndi1 is imported into mammalian mitochondria, most likely by its leader sequence.
Figure 1.
Mitochondrial localization of the Ndi1 enzyme expressed in Chinese hamster CCL16-B2 cells as demonstrated by confocal immunofluorescence microscopy. NDI1-transfected (A, B, and C) and nontransfected (D, E, and F) CCL16-B2 cells were double-labeled with affinity-purified rabbit antibody to S. cerevisiae Ndi1 and human antimitochondria antibody. Secondary detecting reagents were fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (A and D) and rhodamine-conjugated goat anti-human IgG (B and E). (C and F) Overlapping images of A/B and D/E, respectively.
Figure 2.
Immunoblots of mitochondrial fractions from Chinese hamster CCL16-B2 cells by using antibodies against the Ndi1 protein of S. cerevisiae (Right) and the β-subunit of bovine ATP synthase (Left). In both blots, lane 1 is nontransfected cells (100 μg) and lane 2 is NDI1-transfected cells (100 μg). Lane 3 (Left) is bovine mitochondria (1.5 μg). Lane 3 (Right) is the Ndi1 protein (33 ng) containing the leader sequence, which was expressed in and isolated from E. coli. The crude mitochondrial fractions were prepared as follows. The NDI1-transfected and nontransfected cells (approximately 2 × 108 cells) were washed twice with PBS and harvested by trypsinization. The pellet was suspended in 2 ml of isolation buffer containing 210 mM mannitol/70 mM sucrose/1 mM EGTA/5 mM Hepes, pH 7.2/0.2 mM phenylmethanesulfonyl fluoride/0.5% fatty acid-free BSA. The cell suspensions were treated with 0.1 mg/ml of digitonin for 1 min on ice and homogenized in a Dounce homogenizer with a tight pestle (70–100 up/down strokes). The homogenate was centrifuged at 3,000 × g for 5 min at 4°C to remove unbroken cells and nuclei. The supernatant was centrifuged at 10,000 × g for 20 min at 4°C. The pellet was suspended in 0.1 ml of the isolation buffer. This fraction is designated as the crude mitochondrial fraction. Immunoblotting was performed by use of the enhanced chemiluminescence system (Amersham).
Electron Transfer Activities of the Transfected Cells.
To assess whether the yeast NDI1 gene can be useful for repairing complex I defects in mammalian cells, it is required to investigate whether the Ndi1 expressed in these cells is functionally active. Therefore, nontransfected and NDI1-transfected CCL16-B2 cells were assayed for respiratory chain activity. Respiration can be measured with intact cells (27). Digitonin, by binding to cholesterol in the eukaryotic plasma membrane, creates pores through which the soluble components of the cell can be released (34). Because the intracellular membranes have a cholesterol content substantially lower than the plasma membrane, the mitochondria, other cell organelles, and the cytoskeleton are left intact (27). Thus, the permeabilization with digitonin allows ready access by the respiratory substrates and inhibitors (glutamate, succinate, etc.) to be tested. It should be noted, however, that in this assay system added NADH does not contact complex I because NADH/NAD on the cytoplasmic side is unable to penetrate into the matrix. With malate or glutamate as a permeable substrate, the corresponding dehydrogenase generates NADH from NAD in the matrix compartment, which is oxidized by complex I. This assay procedure is reliable and is not disturbed by various diaphorases. In addition, the number of cells required for this assay is not excessive. Therefore, this type of experiment has been carried out by using nontransfected and NDI1-transfected CCL16-B2 cells and parent CCL16 cells.
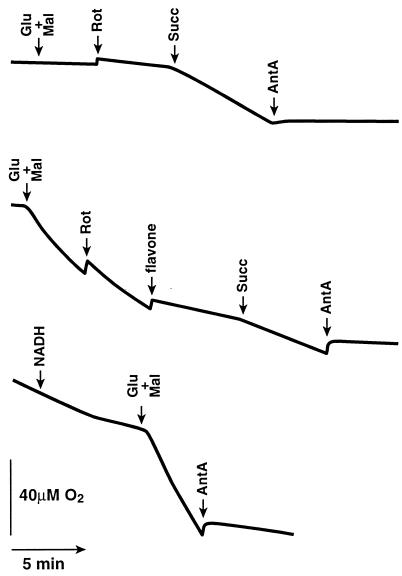
As shown in Fig. 3, the digitonin-permeabilized CCL16-B2 mutant cells did not show any appreciable oxygen consumption in the presence of added malate/glutamate. Rotenone did not affect the respiration. When succinate was added, the respiration was remarkably stimulated. This stimulation was completely inhibited by antimycin A and KCN (data not shown). These results are consistent with previously reported results using mitochondria isolated from CCL16-B2 cells (20). By contrast, the respiration in the digitonin-permeabilized NDI1-transfected CCL16-B2 cells was increased significantly by malate and glutamate, to a level comparable to those activities of the parental CCL16 cells (data not shown). This activity was insensitive to rotenone but sensitive to flavone, a specific inhibitor of the yeast Ndi1 (13), whereas the activities of the parental CCL16 cells are completely inhibited by rotenone as described previously (20). Furthermore, antimycin A and KCN also inhibited this respiration completely. These results indicated that the Ndi1 expressed in mitochondria of CCL16-B2 cells acts as an upstream member of their respiratory chain. In addition, NADH did not increase the respiration of the digitonin-permeabilized NDI1-transfected CCL16-B2 cells, suggesting that the NADH-binding site of the expressed Ndi1 faces the matrix compartment as in the yeast mitochondria. Therefore, it is likely that the signal sequence of the yeast Ndi1 functioned correctly in mammalian cells. This is, however, not surprising because some mammalian signal sequences have been reported to function in import of polypeptides into the yeast mitochondrial matrix phase and vice versa (35, 36).
Figure 3.
Recovery of the NADH oxidase activity in the CCL16-B2 cells transfected with the S. cerevisiae NDI1 gene. (Top) Nontransfected CCL16-B2 cells (1 × 107 cells). (Middle) NDI1-transfected CCL16-B2 cells (1 × 107 cells). (Bottom) NDI1-transfected CCL16-B2 cells (3 × 107 cells). Where indicated, 5 mM glutamate (Glu), 5 mM malate (Mal), 0.5 mM NADH, 5 μM rotenone (Rot), 5 mM succinate (Succ), 5 μM antimycin A (AntA), and 0.5 mM flavone were added. The cells were harvested by trypsinization and resuspended in 1 ml of a medium containing 20 mM Hepes, pH 7.1/250 mM sucrose/10 mM MgCl2. The cells were treated with 50–150 μg of digitonin until more than 90% of the cells are stained by trypan blue. The digitonin-treated cells were washed with the same medium. Oxygen consumption was measured polarographically in 0.6 ml of the buffer containing 20 mM Hepes, pH 7.1/250 mM sucrose/10 mM MgCl2 by using a Clark electrode in a water-jacketed chamber maintained at 37°C.
Effect of Glucose/Galactose on the Cell Growth.
CCL16-B2 mutant cells cannot grow if glucose is omitted from the medium, because cells are totally dependent on glycolysis due to complex I deficiency. When complex I-deficient CCL16-B2 cells are cultured in the presence of galactose instead of glucose, these cells died and detached from the culture plate overnight, because galactose cannot be utilized efficiently for glycolysis. Parent CCL16 cells can satisfy their energy requirement from respiration and oxidative phosphorylation. If complex I deficiency is complemented by the yeast NADH dehydrogenase, the phenotype of CCL16-B2 cells should become similar to that of the parent CCL16 cells and they should be able to utilize energy derived from oxidative phosphorylation for growth. Fig. 4 shows the effects of limiting the carbon source for glycolysis but not for respiration on cell growth of NDI1-transfected CCL16-B2. In the culture medium containing 5 mM glucose (Fig. 4A), NDI1-transfected CCL16-B2 cells can proliferate at rates similar to that of the parent CCL16 cells, whereas the number of CCL16-B2 cells increased for 1 day, but then declined sharply because of cell death. It has been demonstrated that the mutant cells utilize all available glucose during the first day and then become energy starved (21). Galactose is also a fermentable substrate, but the entry into glycolysis via the Leloir pathway is too slow to sustain the cells by glycolysis alone, and ATP has to be produced by oxidative phosphorylation. As anticipated, the NDI1-transfected cells now can grow at low glucose (0.6 mM) as well as parent CCL16 cells. Their growth is stimulated significantly by addition of 5 mM galactose (Fig. 4B). This stimulation by galactose is probably because the cells need a hexose as a carbon source to make ribose and deoxyribose. In contrast, nontransfected cells could not survive under either conditions (Fig. 4B). Taken together, it is apparent that expressed Ndi1 can change the physiological properties of CCL16-B2 cells from total dependence on glycolysis to utilization of respiration and glycolysis. In addition, the NADH-linked respiratory activities of the CCL16-B2 cells appear to be restored by the yeast NDI1 expression to the level of the parental CCL16 cells.
Figure 4.
Effects of glucose (A) and galactose (B) on cell growth of nontransfected and NDI1-transfected CCL16-B2 and parent CCL16. (A) Parent CCL16 cells (105) (□), nontransfected CCL16-B2 cells (▵), and NDI1-transfected CCL16-B2 cells (○) were inoculated in a 5-mM glucose culture medium. (B) Nontransfected (triangles) and NDI1-transfected cells (105) (circles) were inoculated in a 0.6-mM glucose culture medium in the presence (closed symbols) and absence (open symbols) of 5 mM galactose. In the case of NDI1-transfected CCL16-B2 cell culture, 0.5 mg/ml of antibiotic G418 also was present. Cells were cultured at 37°C in a 5% CO2 atmosphere. Cell viability was assessed by trypan blue exclusion, and cell numbers were determined every 24 hr by using a hemocytometer.
DISCUSSION
In a previous paper (12), we have reported that the S. cerevisiae Ndi1 enzyme overexpressed in E. coli acts as a member of the respiratory chain in the prokaryotic host cells, even though E. coli membranes are different from S. cerevisiae inner mitochondrial membranes with regard to quinones and lipid composition. Recognizing Ndi1 to be a versatile enzyme, we considered Ndi1 as a possible candidate for repair of complex I defects in mammalian cells. In this paper, we have demonstrated that the yeast NDI1 gene product has been expressed in mammalian mitochondria. Its function can complement the defect in complex I-deficient Chinese hamster CCL16-B2 cells by restoring a flavone-sensitive NADH oxidase activity, and the capacity for respiration. Sensitivity to antimycin A and KCN indicates that the NADH oxidase activity is coupled to the downstream portion of the mitochondrial electron transport chain (complexes III and IV). In contrast to the original mutant cells, CCL16-B2 expressing Ndi1 can grow in media with low glucose concentrations or with galactose replacing glucose. Both of those conditions require that cells can carry out oxidative phosphorylation. These results indicate that Ndi1 is an excellent candidate for molecular remedy of complex I defects in mammalian mitochondria. In addition, it should be noted that use of the yeast NDI1 gene applies to the complementation of structural and functional defects of complex I subunits encoded not only by mtDNA, but also by nuclear DNA. Although several problems remain to be solved before the use of S. cerevisiae NDI1 gene for this purpose (e.g., stability and level of expression, expression in nonproliferative cells, transfection efficiency, etc.), it may be possible to overcome these problems in the future owing to progress in research of mammalian expression vectors and safe, efficient transfection protocols for gene therapies (37). In addition, we expect that this type of strategy might be applicable for therapies of dysfunction of other enzyme complexes involved in oxidative phosphorylation.
Acknowledgments
We thank Drs. Simon de Vries (Delft University of Technology, The Netherlands) and Carla A. M. Marres for kindly providing plasmids carrying the S. cerevisiae NDI1, Prof. Youssef Hatefi for generously donating the rabbit antibody specific to the β-subunit of the bovine heart ATP synthase, John C. Hamel and Karina Lichtenstein for excellent technical assistance, and Drs. Takahiro Yano and Salvatore Di Bernardo for discussion. This work was supported by U.S. Public Health Service Grants R01DK53244 (to A.M.-Y. and T.Y.) and R01AI39645 (to E.K.L.C.) and an American Cancer Society grant (to I.E.S.). Computer facilities were supported by U.S. Public Health Service Grant M01RR00833 for the General Clinical Research Center. Synthesis of oligonucleotides and DNA sequencing were, in part, supported by the Sam & Rose Stein Endowment Fund. This is publication 11465-MEM from The Scripps Research Institute, La Jolla, CA.
ABBREVIATIONS
- Q
quinone
- complex I
mitochondrial proton-translocating NADH-Q oxidoreductase(s)
- Ndi1
internal rotenone-insensitive NADH-Q oxidoreductase in yeast mitochondria
Footnotes
A 5.5-kbp KpnI/PstI DNA fragment bearing the full-length NDI1 was excised from λ 7,056 (approximately 17 kbp S. cerevisiae DNA inserted) and ligated into KpnI/PstI-cut cloning vector pTZ19U. The resulting plasmid was designated pKP5.5. A 2.3-kbp SalI/EcoRV fragment containing the full-length NDI1 was again isolated from pKP5.5 and ligated into SalI/SmaI-cut cloning vector pTZ18U. The resulting plasmid was designated pRVS2.3.
pHook-2 uses the Rous sarcoma virus promoter to express and display a single-chain antibody against a specific hapten on the surface of transfected cells. The pHook-2 also contains the human immediate early cytomegalovirus promoter in the upstream of a multiple cloning site to express the gene of interest. This plasmid is useful for both of transient and stable transfection.
References
- 1.Buchanan S K, Walker J E. Biochem J. 1996;318:343–349. doi: 10.1042/bj3180343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomyn A, Mariottini P, Cleeter M W J, Ragan C I, Matsuno-Yagi A, Hatefi Y, Doolittle R F, Attardi G. Nature (London) 1985;314:591–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- 3.Chomyn A, Cleeter M W J, Ragan C I, Riley M, Doolittle R F, Attardi G. Science. 1986;234:614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- 4.Hatefi Y. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 5.Yano T, Chu S S, Sled’ V D, Ohnishi T, Yagi T. J Biol Chem. 1997;272:4201–4211. doi: 10.1074/jbc.272.7.4201. [DOI] [PubMed] [Google Scholar]
- 6.Takano S, Yano T, Yagi T. Biochemistry. 1996;35:9120–9127. doi: 10.1021/bi9605853. [DOI] [PubMed] [Google Scholar]
- 7.Yagi T, Yano T, Matsuno-Yagi A. J Bioenerg Biomembr. 1993;25:339–345. doi: 10.1007/BF00762459. [DOI] [PubMed] [Google Scholar]
- 8.Wallace D C. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 9.Robinson B H. Biochim Biophys Acta. 1993;1182:231–244. doi: 10.1016/0925-4439(93)90064-8. [DOI] [PubMed] [Google Scholar]
- 10.Shoffner J M, Wallace D C. Annu Rev Nutr. 1994;14:535–568. doi: 10.1146/annurev.nu.14.070194.002535. [DOI] [PubMed] [Google Scholar]
- 11.Chrzanowska-Lightowlers Z M A, Lightowlers R N, Turnbull D M. Gene Therapy. 1995;2:311–316. [PubMed] [Google Scholar]
- 12.Kitajima-Ihara T, Yagi T. FEBS Lett. 1998;421:37–40. doi: 10.1016/s0014-5793(97)01533-0. [DOI] [PubMed] [Google Scholar]
- 13.de Vries S, Grivell L A. Eur J Biochem. 1988;176:377–384. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]
- 14.Marres C A M, de Vries S, Grivell L A. Eur J Biochem. 1991;195:857–862. doi: 10.1111/j.1432-1033.1991.tb15775.x. [DOI] [PubMed] [Google Scholar]
- 15.de Vries S, Van Witzenburg R, Grivell L A, Marres C A M. Eur J Biochem. 1992;203:587–592. doi: 10.1111/j.1432-1033.1992.tb16587.x. [DOI] [PubMed] [Google Scholar]
- 16.Yagi T. J Bioenerg Biomembr. 1991;23:211–225. doi: 10.1007/BF00762218. [DOI] [PubMed] [Google Scholar]
- 17.Yagi T. Biochim Biophys Acta. 1993;1141:1–17. doi: 10.1016/0005-2728(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 18.Scheffler I E. J Cell Physiol. 1974;83:219–230. doi: 10.1002/jcp.1040830208. [DOI] [PubMed] [Google Scholar]
- 19.Ditta G, Soderberg K, Landy F, Scheffler I E. Somat Cell Genet. 1976;2:331–344. doi: 10.1007/BF01538838. [DOI] [PubMed] [Google Scholar]
- 20.DeFrancesco L, Scheffler I E, Bissell M. J Biol Chem. 1976;251:4588–4595. [PubMed] [Google Scholar]
- 21.Scheffler I M. In: Biochemical Genetics of Respiration-Deficient Mutants of Animal Cells. Morgan M J, editor. New York: Plenum; 1986. pp. 77–109. [Google Scholar]
- 22.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Matsuno-Yagi A, Yagi T. Biochemistry. 1991;30:6422–6428. doi: 10.1021/bi00240a012. [DOI] [PubMed] [Google Scholar]
- 25.Pari G S, Keown W A. Methods Mol Biol. 1997;62:301–306. doi: 10.1385/0-89603-480-1:301. [DOI] [PubMed] [Google Scholar]
- 26.Trounce I A, Kim Y L, Jun A S, Wallace D C. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 27.Hofhaus G, Shakeley R M, Attardi G. Methods Enzymol. 1996;264:476–483. doi: 10.1016/s0076-6879(96)64043-9. [DOI] [PubMed] [Google Scholar]
- 28.Yagi T. Biochemistry. 1987;26:2822–2828. doi: 10.1021/bi00384a025. [DOI] [PubMed] [Google Scholar]
- 29.Devereux J, Haeberli P, Smithies O. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan E K L, Tan E M. J Exp Med. 1987;166:1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung P S C, Coppel R L, Ansari A, Munoz S, Gershwin M E. Semin Liver Dis. 1997;17:61–69. doi: 10.1055/s-2007-1007183. [DOI] [PubMed] [Google Scholar]
- 32.Hofhaus G, Attardi G. EMBO J. 1993;12:3043–3048. doi: 10.1002/j.1460-2075.1993.tb05973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofhaus G, Attardi G. Mol Cell Biol. 1995;15:964–974. doi: 10.1128/mcb.15.2.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granger L D, Lehninger A L. J Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaya G, Kalousek F, Fenton W A, Rosenberg L E. J Cell Biol. 1991;113:65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beard S E, Goodman S I, Bemelen K, Frerman F E. Hum Mol Genet. 1995;4:157–161. doi: 10.1093/hmg/4.2.157. [DOI] [PubMed] [Google Scholar]
- 37.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]