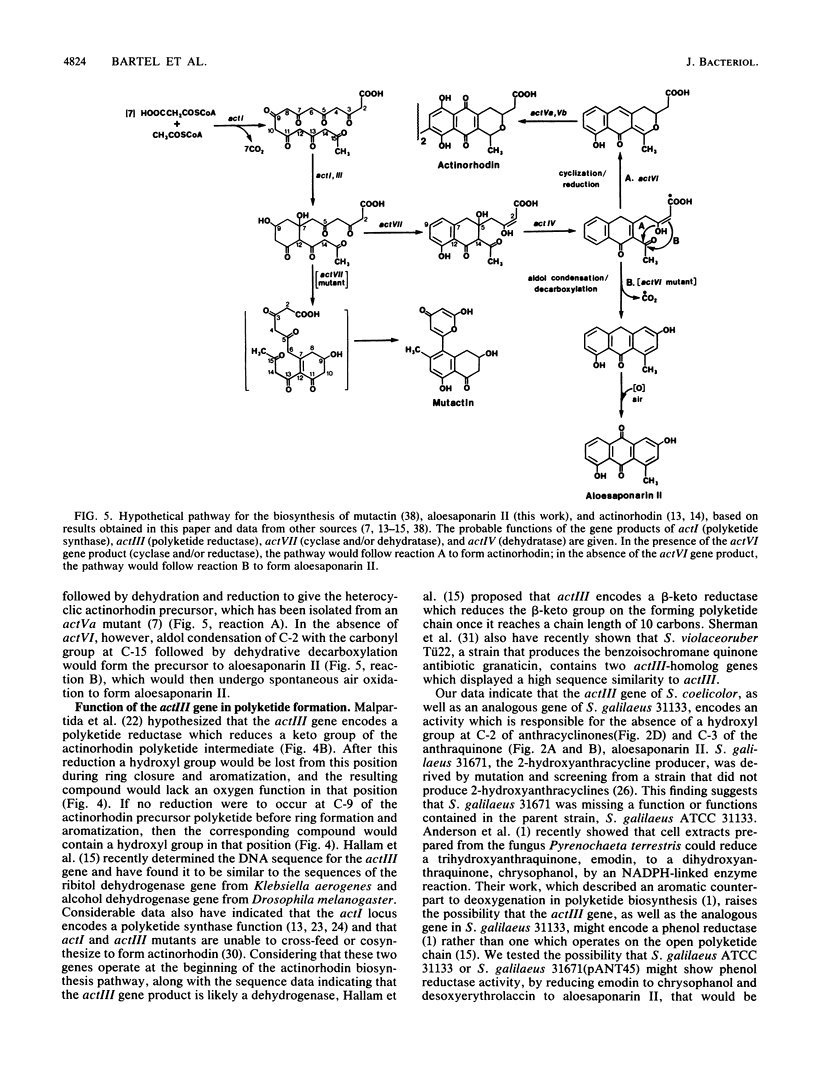
Abstract
Streptomyces galilaeus ATCC 31133 and ATCC 31671, producers of the anthracyclines aclacinomycin A and 2-hydroxyaklavinone, respectively, formed an anthraquinone, aloesaponarin II, when they were transformed with DNA from Streptomyces coelicolor containing four genetic loci, actI, actIII, actIV, and actVII, encoding early reactions in the actinorhodin biosynthesis pathway. Subcloning experiments indicated that a 2.8-kilobase-pair XhoI fragment containing only the actI and actVII loci was necessary for aloesaponarin II biosynthesis by S. galilaeus ATCC 31133. Aloesaponarin II was synthesized via the condensation of 8 acetyl coenzyme A equivalents, followed by a decarboxylation reaction as demonstrated by [1,2-13C2]acetate feeding experiments. S. coelicolor B22 and B159, actVI blocked mutants, also formed aloesaponarin II as an apparent shunt product. Mutants of S. coelicolor blocked in several other steps in actinorhodin biosynthesis did not synthesize aloesaponarin II or other detectable anthraquinones. When S. galilaeus ATCC 31671 was transformed with the DNA carrying the actI, actIII, and actVII loci, the recombinant strain produced both aloesaponarin II and aklavinone, suggesting that the actinorhodin biosynthesis DNA encoded a function able to deoxygenate 2-hydroxyaklavinone to aklavinone. When S. galilaeus ATCC 31671 was transformed with a plasmid carrying only the intact actIII gene (pANT45), aklavinone was formed exclusively. These experiments indicate a function for the actIII gene, which is the reduction of the keto group at C-9 from the carboxy terminus of the assembled polyketide to the corresponding secondary alcohol. In the presence of the actIII gene, anthraquinones or anthracyclines formed as a result of dehydration and aromatization lack an oxygen function on the carbon on which the keto reductase operated. When S. galilaeus ATCC 31671 was transformed with the DNA carrying the actI, actVII, and actIV loci, the recombinant strain produced two novel anthraquinones, desoxyerythrolaccin, the 3-hydroxy analog of aloesaponarin II, and 1-O-methyldesoxyerythrolaccin. The results obtained in these experiments together with earlier data suggest a pathway for the biosynthesis of actinorhodin and related compounds by S. coelicolor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Birch A. J. Biosynthesis of polyketides and related compounds. Science. 1967 Apr 14;156(3772):202–206. doi: 10.1126/science.156.3772.202. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Rudd B. A., Hopwood D. A., Chang C. J., Floss H. G. Biosynthesis of the antibiotic actinorhodin. Analysis of blocked mutants of Streptomyces coelicolor. J Antibiot (Tokyo) 1987 Mar;40(3):340–347. doi: 10.7164/antibiotics.40.340. [DOI] [PubMed] [Google Scholar]
- Dekleva M. L., Strohl W. R. Glucose-stimulated acidogenesis by Streptomyces peucetius. Can J Microbiol. 1987 Dec;33(12):1129–1132. doi: 10.1139/m87-198. [DOI] [PubMed] [Google Scholar]
- Dekleva M. L., Titus J. A., Strohl W. R. Nutrient effects on anthracycline production by Streptomyces peucetius in a defined medium. Can J Microbiol. 1985 Mar;31(3):287–294. doi: 10.1139/m85-053. [DOI] [PubMed] [Google Scholar]
- Deng Z. X., Kieser T., Hopwood D. A. "Strong incompatibility" between derivatives of the Streptomyces multi-copy plasmid pIJ101. Mol Gen Genet. 1988 Oct;214(2):286–294. doi: 10.1007/BF00337723. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Lampel J. S., Strohl W. R. Transformation and transfection of anthracycline-producing streptomycetes. Appl Environ Microbiol. 1986 Jan;51(1):126–131. doi: 10.1128/aem.51.1.126-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hallam S. E., Kieser H. M., Motamedi H., Hutchinson C. R., Butler M. J., Sugden D. A., Warren M., McKillop C., Bailey C. R. Homology between Streptomyces genes coding for synthesis of different polyketides used to clone antibiotic biosynthetic genes. 1987 Feb 26-Mar 4Nature. 325(6107):818–821. doi: 10.1038/325818a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Physical and genetic characterisation of the gene cluster for the antibiotic actinorhodin in Streptomyces coelicolor A3(2). Mol Gen Genet. 1986 Oct;205(1):66–73. doi: 10.1007/BF02428033. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Yoshimoto A., Shibamoto N., Tobe H., Oki T., Naganawa H., Takeuchi T., Umezawa H. New anthracycline metabolites from mutant strains of Streptomyces galilaeus MA144-M1. II. Structure of 2-hydroxyaklavinone and new aklavinone glycosides. J Antibiot (Tokyo) 1981 Aug;34(8):959–964. doi: 10.7164/antibiotics.34.959. [DOI] [PubMed] [Google Scholar]
- Oki T., Kitamura I., Matsuzawa Y., Shibamoto N., Ogasawara T., Yoshimoto A., Inui T., Naganawa H., Takeuchi T., Umezawa H. Antitumor anthracycline antibiotics, aclacinomycin a and analogues. II. Structural determination. J Antibiot (Tokyo) 1979 Aug;32(8):801–819. doi: 10.7164/antibiotics.32.801. [DOI] [PubMed] [Google Scholar]
- Omura S., Ikeda H., Malpartida F., Kieser H. M., Hopwood D. A. Production of new hybrid antibiotics, mederrhodins A and B, by a genetically engineered strain. Antimicrob Agents Chemother. 1986 Jan;29(1):13–19. doi: 10.1128/aac.29.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd B. A., Hopwood D. A. Genetics of actinorhodin biosynthesis by Streptomyces coelicolor A3(2). J Gen Microbiol. 1979 Sep;114(1):35–43. doi: 10.1099/00221287-114-1-35. [DOI] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzman-Engwall K. J., Hutchinson C. R. Multigene families for anthracycline antibiotic production in Streptomyces peucetius. Proc Natl Acad Sci U S A. 1989 May;86(9):3135–3139. doi: 10.1073/pnas.86.9.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Yue S., Motamedi H., Wendt-Pienkowski E., Hutchinson C. R. Anthracycline metabolites of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986 Aug;167(2):581–586. doi: 10.1128/jb.167.2.581-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]