Abstract
A Ca2+-pump ATPase, similar to that in the endoplasmic reticulum, has been located on the outer membrane of rat liver nuclei. The effect of cAMP-dependent protein kinase (PKA) on nuclear Ca2+-ATPase (NCA) was studied by using purified rat liver nuclei. Treatment of isolated nuclei with the catalytic unit of PKA resulted in the phosphorylation of a 105-kDa band that was recognized by antibodies specific for sarcoplasmic reticulum Ca2+-ATPase type 2b. Partial purification and immunoblotting confirmed that the 105-kDa protein band phosphorylated by PKA is NCA. The stoichiometry of phosphorylation was 0.76 mol of phosphate incorporated/mol of partially purified enzyme. Measurement of ATP-dependent 45Ca2+ uptake into purified nuclei showed that PKA phosphorylation enhanced the Ca2+-pumping activity of NCA. We show that PKA phosphorylation of Ca2+-ATPase enhances the transport of 10-kDa fluorescent-labeled dextrans across the nuclear envelope. The findings reported in this paper are consistent with the notion that the crosstalk between the cAMP/PKA- and Ca2+-dependent signaling pathways identified at the cytoplasmic level extends to the nucleus. Furthermore, these data support a function for crosstalk in the regulation of calcium-dependent transport across the nuclear envelope.
It is well established that changes in cytosolic Ca2+ play a central role in the regulation of numerous cytosolic functions. More recently, it has become apparent that Ca2+ also is involved in the control of key nuclear events (for a review see ref. 1). Confocal imaging of intracellular Ca2+ in single cells with fluorescent indicators, as well as the use of electrophysiological techniques, have confirmed that free Ca2+ in the nucleus is regulated independently from free Ca2+ in the surrounding cytoplasm. Furthermore, at least four Ca2+ transporting systems, allowing for an autonomous regulation of Ca2+ levels, have been identified on the nuclear envelope (NE). These include intracellular receptors for Ca2+ signaling molecules such as inositol 1,4,5-trisphosphate (2), inositol 1,3,4,5-tetrakisphosphate (3), and cyclic adenosine diphosphate ribose (4), as well as an ATP-dependent calcium uptake mechanism driven by a sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) corresponding to the SERCA2b isoform (5) that has been located on the outer nuclear membrane (6). A model for the control of nuclear Ca2+ has been proposed, in which the NE lumen plays the role of a dynamic Ca2+ pool (1, 7).
The autonomous regulation of nuclear free Ca2+ has been shown to subserve specific functions. In particular, nuclear Ca2+ is involved in the regulation of gene expression (1, 8, 9). Another function for nuclear Ca2+ is the regulation of protein transport into the nucleus. It has been shown that both nuclear localization signal (NLS)-mediated (10, 11) and NLS-independent (10, 12) protein uptake are regulated by the free Ca2+ concentration in the lumen of the NE.
In the cytoplasm crosstalk between Ca2+- and cAMP-dependent pathways has been well established (13). The cAMP/cAMP-dependent protein kinase (PKA) pathway signals to the nucleus (14, 15). PKA phosphorylates the nuclear transcription factor CREB (cAMP-response element binding protein). Emerging evidence indicates that CREB is also a critical mediator of Ca2+-dependent gene expression (9). It is phosphorylated in response to increased levels of free Ca2+ levels. Ca2+ calmodulin-dependent protein kinase appears to be one of the Ca2+-dependent CREB kinases. Hardingham et al. (8) have shown that CREB functions as a transcription factor responsive to nuclear free Ca2+ levels.
In the study reported here, we have investigated how crosstalk between Ca2+- and cAMP-dependent signaling pathways regulates the transport of macromolecules into the nucleus. Such protein transport not only depends on the free Ca2+ concentration in the lumen of the NE, additionally it is modulated by PKA (16). PKA influences NLS-mediated transport either by direct phosphorylation of the imported protein or through less direct mechanisms (17, 18). PKA also is implicated in the regulation of NLS-independent nuclear protein uptake (19). The phosphorylation of SERCA by PKA at the level of the endoplasmic reticulum (ER) (20–22) suggests that, likewise, nuclear Ca2+-ATPase (NCA) may be a possible target for PKA. Thus, we studied the effect of PKA on NCA and on Ca2+-dependent NLS-independent macromolecule transport. We show that treatment of isolated rat liver nuclei with the catalytic subunit of PKA results in phosphorylation of NCA. We also report that PKA phosphorylation of NCA leads to stimulation of ATP-dependent Ca2+-uptake into the nucleus.
MATERIALS AND METHODS
Materials.
[γ-32P]ATP (specific activity 3,000 Ci/mmol) and 45Ca2+ were obtained from Amersham-Pharmacia. The catalytic subunit of PKA, anti-rabbit IgG antibodies, reactive red-120 agarose, adenyl 5′-yl imidodiphosphate (AMP-PNP), polyoxyethylene 9-lauryl ether (C12E9), and thapsigargin were purchased from Sigma. Okadaic acid was from Calbiochem. Rabbit polyclonal antibodies against SERCA2b were a kind gift from F. Wuytack (University of Leuven, Belgium). Calcium Green-1 (10 kDa and 500 kDa) and lucifer yellow (10 kDa) fluorescent dextrans were purchased from Molecular Probes.
Purification of Rat Liver Nuclei and Preparation of Nuclear Extracts.
Rat liver nuclei were prepared as described (23). The isolated nuclei were suspended in a medium containing 0.25 M sucrose, 4.0 mM MgCl2, and 50 mM Tris⋅HCl, pH 7.5 (buffer A). Final nuclear preparation was free from any microsomal or plasma membrane constituents as tested by the marker enzyme activity (23, 26) and electron microscopy (6). For certain experiments, the final nuclear preparation was suspended at a protein concentration of 10 mg/ml in buffer A supplemented with 150 mM NaCl, 20% glycerol, 2% Triton X-100, 10 μg/ml of leupeptin, and 10 μg/ml of aprotinin. This suspension was allowed to stand 2 hr on ice while stirring gently and centrifuged for 30 min at 12,000 × g. The supernatant constituted the nuclear extract. Protein was determined according to Bradford (24).
Partial Purification of NCA.
The nuclear Ca2+-pump ATPase was partially purified by using reactive red 120-agarose affinity chromatography as described by Coll and Murphy (25). Purified rat liver nuclei at a final concentration of 2 mg/ml of protein were solubilized by using buffer A containing 20 mg/ml of C12E9 (buffer B). Protein was eluted with buffer B containing 400 μM AMP-PNP followed by buffer B supplemented with 2 M NaCl. Ca2+ was omitted from the purification procedure to minimize stimulation of nuclear calpains (26). Møller et al. (27) have reported that the ATPase activity of the solubilized enzyme at low Ca2+ concentrations is partially inactivated. Therefore, the elution profile of NCA was monitored by Western blotting with antibodies specific for SERCA2b. For the determination of Ca2+-ATPase activity, partial purification was performed in the presence of 1 mM CaCl2 and 2 mM phenylmethylsulfonyl fluoride, followed by gel filtration using Sephadex G-50 fine to eliminate AMP-PNP and NaCl.
Ca2+-ATPase Assay.
Ca2+-stimulated ATPase activity was determined by measuring ATP hydrolysis as described by Nelson (28). Basal ATPase activity, measured in the absence of added calcium with 4 mM EGTA, was subtracted from ATP hydrolysis in calcium buffer to yield Ca2+-dependent ATPase activity.
Phosphorylation of Ca2+-ATPase.
Phosphorylation of NCA was carried out at the indicated times in 50 μl of a medium containing 50 mM Tris⋅HCl, pH 7.5, 10 mM MgCl2, and 20 units of PKA catalytic subunit. For nonpurified NCA, isolated nuclei corresponding to 16 μg of protein or 25 μg of nuclear extract protein were used. Protein phosphatase (PP) inhibitors (0.1 μM okadaic acid and 5 mM NaF) were added (29) because PP type 1, which is regulated by PKA (30), is present in considerable amounts in rat liver nuclei (31) together with PP-2C. In some phosphorylation experiments 2 mM EGTA, 0.1 μM CaCl2, (or 10 μM CaCl2), 1 μM thapsigargin, or 50 μM trifluoperazine were included as indicated. To check the degree of endogenous phosphorylation, some nuclear samples were preincubated with 1 unit of alkaline phosphatase/200 μg of protein at 37°C for 10 min. After incubation alkaline phosphatase was removed by placing one volume of sample onto one volume of 50% glycerol and centrifuged at 2,500 × g for 10 min. The pellet was rinsed five times with 1 ml of buffer A. In another set of experiments nuclei were permeabilized with 0.1% digitonin treatment for 30 min on ice, followed by centrifugation on glycerol and rinsing as described above.
For phosphorylation of the partially purified nuclear Ca2+-pump, pooled fractions corresponding to peak I and peak II were concentrated by placing them in dialysis bags, which then were covered with dry polyethylene glycol (7–9 kDa) overnight at 4°C. Phosphorylated proteins were separated on SDS/PAGE (10% polyacrylamide) followed by autoradiography and densitometric analysis.
Determination of Phosphate/Protein Stoichiometry.
Protein contained in the 105-kDa band from nuclear extract or partially purified peak I fraction was estimated by silver nitrate staining of gels and densitometric scanning using BSA as internal standard. 32P incorporated into the same 105-kDa band (corresponding to the autoradiogram) was excised from the gel and digested with H2O2 overnight.
Western Blotting.
Immunoblotting was performed as described elsewhere (32). Immune complexes were detected by using a 5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt/nitroblue tetrazolium visualization solution followed by densitometric scanning of the immunoblots. The antisera used in this study do not immunoprecipitate Ca2+-ATPase. In certain experiments, partialy purified peak I and peak II NCA was concentrated and phosphorylated by PKA in the presence of [γ-32P]ATP as described above. 32P-labeled NCA then was subjected to SDS/PAGE and detected by immunoblotting followed by autoradiography of the same immunoblot according to the method of Sarkadi et al. (33).
45Ca2+ Uptake Studies.
Isolated nuclei were suspended in a calcium uptake medium containing 0.25 M sucrose, 2 mM EDTA, 2 mM EGTA, 4 mM K2HPO4, 4 mM MgCl2, 50 mM Tris⋅HCl, pH 7.5, 1 mM ATP, and various amounts of CaCl2 as determined by Fabiato (34) to give the desired concentrations of external free Ca2+. Traces of 45Ca2+ (2 μCi/ml) were added, and 45Ca2+ uptake was monitored (6).
Assessment of Nucleocytoplasmic Transport.
Transport of intermediate-sized molecules into the nucleus was measured as described (4, 12). Nuclei were loaded with fluorescent dye-coupled dextrans by incubation for 10 min at 37°C with the probe in uptake medium supplemented with varying concentrations of free Ca2+ as described (6). The fluorescent probes (20 μm) used were the 10- and 500-kDa forms of Calcium Green-1 dextran and also the 10-kDa dextran form of the Ca2+-insensitive dye Lucifer yellow. After this step, the nuclei were rinsed by adding to each tube 50 volumes of uptake medium supplemented with the same free Ca2+ concentration as that used for loading, followed by centrifugation for 1 min at 700 × g. The pellet was gently resuspended in 100 μl of Ca2+-deprived uptake medium. Fifty microliters (≈50 μg protein) of this nuclear suspension were deposited on a collagen-coated modified Petri dish (i.e., with the base replaced by glass) and covered with a glass coverslip. Nuclei were allowed to adhere to the Petri dish (20 min) before photographing. Calcium Green-1 fluorescent dextrans were visualized by using a Zeiss LSM-410 laser scanning confocal microscope with an argon-laser light source tuned to λex ≈ 488 nm. Background fluorescence values were subtracted. Pixel values were averaged from within the nucleus and divided by the average pixel value of the bath, and results were expressed as means ± SEM. Ten-kilodalton dextran-coupled Lucifer yellow (maximum λex ≈ 425 nm) was visualized by using classical fluorescence microscopy (Nikon Diaphot TMD-EF).
RESULTS
Phosphorylation of Ca2+-ATPase in Isolated Intact Nuclei with PKA.
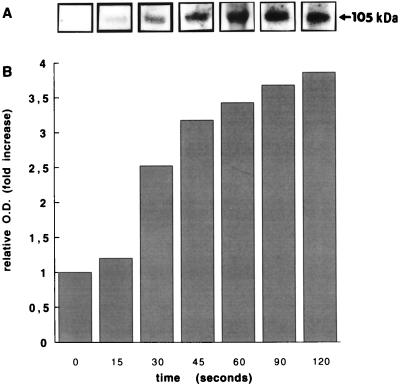
The purity of the nuclear preparation was checked by analyzing marker enzymes (Table 1) and by electron microscopy as described previously (2, 6, 23). Incubation of these nuclei in the phosphorylation medium containing [γ-32P]ATP and the catalytic subunit of PKA resulted in the appearance of several distinct phosphorylated protein bands after SDS/PAGE (data not shown). Among the proteins that underwent significant PKA-mediated phosphorylation was a substrate of molecular mass 105 kDa. The appearance of this band was time dependent, reaching a maximum within 2 min (Fig. 1).
Table 1.
Marker enzyme activity
| Marker enzymes | Liver homogenate
|
Nuclei
|
||
|---|---|---|---|---|
| Sp. act. | Total act. | Sp. act. | Total act. | |
| NAD pyrophosphorylase | 4.04 | 27.3 | 24.3 | 7.06 |
| Mannose-6-phosphatase | 93.3 | 628.0 | 332.0 | 122.0 |
| NADPH cytochrome c reductase | 9.5 | 57.0 | 3.2 | 0.8 |
| 5′-Nucleotidase | 4.1 | 20.5 | 0.21 | 0.04 |
| Cytochrome c oxidase | 12.0 | 60.0 | 0.03 | 0.066 |
NADPH cytochrome c reductase activity was determined by monitoring the reduction of cytochrome c at 550 nm. NAD pyrophosphorylase activity was determined by monitoring the formation of NADH at 340 nm. Mannose-6-phosphatase activity was determined by measuring the absorbance at 730 nm. 5′-Nucleotidase activity, cytochrome c oxidase activity, and NAD pyrophosphorylase activity were determined as described by Masmoudi et al. (23), Malviya et al. (2), and Humbert et al. (6), respectively. Specific activity (sp. act.) is expressed as nmol/min per mg of protein, and the total activity is expressed as μmol/min.
Figure 1.
Time-dependent phosphorylation of NCA. Isolated nuclei were incubated with the catalytic unit of PKA (20 units) for the indicated times as described in Materials and Methods. (A) Representative autoradiogram; the arrow indicates the migration of the 105-kDa protein band. (B) Densitometric analysis of the same autoradiogram.
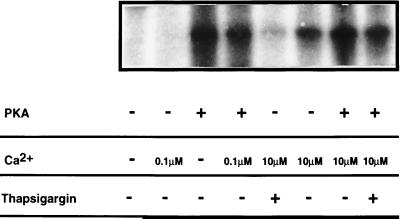
To further characterize the 105-kDa protein band, the phosphorylation reaction was performed under different conditions. First, we studied the effect of incubating purified nuclei with [γ-32P]ATP in the absence of PKA. Under these conditions, if Ca2+ at 10 μM was added, 105-kDa band also was observed (Fig. 2). This band was not affected by adding the calmodulin inhibitor trifluoperazine at a concentration of 50 μM (data not shown), confirming that it is not caused by activation by Ca2+ of endogenous nuclear calmodulin-dependent protein kinase (1). Furthermore, the 105-kDa protein band was sensitive to 1 μM thapsigargin, which strongly inhibited its appearance (Fig. 2) and no phosphorylation was seen if the Ca2+ concentration was decreased to low levels (0.1 μM). These results suggest that the 105-kDa band observed in the absence of PKA corresponds to the formation of a Ca2+-dependent phosphoenzyme intermediate. In the presence of PKA, if 10 μM Ca2+ also was added, the intensity of the 105-kDa protein band was slightly higher than with PKA alone. When 10 μM Ca2+ and 1 μM thapsigargin both were added, the 105-kDa band intensity was the same as that observed with PKA alone (Fig. 2). Therefore, the 105-kDa band induced by incubation with PKA is not because of the formation of a phosphoenzyme intermediate (discussed further below).
Figure 2.
Characterization of NCA phosphorylation. Phosphorylation of isolated nuclei was carried out as described in the legend to Fig. 1. The various conditions used are described in Materials and Methods.
Immunoblotting of Ca2+-ATPase Present in Isolated Nuclei.
Based on its apparent molecular mass and the formation of a thapsigargin-sensitive phosphorylated intermediate, the 105-kDa PKA substrate was identified as NCA. This finding was further confirmed by immunoblotting with antibodies specific for SERCA2b, which recognized a 105-kDa band (data not shown).
Partial Purification of Nuclear 45Ca2+-ATPase.
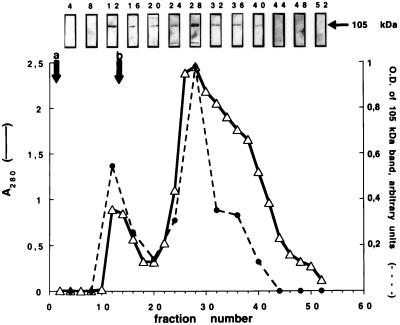
To definitely confirm that the 105-kDa immunoreactive and PKA-phosphorylated bands were indeed identical, we partially purified the nuclear Ca2+-pump ATPase by using reactive red-120 agarose affinity chromatography. Elution of the solubilized nuclear material adsorbed to reactive red 120 agarose with AMP-PNP yielded a peak that contained a single recognizable protein band by SERCA2b antibodies at the expected molecular mass of 105 kDa (Fig. 3). NaCl (2 M) was required for complete elution, thus yielding a second peak that also was enriched in a prominent band at 105 kDa (Fig. 3). In some of the peak II fractions a second immunoreactive species of lower apparent molecular mass was detected, probably corresponding to a proteolytic fragment of NCA. Peak II corresponded to the major protein peak as evidenced by absorbance at 280 nm.
Figure 3.
Partial purification of NCA by affinity chromatography. Reactive red-120 agarose column affinity chromatography was performed as described in Materials and Methods. NCA elution was monitored by immunoblot analysis of aliquots taken from every fourth eluted fraction by using antibodies specific for SERCA2b. A 105-kDa band was observed, which was analyzed by densitometric scanning of the immunoblots (broken line). Two bands were detected in certain fractions corresponding to peak II. Elution of proteins was measured by absorbance at 280 nm (A280, solid line).
Measurement of Ca2+-ATPase activity confirmed that this single-step partial-purification procedure yielded a significantly enriched and active preparation (Table 2). By itself detergent solubilization of nuclei with C12E9 increased specific activity, presumably because of removal of insoluble material by centrifugation. The most significant enrichment in specific activity occurred in peak I fractions, with an increase of more than 150-fold as compared with isolated nuclei.
Table 2.
Summary of partial purification of NCA
| Purification step | Total protein, mg | Specific activity, fmol/mg per min | Total activity, fmol/min | Yield, % |
|---|---|---|---|---|
| Isolated nuclei | 56 | 12.9 | 722 | 100 |
| supernatant | 9.8 | 63 | 617 | 85.5 |
| Peak I | 0.17 | 2,020 | 343 | 47.5 |
| Peak II | 0.75 | 242.9 | 182.2 | 25.2 |
Ca2+-ATPase activity was determined by measuring Pi produced by ATP hydrolysis. For peak I (fractions 9-17 pooled) and peak II (fractions 24-36 pooled), partial purification was performed in the presence of 1 mM CaCl2 and 2 mM phenylmethylsulfonyl fluoride, followed by gel-filtration of pooled fractions using Sephadex G-50 fine to eliminate AMP-PNP and NaCl. Blanks were estimated in the same way except that no protein was added, and values (expressed in fmol/mg per min) were corrected accordingly. Addition of trichloroacetic acid before addition of ATP yielded Pi counts equal to blanks. Basal ATPase activity (with 2 mM EGTA and without added calcium) was subtracted from ATP hydrolysis in calcium buffer to yield Ca2+-dependent ATPase activity.
That the immunoreactive and the PKA-phosphorylated 105-kDa bands were the same was confirmed by simultaneous immunoblotting and autoradiography of partially purified NCA. Concentrated samples from pooled peaks I and II were phosphorylated with PKA and separated on 10% SDS/PAGE followed by immunoblotting with anti-SERCA2b (data not shown). In control experiments performed in the absence of PKA, 32P incorporation into peak I or peak II partially purified NCA was barely detectable (data not shown).
Stoichiometry of NCA Phosphorylation by PKA.
The stoichiometry of phosphorylation of NCA by PKA was calculated to be 0.28 mol of phosphate incorporated/mol NCA when nonpurified intact nuclei were used and 0.76 mol of phosphate incorporated/mol for partially purified peak I NCA. The stoichiometry of 0.30 was observed when nuclei were digitonin-permeabilized before phosphorylation. After alkaline phosphatase treatment of isolated nuclei and removal of excess of phosphatase, PKA phosphorylation gave a stoichiometry of 0.81.
Effect of PKA Phosphorylation on ATP-Dependent Ca2+-Uptake by Isolated Nuclei.
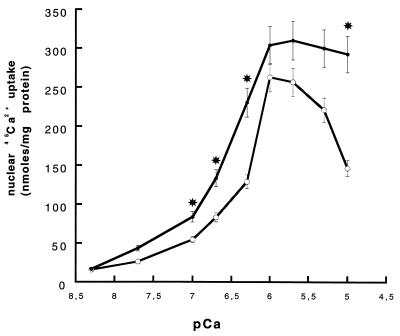
Ca2+ uptake was Ca2+ dependent (Fig. 4) and sensitive to thapsigargin. ATP-dependent Ca2+ uptake was enhanced in nuclei preincubated with PKA (Fig. 4). Stimulation of Ca2+-pumping activity was already apparent at low ionized Ca2+ concentrations. The Ca2+ concentrations required for half-maximal stimulation of Ca2+ uptake were increased 0.4 pCa units from pCa ≈ 6.3 under control conditions to pCa ≈ 6.7 after phosphorylation. Differences were statistically significant for pCa = 7.0 (P < 0.05, u test) and remained significant at higher free Ca2+ concentrations until pCa = 6.0. Maximum Ca2+ uptake was reached at pCa ≈ 6.0 for control nuclei and at pCa ≈ 5.7 for nuclei preincubated with PKA phosphorylation medium. Effect of PKA phosphorylation was dose dependent, becoming apparent only at concentrations of PKA catalytic subunit ≥100 milliunits and reaching maximum levels for 500 milliunits (data not shown).
Figure 4.
Effect of PKA phosphorylation on ATP-dependent 45Ca2+-transport into intact isolated nuclei. Isolated nuclei before (○) and after phosphorylation (•) were incubated at 37°C for 5 min in the presence of 1 mM ATP. Calcium chloride was added into the medium bathing nuclei so as to give the indicated free calcium concentration according to Fabiato (34). Traces of 45Ca2+ were also present in the medium (2 μCi/ml; 1 Ci = 37 Gbq). Ca2+ uptake was terminated by filtering under vacuum over GF/B Whatman glass fiber filters, followed by scintillation counting of the 45Ca2+ trapped on the filters. Values are expressed as means ± SEM, and statistical significance was evaluated by using the Mann–Whitney U test (∗, P < 0.05, control vs. PKA).
Effect of PKA Phosphorylation on Transport into Isolated Nuclei of Intermediate-Size Fluorescent Macromolecules.
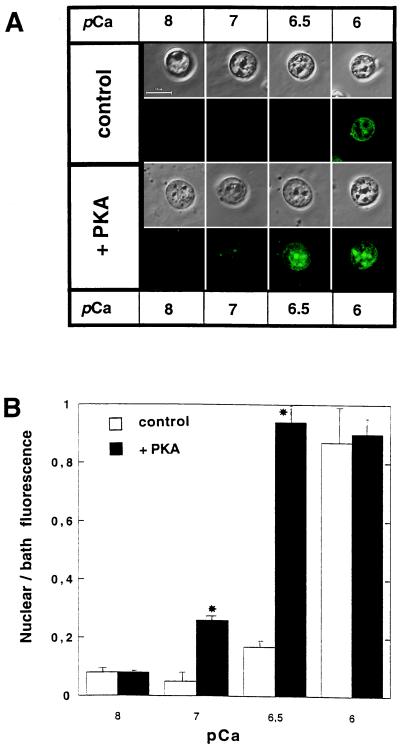
Functional significance of NCA regulation by PKA was assessed by measuring its effect on the transport of intermediate size molecules into the nucleus by using dextran-bound fluorescent indicators. When isolated rat liver nuclei were incubated in the presence of 500-kDa Calcium Green-1dextran, no fluorescence was observed in the nucleoplasm, confirming the integrity of the preparation. On the other hand, 10-kDa Calcium Green-1dextran was transported into the nuclei in a Ca2+-dependent manner (Fig. 5A). Addition of Ca2+ into the medium resulted in a concentration-dependent increase in the fluorescence of isolated nuclei. Similar results have been reported elsewhere (12). In the absence of ATP, transport of this dye into nuclei was not observed irrespective of the free Ca2+ concentration (data not shown). Also, no nuclear transport of the dye occurred in the presence of 1 μM thapsigargin. Thus, ATP-dependent filling of the Ca2+ store in the NE lumen was necessary for transport of the 10-kDa macromolecule. Phosphorylation by PKA accelerated this process (Fig. 5A). The difference between transport into control and phosphorylated nuclei was statistically significant (Fig. 5B) for pCa = 7.0 and pCa = 6.5.
Figure 5.
Transport of 10-kDa Calcium Green-1 dextran into intact isolated nuclei. (A) Micrographs showing Ca2+-dependent loading of nuclear preparation with 10-kDa Calcium Green-1 dextran under control and PKA-phosphorylated conditions. For each condition, fluorescence (λex ≈ 488 nm, λem ≈ 531 nm, Upper) and corresponding phase-contrast (Lower) micrographs are shown. (B) Quantification of confocal fluorescence micrographs. Background fluorescence values were subtracted. Data are presented as mean values ± SEM. Statistical significance was assessed by using the Mann–Whitney U test. (∗, P < 0.05, control vs. PKA).
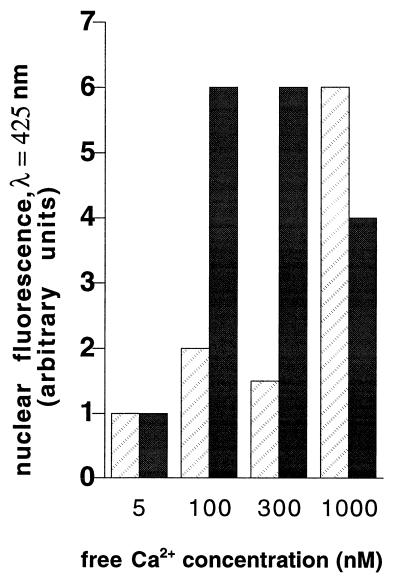
Because the dyes used in these experiments are Ca2+ sensitive, it was necessary to eliminate the possibility that the observed fluorescence intensities resulted from changes in nucleoplasmic Ca2+ concentrations and not from dye transport. However, ATP-dependent Ca2+ filling of the NE lumen also conditioned entry into the nucleoplasm of the 10-kDa dextran form of the Ca2+-insensitive dye Lucifer yellow. Indeed, classical fluorescence microscopy showed that transport of 10-kDa dextran Lucifer yellow into nuclei was Ca2+ dependent, and again this phenomenon was accelerated by PKA phosphorylation (Fig. 6).
Figure 6.
Transport of 10-kDa dextran-coupled Lucifer yellow. Isolated rat liver nuclei were incubated with calcium uptake medium supplemented with 10-kDa dextran-coupled Lucifer yellow at various free calcium concentrations. Fluorescence was visualized with classical optical microscopy (max. λ ≈ 425 nm). Values are depicted in arbitrary units and are derived from two separate experiments with less than 10% variation between independent values. Control (hatched bars) and PKA phosphorylated (solid bars).
DISCUSSION
The SERCAs, together with the plasma membrane Ca2+-ATPase pumps, determine the resting cytoplasmic Ca2+ concentration (35). The NCA participates in the regulation of nuclear Ca2+ levels (1, 7). Here, we report that NCA is a substrate for PKA. The nuclear preparation used in the present study (Table 1) was devoid of ER contamination.
Incubation of isolated intact nuclei with PKA in the presence [γ-32P]ATP results in the rapid appearance of a 105-kDa band. Several arguments confirm that this phosphorylated band corresponds to NCA. An apparent molecular mass of ≈110 kDa was previously reported for the nuclear Ca2+ pump (5). Our results are in reasonable agreement with this value. Another argument is derived from the fact that a 105-kDa protein band in the NE also was labeled with [γ-32P]ATP in the absence of PKA after incubation with 10 μM Ca2+ (Fig. 2). In the absence of PKA the 105-kDa band was no longer detected when Ca2+ was reduced to low concentrations (0.1 μM). This finding suggests that it corresponds to the formation of a high-energy phosphoenzyme intermediate (36). Furthermore, it was abolished after pretreatment with thapsigargin, confirming that it corresponds to a SERCA-type enzyme (37).
The 105-kDa protein band was recognized in isolated rat liver nuclear preparation by polyclonal antibodies raised against SERCA type 2b. SERCA2b is the ubiquitous “housekeeping” isoform found in the ER of all tissues (38). A heterogeneity of SERCA2b-type Ca2+-ATPases has been observed with respect to thapsigargin sensitivity (37, 39). The NCA phosphoenzyme intermediate is found to be thapsigargin sensitive, which is analogous to the thapsigargin-sensitive 100-kDa SERCA phosphorylated intermediate described in bovine adrenal chromaffin cells.
To conclusively demonstrate that the 105-kDa SERCA2b antibody-reactive and PKA-phosphorylated bands are identical, partial purification of NCA was undertaken by using a dye-coupled column. Reactive red-120 agarose affinity chromatography provided reasonably enriched NCA preparations. The major protein band eluted with peak I fractions was the 105-kDa species recognized by anti-SERCA2b antiserum. Complete elution of the enzyme by using nucleotides alone was not possible, and the remaining enzyme attached to the dye matrix was eluted with 2 M NaCl giving a second peak. A second, minor peak, also has been reported during reactive red-120 agarose affinity purification of the ER Ca2+-ATPase (25). However, in the study reported here peak II was much larger (Fig. 3).
Phosphorylation by PKA of concentrated peak I and II samples in the presence of [γ-32P]ATP followed by SDS/PAGE, immunoblotting with SERCA2b polyclonal antibodies, and autoradiography of the same blots showed that this antibody reacts with the 32P-labeled 105-kDa band. This finding confirmed that NCA is a PKA substrate. Møller et al. (27) have reported that the ATPase activity of solubilized SERCA is unstable at low Ca2+ concentrations. To circumvent this problem, enzyme purification was carried out in the presence of 1 mM CaCl2 followed by gel filtration. This procedure yielded an active enzyme (Table 2). It may be noted that the use of detergents runs the risk of perturbing NCA activity, and this use could contribute to the relatively low specific activity measured in the partially purified samples. Immunoblotting and protein purification procedures therefore demonstrate that the 105-kDa band undergoing PKA phosphorylation in isolated rat liver nuclei represents NCA.
The next question that arises is whether nuclear Ca2+-ATPase is directly phosphorylated by PKA or whether PKA phosphorylates an associated protein (40–43). Neyses et al. (20) reported a direct phosphorylation of sarcolemmal SERCA by PKA. Similarly Hawkins et al. (21) have documented a low, but nevertheless, significant level of direct phosphorylation of heart SERCA2a by exogenous PKA. It should be noted that direct phosphorylation of the plasma membrane Ca2+-ATPase by PKA has been demonstrated recently (44). Minimal PKA recognition motifs have been identified in these enzymes (45). Thus, we may argue that NCA is directly phosphorylated by PKA.
We observed a stoichiometry of 0.28 mol of phosphate incorporated/mol of Ca2+-ATPase for the membrane inserted NCA and 0.76 mol of phosphate incorporated/mol for the partially purified peak I enzyme. These results are in reasonable agreement with values reported for SERCA phosphorylation by PKA (20). Partial NCA purification was associated with increased stoichiometry, indicating the availability of additional sites for phosphorylation. Because partially purified enzyme retained its enzymatic activity, this increase cannot be caused by exposure of sites through denaturation.
Because permeabilization of nuclei with digitonin did not affect stoichiometry (0.30), it may be suggested that the phosphorylated sites are located on the part of the pump facing the cytoplasm. Alkaline phosphatase treatment, before phosphorylation, enhanced stoichiometry (0.81) of the membrane-inserted NCA. This finding suggests that an endogenous level of phosphorylating activity, which targets the same sites as those targeted by PKA in vitro, operates in vivo. It may be argued that nearly all of the NCA molecules are available for PKA phosphorylation, except a subpopulation (about 20%) that may be PKA-insensitive NCA. Alternatively, levels of phosphorylation measured may not reflect the maximum phosphorylation potential because of endogenous phosphatases that are not completely inactivated with available inhibitors. With the partially purified NCA, it is most likely that protein phosphatases, eventually copurified, are inhibited. Therefore the highest level of phosphorylation observed with PKA may be attributed to exposure of sites phosphorylated by PKA that are not relevant physiologically.
We studied the functional relevance of NCA phosphorylation by PKA by measuring its effect on ATP-dependent Ca2+ uptake (2). ATP-dependent nuclear 45Ca2+-transport was Ca2+-dependent with a maximum at 1 μM free Ca2+ (Fig. 5). Free Ca2+ levels at which activation of the nuclear Ca2+-pump is maximum are relatively high; this is compatible with a role for NCA (and SERCA in general) in Ca2+ signaling through signal termination (46).
Phosphorylation by PKA had a stimulatory effect on Ca2+ uptake into purified nuclei, with maximum activity shifted to 2 μM free Ca2+. These results are in accordance with early studies (47, 48) showing that cAMP-dependent phosphorylation stimulates Ca2+ pumping into heart microsomes (49). The decrease in Ca2+ uptake into control nuclei at high free Ca2+ (Fig. 4) could be explained by loss of NE integrity, as is observed at high Ca2+ concentrations (50). Incubation under PKA phosphorylating conditions, which prevents this decrease, may be protective.
We further assessed the functional significance of NCA phosphorylation by PKA by measuring the transport of dye-labeled intermediate-size (10 kDa) dextrans into the nucleus (Fig. 5). Stehno-Bittel et al. (12) have shown that, after depletion of Ca2+ from the NE lumen by inositol 1,4,5-trisphosphate or Ca2+ chelators, 10-kDa Calcium Green-1 dextran was excluded from the nucleus. PKA phosphorylation of NCA enhances the transport of 10-kDa Calcium-Green dextran into the nucleus. This indicates the filling of nuclear calcium pool regulating the opening of the nuclear pore complexes. Results with Lucifer-yellow were in reasonable agreement with those obtained by using Calcium-Green dextran (Fig. 6).
The mechanisms underlying the effect of NE intraluminal Ca2+ on transport of intermediate-sized molecules are unknown. Perez-Terzic et al. (51) have shown that depletion of nuclear Ca2+ stores induced conformational changes and closing of the nuclear pore complexer. The influence of NE luminal Ca2+ levels on nuclear protein transport (52) is quite complex. Sweitzer and Hanover (11) described a GTP-independent NLS-dependent nuclear protein transport pathway that is stimulated by Ca2+ and calmodulin.
Phosphorylation of purified nuclei by the PKA catalytic subunit enhanced the transport of dye-coupled 10-kDa dextrans. This result is in agreement with the stimulatory effect of PKA phosphorylation on Ca2+ uptake into purified nuclei. It is also compatible with reports concerning the modulatory effect of PKA phosphorylation on nucleocytoplasmic trafficking (16). PKA has been shown to regulate both NLS-mediated and NLS-independent nuclear protein uptake, through either phosphorylation of the transported protein, of its interacting partner or of components of the transport machinery. The present data suggest that PKA also can modulate Ca2+-dependent macromolecule uptake by regulating NCA Ca2+ pumping activity.
In conclusion, the present data show that PKA phosphorylates and activates the Ca2+-pumping activity of NCA. The control of Ca2+-dependent NLS-independent macromolecule transport appears to be a novel function mediated by phosphorylation of the NCA by PKA. These results represent yet another example of crosstalk between Ca2+- and cAMP-regulated pathways operating at the level of the nucleus.
Acknowledgments
We are indebted to Dr. S. Chasserot-Golaz (U. 338 de Institut National de la Santé et de la Recherche Médicale) for help with confocal imaging and Dr. J.-L. Dupont (U.P.R. 9009 du Centre National de la Recherche Scientifique) for kindly providing modified Petri dishes. Dr. F. Wuytack (Leuven, Belgium) is thanked for the generous gift of anti-SERCA antibodies. S. Ott is acknowledged for secretarial assistance and Dr. K. Langley for editing English usage.
ABBREVIATIONS
- PKA
cAMP-dependent protein kinase
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- NCA
nuclear Ca2+-ATPase
- ER
endoplasmic reticulum
- NE
nuclear envelope
- NLS
nuclear localization signal
- AMP-PNP
adenyl 5′-yl imidodiphosphate
References
- 1.Malviya A N, Rogue P. Cell. 1998;92:17–23. doi: 10.1016/s0092-8674(00)80895-8. [DOI] [PubMed] [Google Scholar]
- 2.Malviya A N, Rogue P, Vincendon G. Proc Natl Acad Sci USA. 1990;87:9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köppler P, Matter N, Malviya A N. J Biol Chem. 1993;268:26248–26252. [PubMed] [Google Scholar]
- 4.Gerasimenko O V, Gerasimenko J V, Tepikin A V, Petersen O H. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 5.Lanini L, Bachs O, Carafoli E. J Biol Chem. 1992;267:11548–11552. [PubMed] [Google Scholar]
- 6.Humbert J P, Matter N, Artault J C, Köppler P, Malviya A N. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- 7.Santella L. J Membr Biol. 1996;153:83–92. doi: 10.1007/s002329900112. [DOI] [PubMed] [Google Scholar]
- 8.Hardingham G E, Chawla S, Johnson S, Bading H. Nature (London) 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh A, Greenberg M. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 10.Greber U F, Gerace L. J Cell Biol. 1995;128:5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweitzer T D, Hanover J A. Proc Natl Acad Sci USA. 1996;93:14574–15579. doi: 10.1073/pnas.93.25.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehno-Bittel L, Perez-Terzic C, Clapham D E. Science. 1995;270:1835–1838. doi: 10.1126/science.270.5243.1835. [DOI] [PubMed] [Google Scholar]
- 13.DeBernadi M A, Brooker G. Proc Natl Acad Sci USA. 1996;93:4577–4582. doi: 10.1073/pnas.93.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy M R. Mol Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faux M C, Scott J D. Trends Biochem Sci. 1997;21:312–315. [PubMed] [Google Scholar]
- 16.Vandromme M, Gauthier-Rouvière C, Lamb N, Fernandez A. Trends Biochem Sci. 1996;21:59–64. [PubMed] [Google Scholar]
- 17.Gauthier-Rouvière C, Vandromme M, Lautredou N, Cai Q Q, Girard F, Fernandez A, Lamb N. Mol Cell Biol. 1995;15:433–444. doi: 10.1128/mcb.15.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra K, Parnaik V K. Exp Cell Res. 1995;216:124–134. doi: 10.1006/excr.1995.1016. [DOI] [PubMed] [Google Scholar]
- 19.Vandromme M, Carnac G, Gauthier-Rouvière C, Fesquet D, Lamb N, Fernandez A. J Cell Sci. 1994;107:613–620. doi: 10.1242/jcs.107.2.613. [DOI] [PubMed] [Google Scholar]
- 20.Neyses L, Reinlib L, Carafoli E. J Biol Chem. 1985;260:10283–10287. [PubMed] [Google Scholar]
- 21.Hawkins C, Xu A, Narayanan N. J Biol Chem. 1994;269:31198–31206. [PubMed] [Google Scholar]
- 22.Reddy L, Jones L R, Pace R C, Stokes D L. J Biol Chem. 1996;271:14964–14970. doi: 10.1074/jbc.271.25.14964. [DOI] [PubMed] [Google Scholar]
- 23.Masmoudi A, Labourdette G, Mersel M, Huang F L, Huang K P, Vincendon G, Malviya A N. J Biol Chem. 1989;264:1172–1179. [PubMed] [Google Scholar]
- 24.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Coll R J, Murphy A J. J Biol Chem. 1984;259:14249–14254. [PubMed] [Google Scholar]
- 26.Mellgren R L, Lu Q. Biochem Biophys Res Commun. 1994;204:544–550. doi: 10.1006/bbrc.1994.2493. [DOI] [PubMed] [Google Scholar]
- 27.Møller J V, Lind K E, Andersen J P. J Biol Chem. 1980;255:1912–1920. [PubMed] [Google Scholar]
- 28.Nelson N. Methods Enzymol. 1980;69:301–313. [Google Scholar]
- 29.Rogue P, Zwiller J, Malviya A N, Vincendon G. Biochem Int. 1990;22:575–582. [PubMed] [Google Scholar]
- 30.Kuret J, Bell H, Cohen P. FEBS Lett. 1983;203:197–202. doi: 10.1016/0014-5793(86)80741-4. [DOI] [PubMed] [Google Scholar]
- 31.Cohen P. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 32.Rogue P, Labourdette G, Masmoudi A, Yoshida Y, Huang F L, Huang K P, Zwiller J, Vincendon G, Malviya A N. J Biol Chem. 1990;265:4161–4165. [PubMed] [Google Scholar]
- 33.Sarkadi B, Enyedi A, Penniston J T, Verma A K, Dux L, Molnár E, Gárdos G. Biochem Biophys Acta. 1988;939:40–46. doi: 10.1016/0005-2736(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 34.Fabiato A. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan D H, Rice W J, Green N M. J Biol Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 36.Fujimori T, Jencks W. J Biol Chem. 1992;267:18446–18474. [PubMed] [Google Scholar]
- 37.Caspersen C, Treiman M. FEBS Lett. 1995;377:31–36. doi: 10.1016/0014-5793(95)01304-0. [DOI] [PubMed] [Google Scholar]
- 38.Gunteski-Hamblin A-M, Greeb J, Shull G E. J Biol Chem. 1988;263:15032–15040. [PubMed] [Google Scholar]
- 39.Wells K M, Abercrombie R F. J Biol Chem. 1998;273:5020–5025. doi: 10.1074/jbc.273.9.5020. [DOI] [PubMed] [Google Scholar]
- 40.Tada M, Katz A M. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- 41.Xu A, Hawkins C, Narayanan N. J Biol Chem. 1993;268:8394–8397. [PubMed] [Google Scholar]
- 42.Toyofuku T, Kurzydlowski K, Narayanan N, MacLennan D H. J Biol Chem. 1994;269:26492–26496. [PubMed] [Google Scholar]
- 43.Odermatt A, Kurzydlowski K, MacLennan D. J Biol Chem. 1996;271:14206–14213. doi: 10.1074/jbc.271.24.14206. [DOI] [PubMed] [Google Scholar]
- 44.Dean W L, Cheng D, Brandt P C, Vanaman T. J Biol Chem. 1997;272:15113–15119. doi: 10.1074/jbc.272.24.15113. [DOI] [PubMed] [Google Scholar]
- 45.Kemp B E, Pearson R B. Trends Biochem Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 46.Lee M G, Xu X, Zeng W, Diaz J, Kuo T H, Wuytack F, Racymaekers L, Muallem S. J Biol Chem. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- 47.Hawkins C, Xu A, Narayanan N. Mol Cell Biochem. 1995;142:131–138. doi: 10.1007/BF00928934. [DOI] [PubMed] [Google Scholar]
- 48.Antipenko A, Spielman A I, Kirchberger M A. J Biol Chem. 1997;272:9371–9373. doi: 10.1074/jbc.272.5.2852. [DOI] [PubMed] [Google Scholar]
- 49.Canoni P, Carafoli E. J Biol Chem. 1981;256:9371–9373. [PubMed] [Google Scholar]
- 50.Subramanian K, Meyer T. Cell. 1997;89:963–971. doi: 10.1016/s0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham D E. Science. 1996;273:1875–1877. doi: 10.1126/science.273.5283.1875. [DOI] [PubMed] [Google Scholar]
- 52.Greber U F, Suomalanian M, Stidwill R P, Bouche K, Ebersold M W, Helenius A. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]