Abstract
Background: As many as 50% of patients with renal cell carcinoma (RCC) will develop systemic metastases. When hepatic metastases from RCC present in a resectable distribution, our group and other groups have previously shown that some patients benefit from curative hepatic resection. In this report we update our own experience and summarize the literature published to date on this topic. Patients and methods: From 1982 to 2005, 19 patients (9 men, 10 women, median age 50 years) with hepatic metastases from RCC were treated with hepatic resection at our institution. In 14 (74%) of the 19 patients the presentation of hepatic metastases was metachronous. Seven (37%) patients had been or were simultaneously treated for extrahepatic metastases. The mean tumor number was 2 and the mean diameter of the largest metastasis was 73 mm. Results: Margin-negative resection was achieved in 17 (89%) of 19 cases. Postoperative morbidity and mortality rates were 32% and 5%, respectively. At a median follow-up interval of 26 months, 15 patients recurred with a mean time to recurrence of 12 months. The 3-year and 5-year disease-free survival rates were 25% and 25%, respectively; 3-year and 5-year overall survival rates were 52% and 26%, respectively, with one patient alive 5 years following first hepatectomy. Study factors which predicted prolonged survivals included male sex and maximum tumor diameter ≤5 cm. Discussion: The overall survival rates in our series (3-year, 52%; 5-year, 26%) and in a complete review of the literature (3-year, 24%; 5-year, 18%) indicate that selected patients with hepatic metastases from RCC benefit from hepatic resection.
Keywords: Renal cancer, liver metastases, hepatic resection, outcomes
Introduction
Malignant renal cancers occur in 3% of adults. These tumors occur three times more frequently in men than in women, with a peak incidence in the sixth to eighth decades of life. The majority of renal cancers are adenocarcinomas, and a majority of these are of the clear cell subtype. As these tumors tend to be aggressive and are resistant to treatment, the prognosis of patients with advanced-stage renal cancer is poor.
At some point in their clinical course, as many as 50% of patients diagnosed with renal cancer will develop systemic metastases. The mechanisms for metastases are twofold. First, these tumors tend to spread via retroperitoneal lymphatics to distant sites, most commonly lung, liver, lymph nodes, and bone. Second, and unique to this malignancy, renal cancers often demonstrate an endovenous growth pattern. They can gain access to the vena cava via the renal veins and develop a tumor thrombus that may extend in the retrohepatic vena cava to the right heart chambers.
Frequently, there is a considerable delay between treatment of the primary renal tumor and development of detectable systemic metastases 1. The lungs are the most common site of renal cancer metastases, accounting for 75% of distant disease. Although 20% of patients will develop hepatic metastases, in very few cases is the liver the only site of distant spread. In fact, the development of hepatic metastases is generally considered a poor prognostic factor and a frequent harbinger of more widespread disease.
Although relatively few patients present with a metastatic disease pattern that is amenable to a curative surgical approach, we and others have previously identified subsets of patients who achieve long-term survival following hepatic resection. A review of the literature identifies 15 reports describing surgical treatment of hepatic metastases from renal cancer published from 1978 to 2005 (Table I) 1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. Combined, these series provide survival data following hepatic resections for renal cancer metastases in 64 patients. Importantly, the 2-year overall survival of this cohort was 40%, which compares favorably to the 2-year overall survival of 10% for all patients who present with metastatic renal carcinoma 16.
Table I. Review of the literature reporting hepatic resection for renal cancer metastases.
| Author | Year | Number of patients | Survival |
|---|---|---|---|
| Foster 2 | 1978 | 5 | Died at 2, 6, 7, 33, and 144 months, respectively |
| Morrow et al. 3 | 1982 | 1 | Alive at 5 years |
| Thompson et al. 4 | 1983 | 1 | Data missing |
| Iwatsuki & Starzl 5 | 1988 | 3 | Data missing |
| Pontes et al. 6 | 1989 | 2 | Data missing |
| Tongaonkar et al. 7 | 1992 | 1 | Died at 10 months |
| Antoniewicz et al. 8 | 1994 | 2 | One died at 8 months, one alive at 24 months |
| Bennett et al. 9 | 1995 | 4 | Two died at 13 and 14 months, two alive at 21 and 32 months |
| Harrison et al. 10 | 1997 | 5 | Three alive at 5 years |
| Stief et al. 1 | 1997 | 13 | Mean survival 16 months |
| Fujisaki et al. 11 | 1997 | 3 | Two died at 10 and 18 months, one alive at 12 months |
| Kawata et al. 12 | 2000 | 4 | Two alive at 24 months |
| Karavias et al. 13 | 2002 | 6 | One died at 1 year, five alive at 2, 3, 5 years |
| Alves et al. 14 | 2003 | 14 | Median survival: 26 months; survival at 1 and 3 years, 69% and 26%, respectively; one alive at 8 years |
| Weitz et al. 15* | 2005 | 11 | Two alive at 24 months |
| Current study† | 2005 | 19 | Median survival: 36 months; survival at 3 and 5 years, 52% and 26%, respectively; one alive at 10 years |
| Total | 1978–2005 | 75‡ | 1-year survival: 46% |
| 3-year survival: 24% | |||
| 5-year survival: 18% |
Also contained in these reports are analyses of prognostic factors. In our report published in 2003, we described a univariate analysis of the clinical features of 14 patients with hepatic metastases from renal cancer treated with hepatic resection 14. In terms of prognostic utility, the three most important factors which predicted improved outcome were as follows: (1) presentation of metastases >24 months following nephrectomy, (2) metastases size <5 cm, and (3) R0 resection. In addition, in patients who had recurrences in the liver following resection those who were candidates for repeat hepatectomy experienced a longer survival than those who were not 14. Likewise, other series have identified a survival advantage for patients who present with a disease-free interval between nephrectomy and diagnosis of their hepatic metastases >24 months and when metastases size is <5 cm 7.
In the current report we update our experience to include five additional patients treated during the past 3 years. Analysis of potential prognostic factors in the expanded cohort of 19 patients both confirms our earlier findings and provides further insight into the natural history of this disease.
Patients and methods
Between June 1982 and September 2005, 19 patients (9 men, 10 women) with metastases from renal tumors were submitted to a liver resection at our institution. Mean patient age was 50 years with a range from 17 to 76 years. In 16 (84%) cases, the primary tumor histology was adenocarcinoma (clear cell subtype), and in the remaining 3 (16%) cases the histology was embryonal. Most patients were diagnosed with metastases long after the treatment of their primary tumor, as evidenced by the mean post-hephrectomy disease-free interval of 53 months (range 9–137 months). Patients with extrahepatic metastases were included if all metastatic disease could be approached with curative intent. Seven (37%) of the 19 patients had been treated previously for extrahepatic metastases or presented with simultaneous resectable extrahepatic disease. Demographic, historical, and pathologic data for this cohort are presented in Table II.
Table II. Characteristics of 19 patients undergoing hepatic resection for renal cancer metastases.
| Factor | n | % | ||
|---|---|---|---|---|
| Age (years) | ||||
| ≤ 30 | 3 | 16% | ||
| 30–60 | 9 | 47% | ||
| > 60 | 7 | 37% | ||
| Sex | ||||
| Male | 9 | 47% | ||
| Female | 10 | 53% | ||
| Primary tumor histology | ||||
| Adenocarcinoma | 16 | 84% | ||
| Embryonic | 3 | 16% | ||
| Timing of metastases diagnosis | ||||
| ≤ 3 months following treatment of primary | 5/19 | 26% | ||
| > 3 months following treatment of primary | 14/19 | 74% | ||
| Median time from treatment of the primary to diagnosis of metastases (range), months | 53 (9–139) | |||
| Number of metastases | ||||
| 1 | 8 | 42% | ||
| 2–3 | 10 | 53% | ||
| > 3 | 1 | 5% | ||
| Median size (range), mm | 73 (20–210) | |||
| Hepatic location | ||||
| Unilobar | 15 | 79% | ||
| Bilobar | 4 | 21% | ||
Statistical considerations
Disease-free and overall survival rates were calculated by the method of Kaplan and Meier. To determine prognostic value, study variables were compared to survivals using log-rank tests.
Results
Operative details
Forty-two percent of patients presented with a solitary hepatic metastasis and in 15 (79%) of the 19 cases the metastases were confined to one hemiliver. The mean size of the largest metastasis was 73 mm (range 20–210 mm). Major hepatectomy, defined as resection of more than 3 liver segments, was required in 14 (74%) of the 19 operations. Anatomic resection alone was performed in 10 cases, while 2 patients were submitted to nonanatomic resection, and the remaining 7 patients required a combined anatomic and nonanatomic resection approach. Margin-negative (R0) resections were achieved in 17 (89%) cases.
No patients died within 30 days of hepatectomy; however, one patient died from infectious complications during the second postoperative month. Seven local complications were observed in a total of six (32%) patients but none required re-intervention. These consisted of biliary fistula in three patients, noninfected perihepatic fluid collection in two, hemorrhage in one, and hepatic insufficiency in one. General complications were observed in 4 (21%) patients. The mean inpatient hospital stay was 13 days with a range from 6 to 27 days.
Clinical follow-up and outcomes
Recurrence and survival data were available for all 19 study patients (Table III). At a mean follow-up interval of 26 months, five (26%) patients remained disease-free. In the remaining 14 (74%) patients, 3 experienced hepatic recurrence only, 5 experienced extrahepatic recurrence only, and 7 experienced both hepatic and extrahepatic recurrence.
Table III. Disease-free and overall survival rates following resection of renal cancer hepatic metastases.
| Parameter | n | % | |
|---|---|---|---|
| Recurrences | |||
| None | 5 | 26 | |
| Hepatic only | 2 | 10 | |
| Extrahepatic only | 6 | 32 | |
| Both hepatic and extrahepatic | 6 | 32 | |
| Status at date of last follow-up | |||
| Alive | 9 | 47 | |
| With recurrence | 5 | 56 | |
| Hepatic only | 0 | ||
| Extrahepatic only | 3 | ||
| Both hepatic and extrahepatic | 2 | ||
| Without recurrence | 4 | 44 | |
| Dead | 10 | 53 | |
| With recurrence | 9 | 90 | |
| Hepatic only | 2 | ||
| Extrahepatic only | 3 | ||
| Both hepatic and extrahepatic | 4 | ||
| Without recurrence | 1 | 10 | |
| Median time from hepatectomy to hepatic recurrence (range), months | 12 (2–27) | ||
| Median time from hepatectomy to extrahepatic recurrence (range), months | 11 (1–50) | ||
| Median, months | 3-year, % | 5-year, % | |
| Survivals | |||
| Disease-free | 13 | 25 | 25 |
| Overall | 36 | 52 | 26 |
| Total alive 5 years following first hepatectomy | 1 | ||
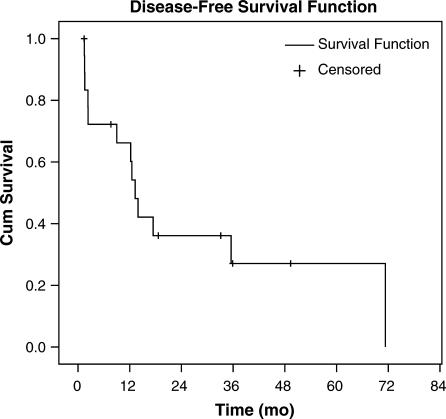
The time to hepatic recurrence ranged from 2 to 27 months, with a mean time of 12 months. Second hepatectomies for hepatic recurrence were performed in three patients, one of whom also underwent a third hepatectomy for recurrence. The time to extrahepatic recurrence ranged from 1 to 50 months with a mean time of 11 months. In two patients, extrahepatic recurrences were amenable to resection. When combined with patients previously or simultaneously treated for extrahepatic disease, in total nine patients underwent at least one extrahepatic resection as part of their treatment during the study interval. The median, 3-year, and 5-year disease-free survival rates following hepatectomy were 13 months, 25%, and 25%, respectively (Figure 1).
Figure 1. .
Kaplan–Meier analysis of disease-free survival for 19 patients following hepatic resection for renal cancer metastases.
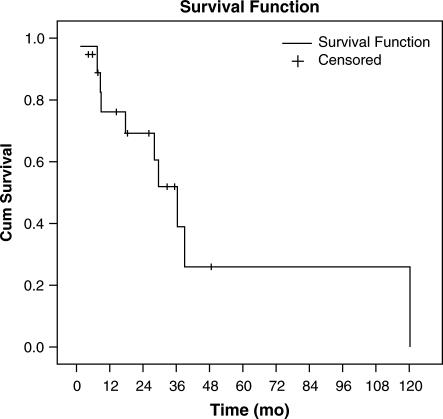
Overall survival data, which were available for all 19 patients, determined that 10 patients died during follow-up. Nine of these 10 patients have succumbed to metastatic renal cancer and 1 patient died of a nononcologic cause. Of the nine patients who are alive following initial hepatectomy, five have experienced recurrence and four have remained disease-free. One patient is alive 5 years following hepatic resection. Combined, these data yield median, 3-year, and 5-year overall survivals following initial hepatic resection of 36 months, 52%, and 26%, respectively (Figure 2).
Figure 2. .
Kaplan–Meier analysis of overall survival for 19 patients following hepatic resection for renal cancer metastases.
Analysis of prognostic factors
Univariate analysis of study variables reveals several associations between prognostic factors and outcome (Table IV). In comparison with female patients (median survival = 17 months), male patients (median survival = 39 months) lived longer following hepatic resection (p=0.014). Patients between 30 and 60 years of age experienced longer survival (median survival = 120 months) than either younger (median survival = 9 months) or older (median survival = 36 months) patients. In addition, patients with tumors ≤5 cm in diameter (median survival = 39 months) tended to live longer than patients with larger tumors (median survival = 30 months) (p = 0.066). Patients with disease-free intervals between treatment of the primary tumor and diagnosis of liver metastases >2 years (median survival = 36 months) tended to live longer following hepatectomy than patients with shorter disease-free intervals (median survival = 28 months). Finally, patients who had recurrences and were treated with rehepatectomy experienced a median survival of 39 months, which was longer than the median survival of patients who did not undergo repeat hepatectomy (median survival = 28 months).
Table IV. Univariate analysis of potential prognostic variables in patients with renal cancer metastatic to the liver.
| Survival rates | ||||||
|---|---|---|---|---|---|---|
| Factor | n | 1-year (%) | 3-year (%) | 5-year (%) | Median (months) | p value |
| Age | ||||||
| ≤ 30 years | 3 | 33 | 33 | – | 9 | 0.35 |
| 30–60 years | 9 | 77 | 57 | 57 | 120 | |
| > 60 years | 7 | 100 | 53 | – | 36 | |
| Sex | ||||||
| Female | 10 | 64 | 24 | – | 18 | 0.014 |
| Male | 9 | 88 | 74 | 49 | 39 | |
| Histology | ||||||
| Adenocarcinoma | 16 | 79 | 47 | 31 | 30 | 0.99 |
| Embryonic | 3 | 67 | 67 | – | 39 | |
| Timing of metastases presentation | ||||||
| Metachronous (>3 months) | 14 | 83 | 49 | 25 | 30 | 0.66 |
| Synchronous (≤3 months) | 5 | 57 | – | – | – | |
| Treatment of the primary to diagnosis of metastases | ||||||
| ≤ 24 months | 11 | 72 | 45 | 45 | 28 | 0.67 |
| > 24 months | 8 | 83 | 63 | – | 36 | |
| Number of metastases | ||||||
| 1 | 8 | 67 | – | – | 23 | 0.13 |
| 2–3 | 10 | 79 | 79 | 38 | 39 | |
| > 3 | 1 | – | – | – | – | |
| Size of largest metastases | ||||||
| ≤ 5 cm | 12 | 100 | 69 | 48 | 39 | 0.066 |
| > 5 cm | 7 | 58 | 40 | – | 30 | |
| Location | ||||||
| Unilateral | 15 | 70 | 52 | 17 | 36 | 0.35 |
| Bilateral | 4 | 100 | 50 | – | – | |
| Prior or simultaneous extrahepatic metastases | ||||||
| Absent | 12 | 82 | 55 | 37 | 39 | 0.33 |
| Present | 7 | 67 | 50 | – | 27 | |
| Extent of hepatectomy | ||||||
| Major (>3 segments) | 14 | 71 | 61 | 30 | 36 | 0.48 |
| Minor | 5 | 100 | – | – | 28 | |
| Margin of resection | ||||||
| R0 | 17 | 80 | 62 | 31 | 36 | 0.16 |
| R1 | 2 | 50 | – | – | 19 | |
| Resection of recurrent hepatic metastases | ||||||
| Absent | 16 | 71 | 50 | – | 28 | 0.44 |
| Present | 3 | 100 | 67 | 33 | 39 | |
Discussion
Patients with metastatic renal cell cancer have few viable therapeutic options. Renal carcinomas and their metastases rarely respond to traditional systemic chemotherapy, radiotherapy, or hormone modulation therapy. Preliminary reports suggest that approximately 10% of patients will respond to immunotherapy composed of interleukin-2 and a higher percentage may respond to novel anti-angiogenic agents, but these approaches remain investigational 17. Although reports of prolonged survival following hepatic resection for renal cancer metastases include highly selected patients, the significantly improved survival experienced by resected patients compared with a general population with metastatic renal cancer (5-year survival 24% vs < 10%, respectively) argues that the treatment rather than the selection of patients with favorable tumor biology is responsible for this effect. Surgical extirpation of resectable renal cancer metastases, therefore, appears to provide the only potential for long-term survival for patients with renal cancer liver metastases.
Unfortunately, despite ‘R0’ hepatic resection, many patients will have recurrences both in the liver and at extrahepatic sites. In addition, no reliable serologic marker of recurrence or disease burden exists. As such, the importance of close clinical follow-up after hepatic resection, to include axial imaging of the thorax and abdomen, cannot be understated. Clinical and radiographic follow-up at 3-month intervals for the first 2 years following resection, and subsequent follow-up at 6-month intervals is recommended.
Our data show that over half of the post hepatectomy recurrences will occur within the first year. In many cases, patients with recurrent metastases in the liver or the lung can be submitted to repeat resection. The finding that the subset of patients with resectable recurrences have longer disease-free and overall survival rates when compared to patients with unresectable recurrences further supports the argument that surgery rather than favorable tumor biology is responsible for improved outcomes.
Previously we have shown that prognostic factors (including disease-free interval >24 months, tumor size <5 cm, and complete resection) can be used to identify patients with renal cancer metastases who most benefit from resection. Our current data, which include analysis of one-third of the reported cases of hepatectomy for metastatic renal cancer, show that male patients, those with small metastases, and those with long intervals between primary tumor therapy and diagnosis of hepatic metastases have the best prognosis following resection. Although recurrences are extremely common, up to half of the patients who share these favorable prognostic features can expect survival intervals of 5 years following resection. For those who do not meet these criteria, survival rates of >2 years are common, particularly when recurrent metastases present in a resectable pattern.
These data strongly support the recommendation that, in the absence of effective systemic therapies, liver resection should be offered to all patients with liver metastases from renal cancer, provided that a complete resection is feasible.
References
- 1.Stief CG, Jahne J, Hagemann JH, Kuczyk M, Jonas U. Surgery for metachronous solitary liver metastases of renal cell carcinoma. J Urol. 1997;158:375–7. [PubMed] [Google Scholar]
- 2.Foster J. Survival after liver resection for secondary neoplasms. Am J Surg. 1978;135:389–94. doi: 10.1016/0002-9610(78)90072-7. [DOI] [PubMed] [Google Scholar]
- 3.Morrow CE, Grage TB, Sutherland DE, Najarian JS. Hepatic resection for secondary neoplasms. Surgery. 1982;92:610–14. [PubMed] [Google Scholar]
- 4.Thompson HH, Tompkins RK, Longmire WP., Jr Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375–88. doi: 10.1097/00000658-198304000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–34. doi: 10.1097/00000658-198810000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pontes JE, Huben R, Novick A, Montie J. Salvage surgery for renal cell carcinoma. Semin Surg Oncol. 1989;5:282–5. doi: 10.1002/ssu.2980050411. [DOI] [PubMed] [Google Scholar]
- 7.Tongaonkar H, Kulkarni J, Kamat M. Solitary metastases from renal cell carcinoma: a review. J Surg Oncol. 1992;5:282–5. doi: 10.1002/jso.2930490111. [DOI] [PubMed] [Google Scholar]
- 8.Antoniewicz A, Krawczyk M, Polanski J, Borowka A, Borkowski A. Resection of the liver in a metastatic disease caused by renal carcinoma. Mater Med Pol. 1994;26:143–4. [PubMed] [Google Scholar]
- 9.Bennett BC, Selby R, Bahnson RR. Surgical resection for management of renal cancer with hepatic involvement. J Urol. 1995;154:972–4. [PubMed] [Google Scholar]
- 10.Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–32. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 11.Fujisaki S, Takayama T, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, et al. Hepatectomy for metastatic renal cell carcinoma. Hepatogastroenterology. 1997;44:817–19. [PubMed] [Google Scholar]
- 12.Kawata N, Hirakata H, Yuge H, Kodama M, Sugimoto S, Yagasaki H, et al. Cytoreductive surgery with liver-involved renal cell carcinoma. Int J Urol. 2000;7:382–5. doi: 10.1046/j.1442-2042.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 13.Karavias DD, Tepetes K, Karatzas T, Felekouras E, Androulakis J. Liver resection for metastatic non-colorectal non-neuroendocrine hepatic neoplasms. Eur J Surg Oncol. 2002;28:135–9. doi: 10.1053/ejso.2001.1221. [DOI] [PubMed] [Google Scholar]
- 14.Alves A, Adam R, Majno P, Delvart V, Azoulay D, Castaing D, et al. Hepatic resection for metastatic renal tumors: is it worthwhile? Ann Surg Oncol. 2003;10:705–10. doi: 10.1245/aso.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D'Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 17.Cooney MM, Remick SC, Vogelzang NJ. Novel agents for the treatment of advanced kidney cancer. Clin Adv Hematol Oncol. 2004;2:664–70. [PubMed] [Google Scholar]