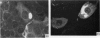
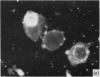
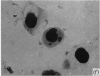
Abstract
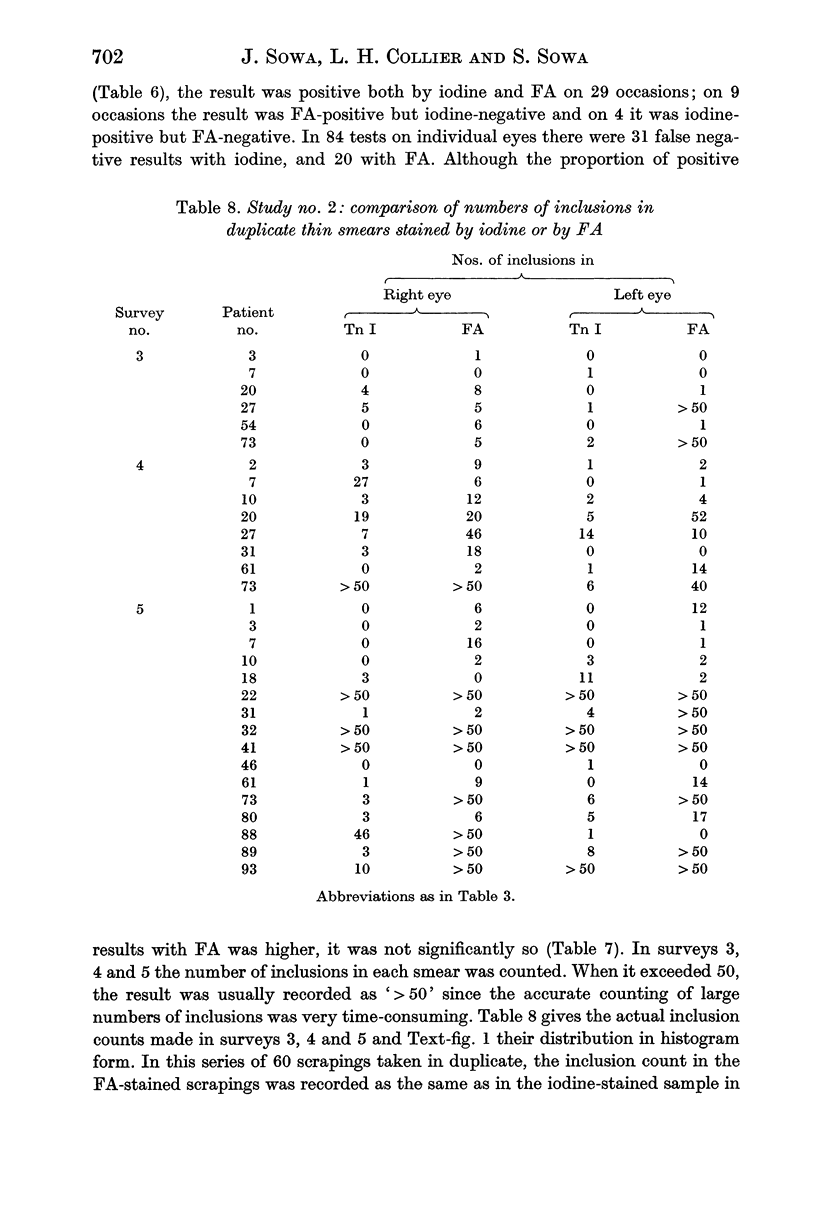
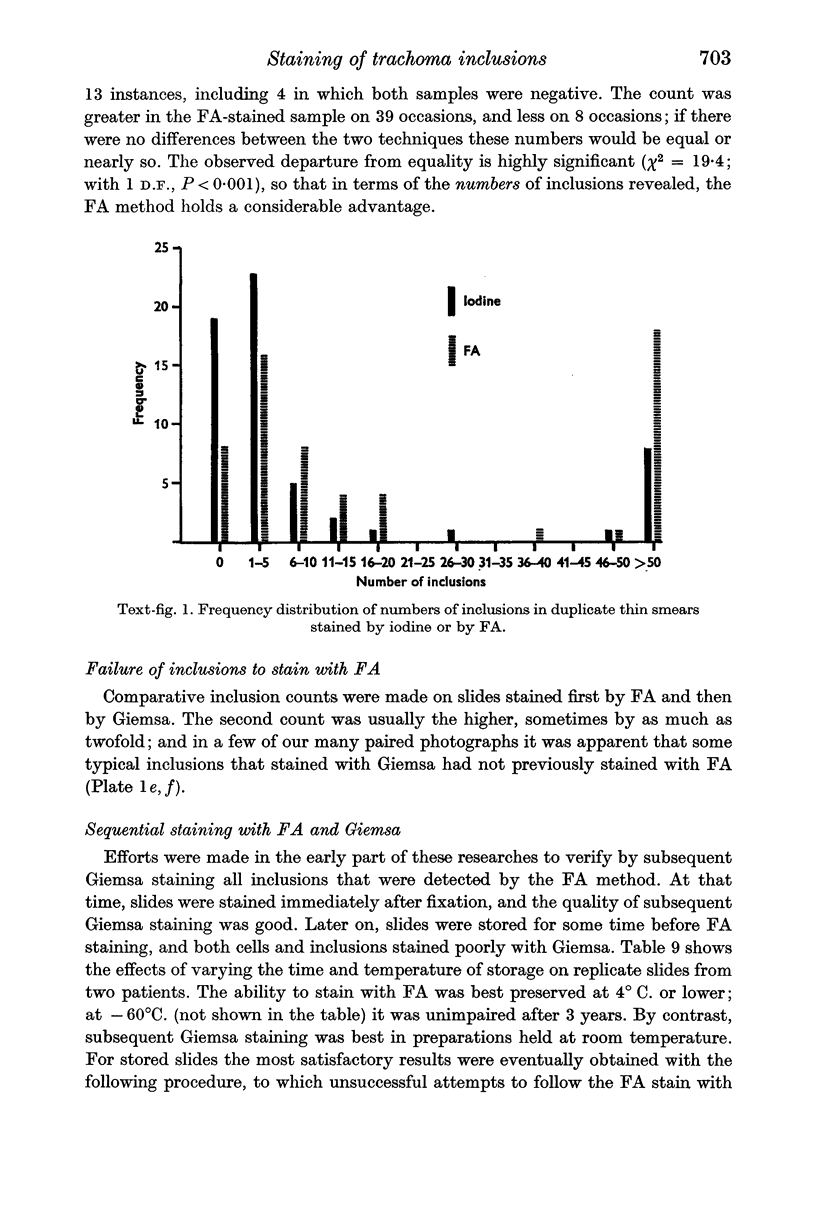
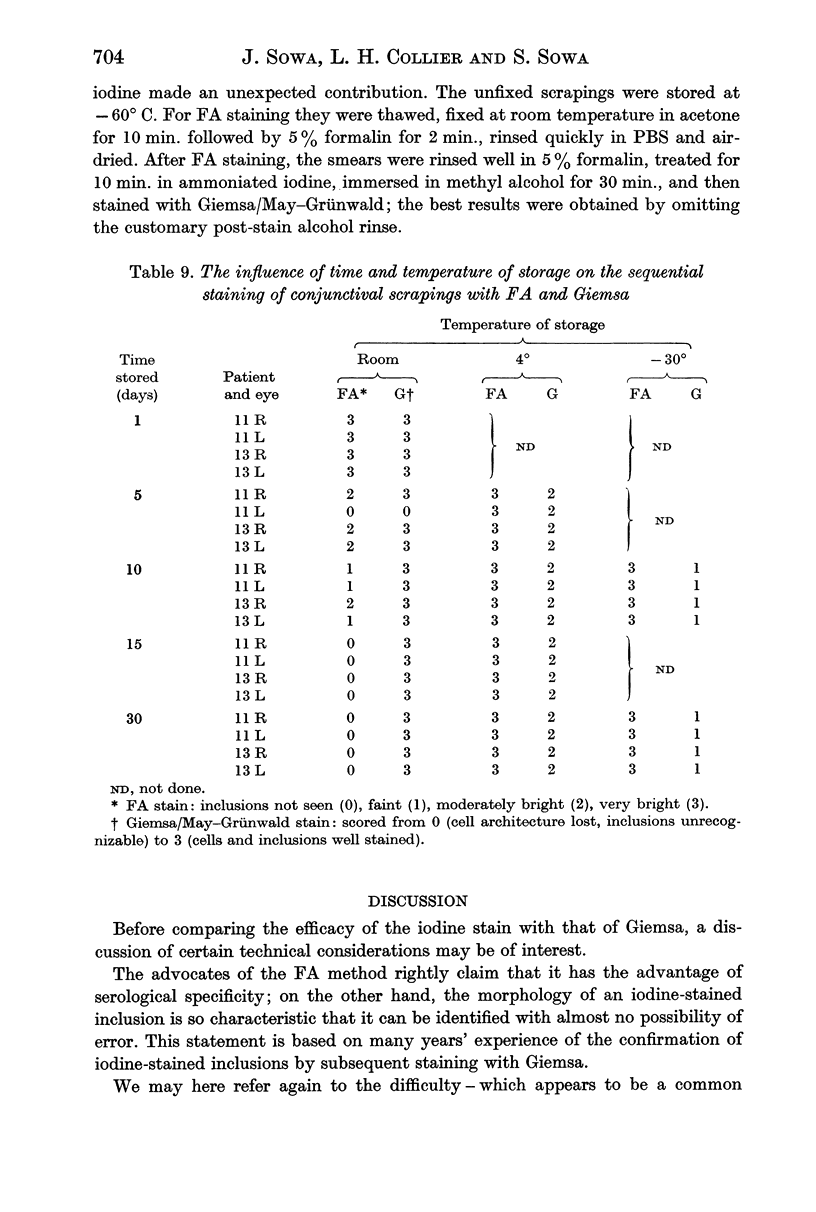
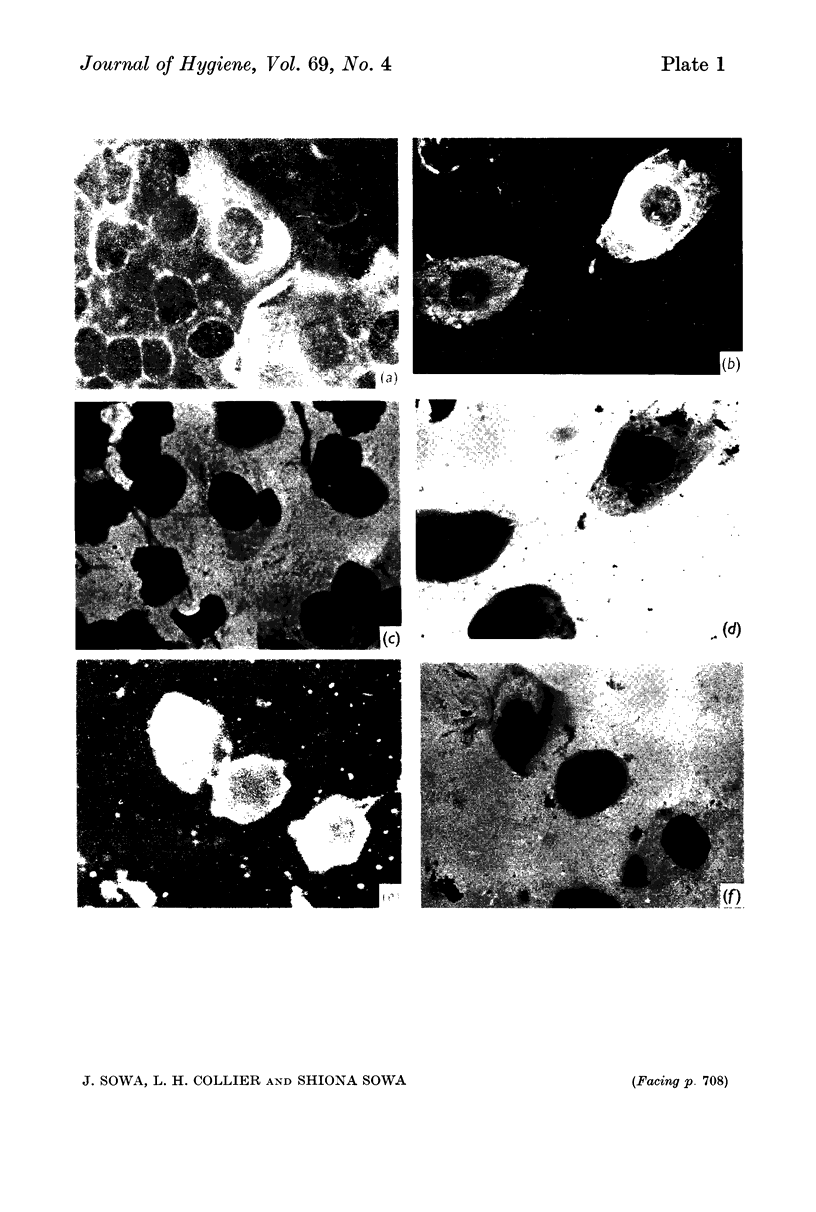
In terms of the rate of positive diagnoses the indirect fluorescent antibody (FA) test was rather more effective than iodine for demonstrating trachoma (TRIC) inclusions in conjunctival scrapings, but the degree of advantage was not statistically significant. In duplicate scrapings stained at random by one or the other method, FA staining yielded the higher inclusion count significantly more often than did iodine. Some inclusions that failed to stain with FA were found on subsequent staining with Giemsa. A method is described for improving the post-FA Giemsa staining of conjunctival smears stored at subzero temperatures. Given adequate facilities, the FA stain is preferable to iodine for demonstrating TRIC inclusions in the conjunctiva; but the iodine method, properly used, holds advantages for field use.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. R., Wang S. P., Grayston J. T. Further classification of TRIC agents from ocular trachoma and other sources by the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1469–1478. doi: 10.1016/0002-9394(67)94133-5. [DOI] [PubMed] [Google Scholar]
- BUCKLEY S. M., WHITNEY E., RAPP F. Identification by fluorescent antibody of developmental forms of psittacosis virus in tissue culture. Proc Soc Exp Biol Med. 1955 Oct;90(1):226–230. doi: 10.3181/00379727-90-21990. [DOI] [PubMed] [Google Scholar]
- Bell S. D., McComb D. E., Nichols R. L., Roca-Garcia M. Studies on trachoma. VII. Isolation of a mixture of type 1 and type 2. Trachoma strains from a child in Saudi Arabia. Am J Trop Med Hyg. 1970 Sep;19(5):842–845. [PubMed] [Google Scholar]
- COLLIER L. H., DUKE-ELDER S., JONES B. R. Experimental trachoma produced by cultured virus. Part II. Br J Ophthalmol. 1960 Feb;44:65–88. doi: 10.1136/bjo.44.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H., DUKE-ELDER S., JONES B. R. Experimental trachoma produced by cultured virus. Br J Ophthalmol. 1958 Dec;42(12):705–720. doi: 10.1136/bjo.42.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H. Experimental infection of baboons with inclusion blennorrhea and trachoma. Ann N Y Acad Sci. 1962 Mar 5;98:188–196. doi: 10.1111/j.1749-6632.1962.tb30543.x. [DOI] [PubMed] [Google Scholar]
- COLLIER L. H. Experiments with trachoma vaccines. Experimental system using inclusion blennorrhoea virus. Lancet. 1961 Apr 15;1(7181):795–800. doi: 10.1016/s0140-6736(61)90119-2. [DOI] [PubMed] [Google Scholar]
- COLLIER L. H., SOWA J. Isolation of trachoma virus in embryonate eggs. Lancet. 1958 May 10;1(7028):993–996. doi: 10.1016/s0140-6736(58)91800-2. [DOI] [PubMed] [Google Scholar]
- Collier L. H., Blyth W. A. Immunogenicity of experimental trachoma vaccines in baboons. I. Experimental methods, and preliminary tests with vaccines prepared in chick embryos and in HeLa cells. J Hyg (Lond) 1966 Dec;64(4):513–528. doi: 10.1017/s0022172400040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier L. H., Blyth W. A. Immunogenicity of experimental trachoma vaccines in baboons. II. Experiments with adjuvants, and tests of cross-protection. J Hyg (Lond) 1966 Dec;64(4):529–544. doi: 10.1017/s0022172400040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier L. H., Sowa S., Sowa J. TRIC-agent neonatal conjunctivitis. Lancet. 1969 Jan 11;1(7585):101–101. doi: 10.1016/s0140-6736(69)91114-3. [DOI] [PubMed] [Google Scholar]
- Collier L. H. The immunopathology of trachoma: some facts and fancies. Arch Gesamte Virusforsch. 1967;22(1):280–293. doi: 10.1007/BF01240523. [DOI] [PubMed] [Google Scholar]
- Collier L. H. The use of graded density filters made by photography in fluorescence microscopy. J Pathol Bacteriol. 1968 Jul;96(1):238–242. doi: 10.1002/path.1700960130. [DOI] [PubMed] [Google Scholar]
- Dawson C. R., Hanna L., Jawetz E. Controlled treatment trials of trachoma in American Indian children. Lancet. 1967 Nov 4;2(7523):961–964. doi: 10.1016/s0140-6736(67)90796-9. [DOI] [PubMed] [Google Scholar]
- GILKES M. J., SMITH C. H., SOWA J. Staining of the inclusion bodies of trachoma and inclusion conjunctivitis. Br J Ophthalmol. 1958 Aug;42(8):473–477. doi: 10.1136/bjo.42.8.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNA L., OKUMOTO M., THYGESON P., ROSE L., DAWSON C. R. TRIC AGENTS ISOLATED IN THE UNITED STATES. X. IMMUNOFLUORESCENCE IN THE DIAGNOSIS OF TRIC AGENT INFECTION IN MAN. Proc Soc Exp Biol Med. 1965 Jul;119:722–728. doi: 10.3181/00379727-119-30282. [DOI] [PubMed] [Google Scholar]
- Hanna L. An evaluation of the fluorescent antibody technic in the diagnosis of trachoma and inclusion conjunctivitis. Rev Int Trach. 1968;45(4):345–359. [PubMed] [Google Scholar]
- JONES B. R., COLLIER L. H., SMITH C. H. Isolation of virus from inclusion blennorrhoea. Lancet. 1959 May 2;1(7079):902–905. doi: 10.1016/s0140-6736(59)91307-8. [DOI] [PubMed] [Google Scholar]
- Jawetz E., Hanna L., Dawson C., Wood R., Briones O. Subclinical infections with TRIC agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1413–1426. doi: 10.1016/0002-9394(67)94125-6. [DOI] [PubMed] [Google Scholar]
- McComb D. E., Bell S. D., Jr The application of an in vitro typing test to TRIC Bedsoniae. Am J Ophthalmol. 1967 May;63(5 Suppl):1429–1437. doi: 10.1016/0002-9394(67)94127-x. [DOI] [PubMed] [Google Scholar]
- NICHOLS R. L., MCCOMB D. E. SEROLOGIC STRAIN DIFFERENTIATION IN TRACHOMA. J Exp Med. 1964 Oct 1;120:639–654. doi: 10.1084/jem.120.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS R. L., McCO MB D. E., HADDAD N., MURRAY E. S. Studies on trachoma. II. Comparison of fluorescent antibody, giemsa, and egg isolation methods for detection of trachoma virus in human conjunctival scrapings. Am J Trop Med Hyg. 1963 Mar;12:223–229. [PubMed] [Google Scholar]
- NICHOLS R. L., McCOMB D. E. Immunofluorescent studies with trachoma and related antigens. J Immunol. 1962 Oct;89:545–554. [PubMed] [Google Scholar]
- Nichols R. L., Bobb A. A., Haddad N. A., McComb D. E. Immunofluorescent studies of the microbiologic epidemiology of trachoma in Saudi Arabia. Am J Ophthalmol. 1967 May;63(5 Suppl):1372–1408. doi: 10.1016/0002-9394(67)94123-2. [DOI] [PubMed] [Google Scholar]
- REEVE P., TAVERNE J. Observatios on the growth of trachoma and inclusion blennorrhoea viruses in embryonate eggs. J Hyg (Lond) 1963 Mar;61:67–75. doi: 10.1017/s0022172400020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa S., Sowa J., Collier L. H., Blyth W. A. Trachoma vaccine field trials in The Gambia. J Hyg (Lond) 1969 Dec;67(4):699–717. doi: 10.1017/s0022172400042157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa S., Sowa J., Collier L. H. Investigation of neonatal conjunctivitis in the Gambia. Lancet. 1968 Aug 3;2(7562):243–247. doi: 10.1016/s0140-6736(68)92352-0. [DOI] [PubMed] [Google Scholar]
- VOZZA R., BALDUCCI D. The technique of fluorescent antibodies in opthalmology. A study of herpes simplex and vaccine keratoconjunctivitis and human trachomatous infection. Am J Ophthalmol. 1961 Jul;52:72–77. [PubMed] [Google Scholar]