Abstract
Background. Immunomodulation may represent a potential way to improve surgical outcome. These types of interventions should be based on detailed knowledge of the underlying mechanisms involved. The aim of the present review is to summarize some experience on the acute phase response, potential ways of intervention and experiences from critical illness and HPB disease. Discussion. Mechanisms of the acute phase response are discussed including the individual parameters and local changes that take part. Mechanisms involved in failure of the gut barrier are presented and include changes in gut barrier permeability, effects on gut-associated immunocompetent cells, and systemic implications. As examples of HPB disease, mechanisms of the acute phase response and potential ways of intervention in obstructive jaundice and acute pancreatitis are discussed. Nutritional pharmacology and lessons learned from immunomodulation and immunonutrition in critical illness and major abdominal surgery, including upper GI and HPB surgery, are referred to. Overall, immunomodulation represents a potential tool to improve results but requires a thorough mapping of underlying mechanisms in order to achieve individualized treatment or prevention based on patients' specific needs.
Keywords: immunomodulation, surgery, critical illness, acute phase response, cytokines, immunonutrition
Introduction
Immunomodulation has been suggested as one way of optimizing the course of surgical patients, both as part of the perioperative management in elective surgical patients and in acute disease and critical illness. The concept has also been included in nutritional management and termed immunonutrition. Modulation of the acute phase response and immune function, however, requires a detailed knowledge about the acute inflammatory response and its mechanisms in order to understand and optimize selective intervention. This review discusses some of the mechanisms involved in the acute inflammatory response, that have the potential for intervention. It is understood that we still lack substantial information in order to treat and prevent by modulating the immune response in the most optimal and “tailored” fashion. However, some experience already exists as regards at which time point and with what agents we potentially can intervene in different disease processes, and lessons learned from treatment in critical illness are of value. Immunomodulation in HPB disease and surgery will also be discussed.
Surgery and the acute phase response
Major surgery results in an acute phase response where the magnitude of the response usually correlates with the extent of the surgical trauma. The surgical intervention also results in a transient immunosuppression and potential alterations in gastrointestinal tract function. In the normal situation, these alterations are restored within a few days unless complications occur 1.
The immune response caused by the surgical intervention may be followed by an excessive inflammatory response and paralysis of cell-mediated immunity. This effect of major surgery could be responsible for the increased susceptibility to subsequent septic complications 2. It has been shown that laparoscopic surgery results in diminished tissue trauma as compared with open surgery 3. Decreased surgical trauma by minimal invasive surgery together with, for example, optimal pain control, early enteral nutrition and mobilization, brought together as “facilitated” or “fast track surgery”, probably diminishes the operative stress and acute inflammatory response (Figure 1) 4.
Figure 1. .
Surgery and the immune response.
The acute phase and inflammatory response following surgical trauma in critical illness seem to depend on the net of the pro- and anti-inflammatory responses that occur simultaneously. In critical illness and after major surgical trauma in general the overall response seems to be a hyperinflammatory response, which could include the development of the systemic inflammatory response syndrome (SIRS) that might proceed to the development of “early” multiple organ dysfunction syndrome (MODS). Later on during the course of disease, a net hypoinflammatory state may occur, sometimes termed the compensatory anti-inflammatory response syndrome (CARS). During this phase, a supervening infection or sepsis could further impair the condition and contribute to the development of “late” MODS 5.
The acute phase response
The acute phase response is the result of the action of pro- and anti-inflammatory factors as mentioned above. A number of pro-inflammatory mediators are known to play a major role in the complex acute phase response, among others the intracellular pro-inflammatory regulator nuclear factor kappa B (NF-κB), as well as cytokines like tumor necrosis factor (TNF)κ, interleukin (IL)-1, IL-6 and IL-8, and platelet activating factor. Upregulation of various adhesion molecules is also involved in the acute phase response and participates in the potential development of organ failure. Contributory factors also include endotoxin, oxygen free radicals, nitric oxide and histamine as well as enzymes like phospholipase A2, especially when released from neutrophils that have migrated through the endothelial barrier into the tissues of various organs, thereby causing tissue injury and organ failure. Overall, the magnitude of the acute phase response and the cytokine and mediator release seems to correlate well with the potential development of systemic complications and remote organ failure, as has been shown both experimentally and in the clinical setting 6,7,8,9.
Local challenges may be followed by systemic effects, where the changes that occur merely depend on the magnitude of the local injury. Furthermore, local preventive and therapeutic interventions may also contribute to a normalization of the systemic response. For example, a gut-pulmonary axis been described where local injury to the gut will affect the lungs 10,11,12. Similarly, a gut-liver axis has been described in which obstructive jaundice negatively affects gut barrier function and activation of host immune function 13.
Gut barrier failure and sepsis
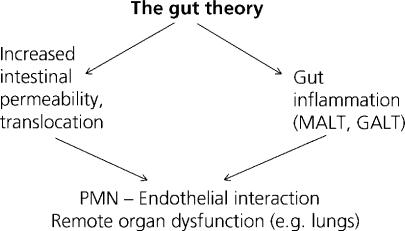
The gastrointestinal tract has been called “the undrained abscess” of multiple organ failure 14. The gut barrier has mostly been considered as a defense against permeability and the permeation of bacteria and toxins normally contained within the intestinal lumen. However, gut barrrier failure has been shown not only to represent an increase in intestinal permeability and translocation, but also concerns the activation of immunocompetent cells within the gut wall and associated lymph nodes. Overall, mucosa-associated lymphoid tissue (MALT) and gut-associated lymphoid tissue (GALT) probably represent one of our largest immunological “organs” 15. The combination of increased barrier permeability and gut inflammation may end up in the interaction between polymorphonuclear leukocytes and the endothelial lining, potentially resulting in remote organ dysfunction, e.g. in the lungs (Figure 2).
Figure 2. .
Gut barrier failure includes both an increase in intestinal permeability and a gut inflammatory state. MALT, mucosa-associated lymphoid tissue; GALT, gut-associated lymphoid tissue; PMN, polymorphonuclear leukocytes.
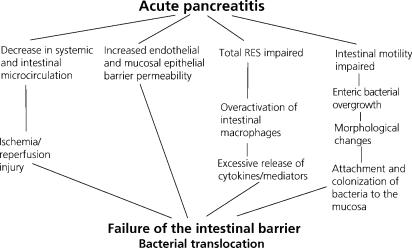
The complexicity underlying gut barrier failure is illustrated when summarizing some pathophysiological mechanisms involved in intestinal barrier failure in acute experimental pancreatitis (Figure 3) 15. As can be seen, the complexicity in gut barrier dysfunction is substantial and involves a number of factors that individually represent potential targets for both preventive and therapeutic measures 15,16.
Figure 3. .
Interactive mechanisms underlying the development of intestinal barrier failure in acutew experimental pancreatitis. RES, reticuloendothelial system.
Why no magic bullets modulating the acute phase response?
A number of interventions have been tried individually in the clinical setting, based on successful experimental findings. Regrettably, the overall result has been a failure in demonstrating a beneficial clinical outcome with these types of interventions. Reasons for this might be the fact that there seems to be a limited window for potential therapeutic intervention, especially in critical illness, with complex mechanisms from the very onset and a rapid progression of the disease. Thus, treatment attempts at blocking various individual pro-inflammatory responses have failed. Moreover, we still lack a proper mapping of the actual course of the acute phase response, knowledge which could represent a basis for obtaining a more tailored and individualized type of treatment.
Potential interventions include gut protective strategies, modulation of SIRS, endothelial barrier dysfunction and MODS and the potential role of immunonutrition, in which case the route of administration and the addition of individual supplements with beneficial effects (“nutritional pharmacology”) have to be addressed.
Concerning gut protection, the permeability as well as the gut inflammatory response have been addressed. The importance of luminal nutrition has to be emphasized, as enterocytes depend on glutamine to a large extent and colonocytes depend on short-chain fatty acids as energy source 17. No parenteral supplementation will thus fully compensate for the lack of luminal nutrition and absence of these specific “nutrients”.
Modulation of SIRS, endothelial barrier dysfunction and MODS may be achieved by for example the administration of anti-oxidants. Other potential ways of obtaining this could be by blocking pro-inflammatory cytokines, the administration of platelet activating factor antagonists, antibodies against adhesion molecules or agents binding endotoxin. Immunonutrition and the route of administration of nutrition have to be addressed, where the importance of gut luminal nutrition should be emphasized. Factors to consider are patients' basic nutritional requirements and additives if, for example, malnutrition, severe catabolism, complications, intensive care treatment, etc., exist. The administration of probiotics or synbiotics could also be of benefit. The role of key nutrients, often as supplements to nutritional formulas, on the acute inflammatory response, immune competence and gastrointestinal function has to be further investigated.
Obstructive jaundice--effects on immune function and outcome
Obstructive jaundice has been reported to be associated with an impaired immune function, including both the systemic and local defense. Alterations have been reported in gut barrier function, as well as the existence of endotoxemia. Overall, an increase in the incidence of postoperative complications including sepsis and infection has been seen in obstructive jaundice 18,19. Host defense in subjects with obstructive jaundice is influenced in multiple ways. The overall reticuloendothelial system (RES) seems to be impaired and Kupffer cell function is altered. An impaired cellular immunity, including both the fixed reticuloendothelial system and macrophages has been reported, as well as a decrease in natural killer cell activity. Biliary obstruction has also been associated with an increase in cytokine release, including TNFα, IL-6 and IL-8, and upregulation of adhesion molecules.
Biliary obstruction results in an increase in intestinal permeability, upregulation of HLA-DR expression on enterocytes and GALT, suggesting an immune activation. There also seems to be an increase in the acute phase response and in circulating anti-endotoxin core antibodies. Following internal biliary drainage, a normalization of intestinal permeability occurs 20.
Additional surgery exaggerates the impaired gut barrier function and activation of host immune function seen in patients with obstructive jaundice, implying caution in order to minimize the risk of developing systemic complications 13. The endotoxemia noted in biliary obstruction is considered to be due to a number of coinciding changes including an increase in intestinal permeability and a lack of bile flow to the gastrointestinal tract, with concomitant absence of bile salt neutralization of endotoxin. A decrease in the elimination of endotoxin could also be due to altered Kupffer cell function. Altogether this favours the occurrence of endotoxemia in jaundice and can be summarized as disturbances in the homeostasis of the suggested gut-liver axis 21. A number of interventions have been tried to improve immune function in obstructive jaundice. For example, preoperative lactulose administration in biliary obstruction seems effective in experimental biliary obstruction 22,23. Lactulose was also of benefit when administered together with deoxycholate for the prevention of postoperative renal dysfunction in jaundiced patients 24. Mechanisms explaining the effect of lactulose may be inactivation of gut-derived endotoxin and endoxotin-induced TNF production 25,26.
The impaired RES function in obstructive jaundice is restored after internal biliary drainage but the recovery phase is prolonged. Also, intestinal permeability slowly recovers after internal biliary drainage. For both alterations, a period of up to 5 weeks seems to be required to regain normal function 20,27.
This prolonged recovery has been a contributing factor to questioning the routine use of preoperative biliary stenting in jaundiced patients 28. Preoperative biliary drainage has even been reported to increase complications, including infectious complications, intra-abdominal abscess formation and postoperative death, in patients subjected to pancreaticoduodenectomy 29.
Immunomodulation has been tried in obstructive jaundice. Experimentally, the administration of an immunostimulating compound (muramyl dipeptide) improved RES recovery in jaundiced rats treated with internal biliary decompression 30,31. Immunomodulation by the use of muramyl tripeptide phosphatidyl-ethanolamine inhibited bacterial translocation, probably by activation of mucosal macrophages in experimental biliary obstruction 32.
Acute pancreatitis and the inflammatory response
Acute pancreatitis is a pronounced pro-inflammatory condition, especially in fulminant cases. Central mechanisms include the existence of ischemia and reperfusion injury and increased endothelial barrier permeability. Experimentally, treatment with, for example, platelet activating factor inhibitors, antibodies against adhesion molecules and tromboxane A2, has been effective against microcirculatory and endothelial barrier permeability derangements 33,34.
Oxygen free radicals play a crucial pathophysiological role, especially during the early stages of acute pancreatitis and are produced in response to ischemia/reperfusion. Antioxidants have partly been effective in experimental acute pancreatitis, decreasing the magnitude of endothelial barrier dysfunction in remote organs. Clinical data supporting an effect by antioxidant administration alone is, however, lacking 35,36,37.
Immunomodulation may also be achieved by the administration of steroids. Beneficial effects have been reported in experimental pancreatitis following administration of steroids 38 but this has not been reproduced clinically 39. The use of glucocorticoids may, however, be of value, as part of a multimodal treatment strategy, as they suppress the inflammatory response, potentially by the inhibition NF-κB, a dominant intracellular regulator of the pro-inflammatory response 40,41. Experimentally, specific inhibition of NF-κB has improved outcome 42,43,44.
Cytokine release, including IL-6 and IL-8, has been identified as an early predictor of severity in acute pancreatitis 45,46,47. Treatment with IL-10 and selective inhibition of IL-1 β and IL-8 have been shown to be of benefit in experimental acute pancreatitis 48,49,50. The expression of adhesion molecules is central in the development of endothelial barrier dysfunction, regulates transmigration of neutrophils and concomitant development of organ dysfunction.
Experimentally, treatment with antibodies against adhesion molecules like ICAM-1 and PECAM-1 has been effective 51,52,53,54. Combining various agents in a “multimodal treatment” directed against various pathophysiological mechanisms in acute pancreatitis has been tried experimentally. The combination of the broad-acting antioxidant N-acetylcysteine, the PAF inhibitor lexipafant and monoclonal antibodies against the adhesion molecule PECAM-1 was effective when administered in animals with ongoing organ failure in a model of taurodeoxycholate-induced acute pancreatitis. By this treatment, the acute phase response and organ dysfunction decreased, and gut barrier failure and translocation could be prevented 55. Clinical evidence for the effectiveness of this type of “cocktail regimen” is still not available.
Anticoagulation and anti-inflammation
Attention has recently been paid to the anti-inflammatory properties possessed by various anticoagulating agents. Activated protein C blunts the anti-inflammatory response to sepsis 56 and the use of recombinant activated protein C has been shown to reduce mortality in severe sepsis 57. Anti-inflammatory properties have also been proposed when inhibiting other factors of the coagulation cascade, like inhibition of tissue factor pathway inhibitor, factor Xa and factor VII 58,59. This field of potential intervention is presently under investigation and the future will tell us about the value of this strategy, taking the increased risk of bleeding into account.
Nutritional pharmacology
Arginine
The amino acid arginine possesses cytoprotective effects in ischemia and reperfusion and can form nitric oxide, citrulline, ornithine, growth factors, etc., and exerts numerous beneficial effects on the immune system. Arginine induces secretion of numerous hormones like pituitary growth hormone, insulin-like growth factor IgF-1, insulin, vasopressin, catecholamines, and somatostatin. Arginine also inhibits NF-κB translocation and decreases the release of IL-6, TNFα, IL-18, blocks adhesion molecules and inhibits lipid peroxidation 60.
Glutamine
Glutamine is a non-essential glycogenic amino acid and the preferred fuel for lymphocytes, enterocytes, and neutrophils. Glutamine also improves neutrophil, lymphocyte, and intestinal function 61. This amino acid also maintains a normal GALT function and respiratory immunity 60.
Omega 3 Fatty Acids
Omega 3 polyunsaturated fatty acids (PUFA) represent essential fatty acids that possess immunomodulating effects due to their rapid incorporation into cell membranes. Thereby, they have influence on membrane stability, fluidity, cell mobility, and intra-cellular signaling pathways, as well as gene expression and cell differentiation. Omega 3 fatty acids are claimed to provide protection against infection 62. Omega 3 fatty acids regulate the immune response by increasing membrane fluidity, introducing free radical lipid peroxide and by providing precursors in eicosanoid metabolism.
Nucleotides
Immunosuppression may partly be caused by nucleotide restriction. T-cell-dependent antibody production and lymphocyte function also seem to depend on nucleotide supplementation 63.
Lessons learned from immunomodulation and immunonutrition in critical illness and in association with major surgery
High-dose parenteral glutamine supplementation was followed by a reduction in infectious complications and shortened hospital stay in surgical patients and reduced complications and mortality in critically ill patients 64. Glutamine-containing parenteral nutrition in critically ill ICU patients unable to receive enteral nutrition improved survival as evaluated after 6 months and reduced hospital costs per survivor 65. A glutamine-containing enteral feed in the ICU reduced costs per survivor by 30% 66.
As mentioned above, the use of activated protein C reduces mortality in patients with severe sepsis 57. The use of low-dose steroids decreases mortality in patients with septic shock and proven adrenalin insufficiency 67. Intensive insulin therapy, maintaining “normal” glucose levels, reduced mortality in intensive care, especially deaths due to multiple organ failure in patients with a proven septic focus 68. Perioperative omega 3 fatty acids downregulate the inflammatory response, diminish postoperative immunosuppression and shorten postoperative intensive care. Furthermore, the incidence of severe infections is reduced 69.
Immunonutrition (including arginine, glutamine, nucleotides, and omega 3 fatty acids) decreases infectious complications and the length of hospital stay in the critically ill, and especially in surgical patients. There does not, however, seem to be any effect of immunonutrition on mortality 70,71,72,73,74. An important observation in a recent review is that mortality tends to increase in a subgroup of critically ill patients, especially when using products other than those high in arginine 75. The reason for this is to be speculated upon but further emphasis may be directed at a more tailored type of immunomodulation, taking the actual immune function of the individual patient into consideration.
Perioperative immunonutrition in upper GI and HPB surgery
Preoperative immunonutrition for 5 days has been reported to improve outcome, including an improved immunometabolic response, a decreased infection rate, and reduced treatment costs of complications. There seems to be no additional benefit of postoperative prolongation of the immunonutrition 76,77.
In acute pancreatitis, the use of a platelet-activating factor antagonist initially seemed promising with a decrease in the incidence of organ failure and organ failure score 78,79, while later studies could not confirm the beneficial effects of lexipafant alone in acute pancreatitis 80,81.
In acute pancreatitis, early enteral nutrition seems feasible and reduces costs (at least of the nutritional supply), reduces septic complications, and the inflammatory response 82,83,84,85. Overall, these studies have limitations due to a small number of included patients, a delay prior to initiation of the enteral nutrition, varying severity of pancreatitis, and a distal tube positioning. The supplementation with lacto-bacilli and fibers in early enteral nutrition reduced the incidence of pancreatic sepsis and the need for surgical intervention in patients with prognostic severe acute pancreatitis 86.
For pancreatic cancer, the condition seems to be associated with a frequent pro-inflammatory response with increases in, for example, CRP levels. A predominant problem in patients with irresectable pancreatic cancer, or recurrence after radically attempted resection, is cancer cachexia which seems to depend on the acute phase response 87. A positive effect on the acute phase response, cancer-associated cachexia and quality of life has been reported following the administration of EPA (eicosapentaenoic acid, an omega 3 PUFA) 88. Further support for a pro-inflammatory response in pancreatic cancer is the finding of an increased expression of cyclooxygenase (COX)-2 in pancreatic cancer and that resting energy expenditure decreased, implying a reduction in acute phase protein production, with ibuprofen administration in pancreatic cancer patients 89,90.
Conclusion and future aspects
In general, it seems that a limited window for intervention exists for modulation of the acute inflammatory response due to its complexity. Multiple mediators are involved and thereby a multimodal approach seems reasonable. From a nutritional point of view, emphasis should be put on the importance of enteral nutrition. Preoperative immunomodulation is probably becoming more established and could be offered as an oral formula. Additional specific nutrients and compounds are awaited, the indications for which should be based on the knowledge derived from a detailed mapping of the dynamics of the acute phase response, rendering possibilities for a future tailored and individualized treatment.
Acknowledgments
The present review is based on an invited lecture (RA) at the 5th European Congress of the IHPBA in Istanbul, Turkey, 2003.
References
- 1.O'Flaherty L, Bouchier-Hayes DJ. Immunonutrition and surgical practice. Proc Nutr Soc. 1999;58:831–7. doi: 10.1017/s0029665199001123. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6:298–305. doi: 10.1186/cc1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg. 2000;231:506–11. doi: 10.1097/00000658-200004000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome (review) Am J Surg. 2002;183:630–41. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 5.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–12. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 6.de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996;83:349–53. doi: 10.1002/bjs.1800830317. [DOI] [PubMed] [Google Scholar]
- 7.McKay CJ, Gallagher G, Brooks B, Imrie CW, Baxter JN. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996;83:919–23. doi: 10.1002/bjs.1800830712. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa M. Acute pancreatitis and cytokines: “second attack” by septic complication leads to organ failure. Pancreas. 1998;16:312–15. [PubMed] [Google Scholar]
- 9.Lundberg AH, Granger DN, Russell J, et al. Quantitative measurement of P- and E-selectin adhesion molecules in acute pancreatitis: correlation with distant organ injury. Ann Surg. 2000;231:213–22. doi: 10.1097/00000658-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134:1333–41. [PubMed] [Google Scholar]
- 11.Adams CA, Jr, Sambol JT, Xu DZ, Lu Q, Granger DN, Deitch EA. Hemorrhagic shock induced up-regulation of P-selectin expression is mediated by factors in mesenteric lymph and blunted by mesenteric lymph duct interruption. J Trauma. 2001;51:625–32. doi: 10.1097/00005373-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Adams JM, Hauser CJ, Adams CA, Jr, Xu DZ, Livingston DH, Deitch EA. Entry of gut lymph into the circulation primes rat neutrophil respiratory burst in hemorrhagic shock. Crit Care Med. 2001;29:2194–8. doi: 10.1097/00003246-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Parks RW, Halliday MI, McCrory DC, et al. Host immune responses in intestinal permeability in patients with jaundice. Br J Surg. 2003;90:239–45. doi: 10.1002/bjs.4029. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Ann Surg. 1993;218:111–19. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson R, Andersson E, Axelsson J. Gut barrier and gut inflammatory states in acute pancreatitis. Archives of Hellenic Medicine. 2003;20(Suppl A):61–7. [Google Scholar]
- 16.Andersson R, Wang XD. Gut barrier dysfunction in experimental acute pancreatitis. Ann Acad Med Singapore. 1999;28:141–6. [PubMed] [Google Scholar]
- 17.Eckerwall G, Andersson R. Early enteral nutrition in severe acute pancreatitis-a way of providing nutrients, gut barrier protection, immunomodulation or all of them? Scand J Gastroenterol. 2001;36:449–58. doi: 10.1080/003655201750153179. [DOI] [PubMed] [Google Scholar]
- 18.Lai ECS, Mok FPT, Fan ST, Lo CM, Chu KM, Liu CL. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–8. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 19.Kimmings AN, van Deventer SJ, Obertop H, Rauws EAJ, Gouma DJ. Inflammatory and immunologic effects of obstructive jaundice: pathogenesis and treatment. J Am Coll Surg. 1995;181:567–81. [PubMed] [Google Scholar]
- 20.Welsh FK, Ramsden CW, Maclellan K, et al. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;27:205–12. doi: 10.1097/00000658-199802000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clementz WDB, McCaigue MD, Halliday I, Barclay GR, Rowlands BJ. Conclusive evidence of endotoxemia in biliary obstruction. Gut. 1998;42:293–9. doi: 10.1136/gut.42.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greve JW, Gouma DJ, Soeters PB, Buurman WA. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology. 1990;98:478–85. doi: 10.1016/0016-5085(90)90841-n. [DOI] [PubMed] [Google Scholar]
- 23.Bemelmans MHA, Greve JW, Gouma DJ, Buurman WA. Increased concentrations of tumor necrosis factor (TNF) in soluble TNF receptors in biliary obstruction in mice, soluble receptors as prognostic factors for mortality. Gut. 1996;38:447–53. doi: 10.1136/gut.38.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pain JA, Cahill CJ, Gilbert JN, Johnson CD, Trapnell JE, Bailey ME. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: a multicenter study of bile salts and lactulose. Br J Surg. 1991;78:467–9. doi: 10.1002/bjs.1800780425. [DOI] [PubMed] [Google Scholar]
- 25.Pain JA, Bailey ME. Experimental and clinical study of lactulose in obstructive jaundice. Br J Surg. 1986;73:775–8. doi: 10.1002/bjs.1800731003. [DOI] [PubMed] [Google Scholar]
- 26.Greve JW, Gouma DJ, Soeters PB, Buurman WA. Lactulose inhibits endotoxin-induced tumour necrosis factor production by monocytes. Gut. 1990;31:198–203. doi: 10.1136/gut.31.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–9. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 28.Sewnath ME, Birjmohun RS, Rauws EAJ, Huibregtse K, Obertop H, Gouma DJ. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001;192:726–34. doi: 10.1016/s1072-7515(01)00819-5. [DOI] [PubMed] [Google Scholar]
- 29.Povosky SB, Karpeh MS, Conlon KC, Blumgart LH, Brennan MF. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230:131–42. doi: 10.1097/00000658-199908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pain JA, Collier DS, Ritson A. Reticuloendothelial system phagocytic function in obstructive jaundice and its modification by e-muramyl dipeptide analog. Eur Surg Res. 1987;19:16–22. doi: 10.1159/000128676. [DOI] [PubMed] [Google Scholar]
- 31.Ding JW, Andersson R, Hultberg B, Soltesz V, Bengmark S. Modification of reticuloendothelial function by muramyl dipeptide-encapsulated liposomes in jaundiced rats treated with biliary decompression. Scand J Gastroenterol. 1993;28:53–62. doi: 10.3109/00365529309096045. [DOI] [PubMed] [Google Scholar]
- 32.Ding JW, Andersson R, Soltesz V, et al. Inhibition of bacterial translocation in obstructive jaundice by muramyl tripeptide phosphatidyl-ethanolamine in the rat. J Hepatol. 1994;20:720–8. doi: 10.1016/s0168-8278(05)80141-2. [DOI] [PubMed] [Google Scholar]
- 33.Andersson R, Sun ZW, Wang XD, Deng XM, Soltesz V, Ihse I. Effect of a platelet-activating factor antagonist on pancreatitis-associated gut barrier dysfunction in rats. Pancreas. 1998;17:107–19. doi: 10.1097/00006676-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Menger MD, Plusczyk T, Vollmar B. Microcirculatory derangements in acute pancreatitis. J Hepatobil Pancreat Surg. 2001;8:187–94. doi: 10.1007/s005340170015. [DOI] [PubMed] [Google Scholar]
- 35.Wang XD, Deng XM, Haraldsen P, Andersson R, Ihse I. Antioxidant and calcium channel blockers counteract endothelial barrier injury induced by acute pancreatitis in rats. Scand J Gastroenterol. 1995;30:1129–36. doi: 10.3109/00365529509101619. [DOI] [PubMed] [Google Scholar]
- 36.Schultz HU, Niederau C, Klonowski-Stumpe H, Hallangk W, Luthen N, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736–50. [PubMed] [Google Scholar]
- 37.Bonham MJ, Abu-Zidan FM, Simovic MO, et al. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999;86:1296–301. doi: 10.1046/j.1365-2168.1999.01182.x. [DOI] [PubMed] [Google Scholar]
- 38.Barzilai A, Ryback BJ, Medina JA, Toth L, Dreiling A. The morphological changes of the pancreas in the hypovolemic shock and the effect of pretreatment with steroids. Int J Pancreatol. 1987;2:23–32. doi: 10.1007/BF02788346. [DOI] [PubMed] [Google Scholar]
- 39.Budzynska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. The prospective, randomized placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766–72. doi: 10.1055/s-2001-16520. [DOI] [PubMed] [Google Scholar]
- 40.Matsumura M, Kakishita H, Suzuki M, Banba N, Hattori Y. Dexamethasone suppresses iNOS gene expression by inhibiting NFkappaB in vascular smooth muscle cells. Life Sci. 2001;69:1067–77. doi: 10.1016/s0024-3205(01)01196-1. [DOI] [PubMed] [Google Scholar]
- 41.Van Leeuwen HJ, van der Bruggen T, van Asbeck BS, Boereboom FT. Effect of corticosteroids on nuclear factor-kappa B activation and hemodynamics in late septic shock. Crit Care Med. 2001;27:1074–7. doi: 10.1097/00003246-200105000-00041. [DOI] [PubMed] [Google Scholar]
- 42.Dunn JA, Li C, Ha T, Kao RL, Browder W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am Surg. 1997;63:1036–43. [PubMed] [Google Scholar]
- 43.Gukovsky I, Gukovskaya AS, Blenman TA, Zaninovic V, Pandol SJ. Early NF-kappa B activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 44.Satoh A, Shimosegawa T, Fujita M, et al. Inhibition of nuclear factor-kappa B activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–8. doi: 10.1136/gut.44.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leser HG, Gross V, Scheibenbogen C, Heinisch A, et al. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991;101:782–5. doi: 10.1016/0016-5085(91)90539-w. [DOI] [PubMed] [Google Scholar]
- 46.Viedma JA, Perez-Mateo M, Dominguez JE, Carballo F. The role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein in phospholipase A. Gut. 1992;33:1262–7. doi: 10.1136/gut.33.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berney T, Gasche Y, Robert J, et al. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999;18:371–7. doi: 10.1097/00006676-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Rongione AJ, Kusske AM, Reber HA, Ashley SW, McFadden DW. Interleukin-10 reduces circulating levels of serum cytokines in experimental pancreatitis. J Gastrointest Surg. 1997;1:159–66. doi: 10.1016/s1091-255x(97)80104-7. [DOI] [PubMed] [Google Scholar]
- 49.Osman MO, Kristensen JU, Jacobsen NO, et al. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut. 1998;43:232–9. doi: 10.1136/gut.43.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paszkowski AS, Rau B, Mayer JM, Moller P, Beger HG. Therapeutic application of kaspase-1/interleukin-1κ-converting enzyme inhibitor decreases death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68–76. doi: 10.1097/00000658-200201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werner J, Zgragen K, Fernandez-del Castillo CE, Lewandrowski KB, Compton CC, Warshaw AL. Specific therapy for local and systemic complications of acute pancreatitis with monoclonal antibodies against ICAM-1. Ann Surg. 1999;226:834–42. doi: 10.1097/00000658-199906000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XD, Sun ZW, Börjesson A, Andersson R. Inhibition of platelet-activating factor, intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 reduces experimental pancreatitis-associated gut endothelial barrier dysfunction. Br J Surg. 1999;6:411–16. doi: 10.1046/j.1365-2168.1999.01028.x. [DOI] [PubMed] [Google Scholar]
- 53.Frossard JL, Saluja A, Bhagat L, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- 54.Hartwig W, Jimenez RE, Fernández-del Castillo C, Kelliher A, Jones R, Warshaw AL. Expression of adhesion molecule Mac-1 and L-selectin on neutrophils in acute pancreatitis is protease- and complement-dependent. Ann Surg. 2001;333:371–8. doi: 10.1097/00000658-200103000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haraldsen P, Sun ZW, Borjesson A, Olanders K, Lasson A, Andersson R. Multimodal management-of value in fulminant acute pancreatitis? Pancreatology. 2003;3:14–25. doi: 10.1159/000069148. [DOI] [PubMed] [Google Scholar]
- 56.Bernard GR, Vincent JL, Laterre PF, et al. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 57.Esmon CT. The normal role of activated protein C in maintaining homeostasis and its relevance to critical illness. Crit Care. 2001;5:S7–S12. doi: 10.1186/cc1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creasey AA, Reinhart K. Tissue factor pathway inhibitor activity in severe sepsis. Crit Care Med. 2001;29((7 Suppl)):S126–S129. doi: 10.1097/00003246-200107001-00038. [DOI] [PubMed] [Google Scholar]
- 59.Akahane K, Okamoto K, Kikuchi M, et al. Inhibition of factor Xa suppresses the expression of tissue factor in human monocytes and lipopolysaccharide-induced endotoxemia in rats. Surgery. 2001;130:809–18. doi: 10.1067/msy.2001.116452. [DOI] [PubMed] [Google Scholar]
- 60.Alexander JW. Nutritional pharmacology in surgical patients. Am J Surg. 2002;183:349–52. doi: 10.1016/s0002-9610(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 61.Rhoads JM, Argenzio RA, Chen W, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943–G953. doi: 10.1152/ajpgi.1997.272.5.G943. [DOI] [PubMed] [Google Scholar]
- 62.Alexander JW. Immunonutrition: the role of omega-3 fatty acids. Nutrition. 1998;14:627–33. doi: 10.1016/s0899-9007(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 63.Jyonouchi H. Nucleotide actions on humoral immune responses. J Nutr. 1994;124(1 Suppl):138S–143S. doi: 10.1093/jn/124.suppl_1.138S. [DOI] [PubMed] [Google Scholar]
- 64.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022–9. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295–302. [PubMed] [Google Scholar]
- 66.Jones C, Palmer TA, Griffiths RD. A randomized clinical outcome study of critically ill patients given glutamine-supplemented enteral nutrition. Nutrition. 1999;15:108–15. doi: 10.1016/s0899-9007(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 67.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 68.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 69.Mayer K, Grimm H, Grimminger F, Seeger W. Parenteral nutrition with n-3 lipids in sepsis. Br J Nutr. 2002;87(Suppl 1):S69–S75. doi: 10.1079/bjn2001458. [DOI] [PubMed] [Google Scholar]
- 70.Gianotti L, Braga M, Vignali A, et al. Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Arch Surg. 1997;132:1222–30. doi: 10.1001/archsurg.1997.01430350072012. [DOI] [PubMed] [Google Scholar]
- 71.Senkal M, Mumme A, Eickhoff U, et al. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit Care Med. 1997;25:1489–96. doi: 10.1097/00003246-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med. 1999;27:2799–805. doi: 10.1097/00003246-199912000-00032. [DOI] [PubMed] [Google Scholar]
- 73.Gianotti L, Braga M, Frei A, Greiner R, Di Carlo V. Health care resources consumed to treat postoperative infections: cost saving by perioperative immunonutrition. Shock. 2000;14:325–30. doi: 10.1097/00024382-200014030-00015. [DOI] [PubMed] [Google Scholar]
- 74.McCowen KC, Bistrian BR. Immunonutrition: problematic or problem solving? Am J Clin Nutr. 2003;77:764–70. doi: 10.1093/ajcn/77.4.764. [DOI] [PubMed] [Google Scholar]
- 75.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–53. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 76.Senkal M, Zumtobel V, Bauer KH, et al. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg. 1999;134:1309–16. doi: 10.1001/archsurg.134.12.1309. [DOI] [PubMed] [Google Scholar]
- 77.Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805–14. doi: 10.1067/msy.2002.128350. [DOI] [PubMed] [Google Scholar]
- 78.Kingsnorth AN, Galloway SW, Formela LJ. Randomized, double-blind phase II trial of Lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg. 1995;82:1414–20. doi: 10.1002/bjs.1800821039. [DOI] [PubMed] [Google Scholar]
- 79.McKay CJ, Curran F, Sharples C, Baxter JN, Imrie CW. Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg. 1997;84:1239–43. [PubMed] [Google Scholar]
- 80.Johnson CD, Kingshorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–9. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larvin M, Ammori B, McMahon J, et al. A double-blind, randomised, placebo-controlled multi-center trial to evaluate the efficacy and safety of two doses of lexipafant in acute pancreatitis therapy [Abstract] Pancreatology. 2001;1:279–80. [Google Scholar]
- 82.Kjeldsen BJ, Kronborg O, Fenger C, Jorgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–9. [PubMed] [Google Scholar]
- 83.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–5. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakad A, Piessevaux H, Marot JC, et al. Is early enteral nutrition in acute pancreatitis dangerous? About 20 patients fed by an endoscopically placed nasogastrojejunal tube. Pancreas. 1998;17:187–93. doi: 10.1097/00006676-199808000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Olah A, Pardavi G, Belagyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18:259–62. doi: 10.1016/s0899-9007(01)00755-9. [DOI] [PubMed] [Google Scholar]
- 86.Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–7. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 87.Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–8. doi: 10.1007/pl00012351. [DOI] [PubMed] [Google Scholar]
- 88.Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81:80–6. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wigmore SJ, Falconer JS, Plester CE, et al. Ibuprofen reduces energy expenditure and acute-phase protein production compared with placebo in pancreatic cancer patients. Br J Cancer. 1995;72:185–8. doi: 10.1038/bjc.1995.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merati K, said Siadaty M, Andea A, et al. Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol. 2001;24:447–52. doi: 10.1097/00000421-200110000-00007. [DOI] [PubMed] [Google Scholar]