Abstract
Background. The most common site of colorectal extra-abdominal metastases is the lung. The relative risk of lung metastases after resection and cryotherapy has not previously been compared. Methods. All patients underwent an extensive preoperative staging including clinical examination, abdominal computed tomography (CT) and abdominal angio-CT to assess their hepatic disease. Two groups of patients were compared in this study (hepatic resection alone and hepatic cryotherapy with or without resection). A retrospective analysis of prospectively collected data was performed to assess the incidence and disease-free interval of pulmonary metastasis after surgical treatment of colorectal liver metastasis. Results. This paper clearly shows two differences regarding pulmonary metastases between patients treated with resection only and cryotherapy with or without resection. Among the 10 clinical variables, cryotherapy had the greatest correlation with pulmonary metastases (p=0.004). A patient who undergoes hepatic resection only has a probability of 35% for developing pulmonary recurrence, compared with 51% following cryotherapy. Cryotherapy was also independently associated with shorter pulmonary disease-free interval (p=0.036). Conclusion. There clearly is a higher risk of pulmonary metastasis after cryotherapy than after resection, whether this is related to selection of patients or a direct deleterious procedural effect requires more study.
Introduction
Colorectal cancer is a major cause of cancer-related mortality; 40% of these patients will develop hepatic metastases 1. Liver resection has been regarded as the only curative treatment for colorectal liver metastases, yielding 5-year survival of 20–30% 2,3,4. However, only 25% of such patients are suitable for liver resection, due to extensive hepatic disease, extra-hepatic spread at presentation or the patients poor performance status 5,6,7.
Hepatic cryotherapy was the first widely used ablative technique 5 for colorectal liver metastases and is a relatively safe and effective alternative treatment for colorectal liver metastases, achieving a 5-year survival of 20% 8. We have also used the combined treatment modality of liver resection and cryotherapy for residual disease in the contralateral lobe (contralobe cryotherapy) and this has produced similar survival results to hepatic resection alone 9,10. Edge-cryotherapy for close margins appears to substantially reduce the risk of recurrence and achieves 20% 5-year survival even in patients with involved margins 11. These surgical techniques and others allow us to offer potentially curative surgery to a greater proportion of patients with liver-only disease and the survival is much superior to that achieved with regional or systemic chemotherapy.
The most common site of colorectal extra-abdominal metastases is the lung. The risk of lung metastases after resection and cryotherapy has not previously been reported. These procedures have fundamental differences. We hypothesized that a higher pulmonary recurrence rate after hepatic cryotherapy might be seen as a result of intravascular dissemination due to detachment of viable tumour emboli during cryo-probe introduction and after thawing. We also hypothesized that the greater cytokine release following cryotherapy could up-regulate the expression of adhesion molecules in vascular endothelium, thus favouring tumour cell implantation and growth of metastases. The aim of this study was to compare the incidence of pulmonary metastases after hepatic resection with that after hepatic cryotherapy.
Materials and methods
Patient selection
Between April 1990 and January 2003, 906 patients had a laparotomy with the intention of surgical treatment of colorectal liver metastases in our institution. In all, 430 patients underwent hepatic arterial catheter insertion for regional chemotherapy only; 78 patients had cryotherapy applied to the liver resection edge due to an involved or inadequate resection margin; 47 patients had cryotherapy and/or resection with palliative intent because of the presence of extrahepatic disease or extensive intrahepatic disease, which was not detected during preoperative work-up; 53 patients had other types of ablative or palliative surgical therapy including radiofrequency ablation. The remaining 298 patients underwent surgical treatment with curative intent and formed the basis of this report. Among them, 160 patients had liver resection, 74 patients had combined treatment of resection and contralobe cryotherapy and 64 had cryotherapy alone. Patients were considered for liver resection on the basis of the following criteria: (1) absence of extrahepatic disease; (2) preservation of adequate functional liver tissue. Patients were considered for cryotherapy alone or in combination with resection on the basis of the following criteria: (1) absence of extrahepatic disease; (2) bilobar liver disease beyond the reach of resection to achieve total clearance in which cryotherapy allowed focal destruction of tumours and preservation of functional liver tissue (Note that the number of colorectal liver metastases per se was not a criterion for deciding whether the patient would receive resection, cryotherapy or both, because the authors believe that number is not a strong prognostic determinant for survival 8).
Preoperative management
All of these patients underwent an extensive preoperative work-up including clinical examination, abdominal computed tomography (CT), chest CT, pelvic CT and bone scan to exclude extrahepatic disease. Positron emission tomography (PET) was not routinely available. If liver resection or cryotherapy was indicated, abdominal angio-CT was used for assessment of hepatic involvement (number, size and localization) and resectability. Measurements of liver function and preoperative carcinoembryonic antigen (CEA) level were carried out. A preoperative biopsy of the hepatic tumour was not usually performed.
Operative technique
The initial right subcostal incision was performed to exclude extrahepatic malignancy. Any suspicious lymph node or peritoneal nodule was biopsied for frozen section histology. A thorough examination of the liver was performed both by palpation and by intraoperative ultrasonography (IOUS) to confirm the number, size, location (in relation to hepatic and portal veins) and echogenicity of the tumours. When liver resection or cryotherapy was feasible, the incision was extended to bilateral subcostal or triradiate incision and the liver was fully mobilized.
Liver resection was performed using an ultrasonic dissector (Sumisonic ME-2210; Sumitomo Bakelite Co., Japan or Selector Spembly UK), with portal inflow occlusion only in selected cases to minimize blood loss or improve the efficiency of cryotherapy. Resection was used preferentially for large tumours, because of high recurrence rates with cryotherapy 12 and the relationship of cryo-volume to cryo-shock 13.
Cryotherapy was performed using the LCS 3000 liquid nitrogen system (Spembly, Andover, UK) or the Erbe system (Tubingen, Germany). For the superficial lesions, a spike probe was inserted into the centre of the lesion under direct vision. For the deep intra-parenchymal lesions, a Seldinger-type technique was used by placing an ultrasound (US)-guided spinal needle in the tumour, followed by insertion of cryo-probe(s) 5. Intraoperative US (IOUS) was used to monitor ice-ball formation and in particular its extension beyond the tumour in all planes by a margin of 1 cm 14,15. In most cases, we allow thawing of the outer rim of the ice-ball by approximately 1 cm prior to refreezing (partial double freeze-thaw cycle) to increase the lethality of freezing, because complete thawing would considerably increase the operative time and, more importantly, complete thawing and refreezing is causally associated with the cryo-shock phenomenon 5,14,16,17, After thawing, the probe was withdrawn gently and some gelfoam was packed in the cryoprobe track to minimize bleeding. Cryolesions often crack and any subsequent bleeding was easily managed by suturing. Hepatic arterial catheter insertions were routinely placed and details of the technique are described elsewhere 18,19.
Postoperative management
Adjuvant hepatic arterial chemotherapy with 1 g 5-fluorouracil per 24 hours for 4 days every 2 weeks, was commenced within 1 month of the cryosurgery. Patients were reviewed 1 month after their surgery and at 3-monthly intervals thereafter. This included clinical examination and measurement of CEA level. Any rise in CEA levels was investigated by CT to identify recurrent disease. In patients with a serum CEA level of <5 ng/ml preoperatively (non-CEA secretors) abdominal CT was performed every 3 months. All patients were followed up until January 2004 or until death occurred. A minority of patients who were privately insured received 5-fluorodeoxy-uridine (FUDR) rather than 5-FU. If FUDR was to be used, we would recommend waiting at least 1 month before starting the therapy, otherwise bilomas are likely to develop in the cryo-site(s) 20. FUDR was given at 0.18 mg/kg for 2 weeks on and 2 weeks off. Systemic chemotherapy was only given to the patients who developed extrahepatic metastases.
Statistical analysis
The study outcome of presence or absence of pulmonary metastases was analysed by treatment groups and available clinical factors. For univariate analysis, χ2 (or Fisher's exact) test was used for categorical factors, Wilcoxon rank test was used for the comparison of difference in means of continuous factors. Logistic regression was used for the multivariate analysis of study outcome. All combinations of the selected clinical variables were compared in order to obtain the best prediction of outcome. The disease-free survival analysis was performed using Kaplan-Meier method and the log-rank test was used for the comparison of survival between groups. Multivariate analysis for disease-free interval was performed using a Cox regression (Cox proportional hazards model) with forward stepwise selection of covariates and with enter and remove limits of p<0.05 and p>0.10, respectively. These statistical analyses were performed using SPSS for Windows (Version 11.5; SPSS GmbH, Munich, Germany). A significant difference was assumed for p values <0.05.
Results
Between April 1990 and January 2003, 298 patients with colorectal hepatic metastases underwent surgical treatment with curative intent in our liver unit. The minimum length of follow-up was 12 months. One hundred and sixty patients had hepatic resection alone; 74 patients had resection and contralobe cryotherapy and 64 patients had cryotherapy only. One hundred and forty-one patients (47%) remained alive. The overall median follow-up was 26 months (range 12–110 months). The median follow-up was 26 months (range 12–85) in the resection group and 26 months (range 12–110) in the cryotherapy with or without resection group. The mean length of follow-up was 28 and 31 months for the resection and cryotherapy groups, respectively. One hundred and twenty-seven patients (43%) had developed pulmonary recurrence at the last time of contact. The distribution of nine clinical variables among these two groups is shown in Table I and analysed using χ2 test or Wilcoxon rank test. It showed that International Union Against Cancer TNM staging of the primary tumor (p=0.001), synchronous vs metachronous (p=0.000), number (p=0.000) and size (p=0.000) of colorectal liver metastases were statistically different between the two groups.
Table I. The distribution of nine clinical variables in the resection only group and the cryotherapy with or without resection group (n=298).
| Parameter | Resection only |
Cryotherapy±resection |
|||
|---|---|---|---|---|---|
| Clinical factors | n=160 | % | n=138 | % | p value |
| Gender | 0.581 | ||||
| Male | 90 | 56 | 82 | 59 | |
| Female | 70 | 44 | 56 | 41 | |
| Age (years) | 0.165 | ||||
| ≤ 60 | 66 | 41 | 68 | 49 | |
| > 60 | 94 | 59 | 70 | 51 | |
| UICC TNM staging | 0.001 | ||||
| Stage I and II | 53 | 33 | 25 | 18 | |
| Stage III | 55 | 34 | 37 | 27 | |
| Stage IV | 37 | 23 | 60 | 44 | |
| Unknown | 15 | 9 | 16 | 11 | |
| Primary histological differentiation | 0.439 | ||||
| Well/moderate | 115 | 72 | 95 | 69 | |
| Poor | 20 | 12 | 14 | 10 | |
| Unknown | 25 | 16 | 29 | 21 | |
| Treatment time of liver metastases | 0.000 | ||||
| Synchronous | 15 | 9 | 60 | 43 | |
| Metachronous | 145 | 91 | 78 | 57 | |
| No. of liver metastases | 0.000 | ||||
| 1 | 91 | 57 | 12 | 9 | 0.000* |
| 2–3 | 55 | 34 | 53 | 38 | |
| > 3 | 14 | 9 | 73 | 53 | |
| Largest size of liver metastases | 0.000 | ||||
| 1–3 cm | 42 | 26 | 91 | 66 | 0.000* |
| 4–6 cm | 55 | 34 | 34 | 25 | |
| > 7 cm | 63 | 40 | 13 | 9 | |
| Preoperative CEA level (ng/ml) | 0.164 | ||||
| ≤ 5.0 | 43 | 27 | 34 | 24 | |
| 5.01–100.0 | 81 | 51 | 59 | 43 | |
| > 100.0 | 22 | 14 | 22 | 16 | |
| Unknown | 14 | 9 | 23 | 17 | |
| Postoperative CEA level (ng/ml) | 0.840 | ||||
| ≤ 5.0 | 121 | 76 | 104 | 75 | |
| > 5.0 | 25 | 15 | 24 | 17 | |
| Unknown | 14 | 9 | 10 | 7 | |
* Analysed using Wilcoxon rank test.
Mortality and morbidity rates
One patient (0.3%) died of myocardial infarction in the hospital within 30 days postoperatively. Intraoperative haemorrhage that required transfusion of more than four units of packed red blood cells occurred in 18 patients (11%) in the resection group and in 7 patients (5%) in the cryotherapy with or without resection group. The total postoperative morbidity rate was 29% (87 of 298 patients). Postoperative complications included wound infection (n=21), intra-abdominal abscess (n=26), adhesional bowel obstruction (n=5), biloma or fistula (n=16), bile leak (n=6), sclerosing cholangitis (n=7), respiratory complications (n=28), cardiac complications (n=6) and renal complications (n=13).
Survival
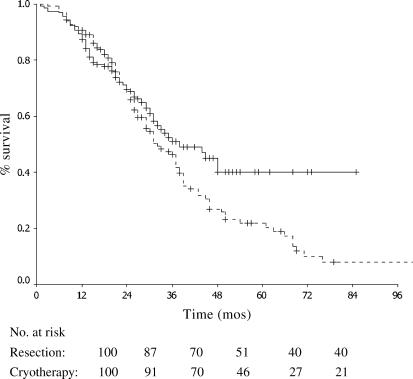
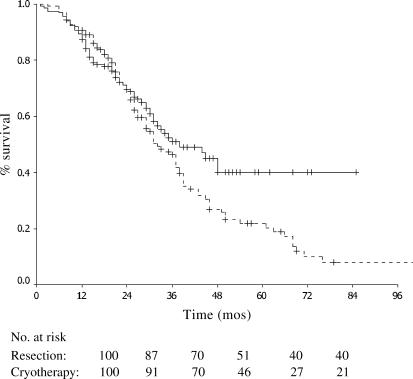
The median survival was 38 months in the resection group vs 32 months in the cryotherapy±resection group. The 1-, 3- and 5-year survivals were 87%, 51% and 35% in the resection group vs 91%, 46% and 22% in the cryotherapy±resection group, respectively (Figure 1).
Figure 1. .
Survival in patients with colorectal liver metastases treated with resection only (solid line, n=160) and cryotherapy with or without resection (dashed line, n=138) (p=0.076).
Incidence of colorectal pulmonary metastases
The correlation of clinical factors and incidence of pulmonary metastases in 298 patients with surgically treated colorectal liver metastases is indicated in Table II. Statistical analysis was performed using χ2 test and Wilcoxon rank test. Univariate analysis showed that the only clinical factor that was independently associated with a higher incidence of pulmonary metastases was the use of cryotherapy with or without resection (p=0.004). Logistic regression showed that operation type (resection vs cryotherapy with or without resection) had the greatest magnitude of odds ratio of outcome. Number of lesions and other factors were not associated with development of lung metastasis. A patient who underwent hepatic resection only had a probability of 35% of developing pulmonary recurrence, compared with 51% if they had cryotherapy. The incidence of concurrent metastatic disease with pulmonary metastases is demonstrated in Table III.
Table II. The correlation of clinical factors and incidence of pulmonary metastases in 298 patients with surgically treated colorectal liver metastases.
| Factors | Pulmonary recurrence |
||||
|---|---|---|---|---|---|
| Yes |
No |
||||
| Clinical factors | n=127 | % | n=171 | % | p value |
| Gender | 0.434 | ||||
| Male | 70 | 55 | 102 | 60 | |
| Female | 57 | 45 | 69 | 40 | |
| Age (years) | 0.165 | ||||
| < 60 | 63 | 50 | 71 | 42 | |
| ≤ 60 | 64 | 50 | 100 | 56 | |
| UICC TNM staging | 0.305 | ||||
| Stage I and II | 29 | 23 | 49 | 29 | |
| Stage III | 44 | 35 | 48 | 28 | |
| Stage IV | 44 | 35 | 53 | 31 | |
| Unknown | 10 | 8 | 21 | 12 | |
| Primary histological differentiation | 0.752 | ||||
| Well/moderate | 89 | 70 | 121 | 71 | |
| Poor | 13 | 10 | 21 | 12 | |
| Unknown | 25 | 20 | 29 | 17 | |
| Treatment time of liver metastases | 0.582 | ||||
| Synchronous | 34 | 27 | 41 | 24 | |
| Metachronous | 93 | 73 | 130 | 76 | |
| No. of liver metastases | 0.300 | ||||
| 1 | 42 | 33 | 61 | 36 | 0.140* |
| 2–3 | 42 | 33 | 66 | 39 | |
| > 3 | 43 | 34 | 44 | 25 | |
| Largest size of liver metastases | 0.427 | ||||
| 1–3 cm | 53 | 42 | 80 | 47 | 0.656* |
| 4–6 cm | 43 | 33 | 46 | 27 | |
| > 7 cm | 31 | 24 | 45 | 26 | |
| Preoperative CEA level (ng/ml) | 0.081 | ||||
| ≤ 5.0 | 24 | 19 | 53 | 31 | |
| 5.01–100.0 | 62 | 49 | 78 | 46 | |
| > 100.0 | 21 | 16 | 23 | 13 | |
| Unknown | 20 | 16 | 17 | 10 | |
| Postoperative CEA level (ng/ml) | 0.201 | ||||
| ≤ 5.0 | 95 | 75 | 130 | 76 | |
| > 5.0 | 25 | 20 | 24 | 14 | |
| Unknown | 7 | 5 | 17 | 10 | |
| Operation type | 0.004 | ||||
| Resection only | 56 | 44 | 104 | 61 | |
| Cryotherapy±resection | 71 | 56 | 67 | 39 | |
* Analysed using Wilcoxon rank test.
Table III. The incidence of concurrent metastatic disease with pulmonary recurrence.
| Resection only |
Cryotherapy±resection |
|||
|---|---|---|---|---|
| Overall pulmonary recurrence | n=56 | % | n=71 | % |
| Lung only | 20 | 36 | 22 | 31 |
| Lung and liver | 25 | 45 | 39 | 55 |
| Lung and other extrahepatic sites | 7 | 12 | 2 | 3 |
| Lung, liver and other extrahepatic sites | 4 | 7 | 8 | 11 |
| Lung and subsequently liver recurrence | 15 | 27 | 29 | 41 |
| Lung and subsequently other extrahepatic sites | 8 | 14 | 8 | 11 |
Disease-free interval (DFI) of colorectal pulmonary metastases
Ten clinical factors in Table II were entered into the Cox regression model (Table IV). It demonstrated that low preoperative CEA level (≤5.0 ng/ml) (p=0.005) and the use of resection alone (p=0.036) were independently associated with longer disease-free interval in-the lung. Figure 2 showed the difference in time until recurrence of pulmonary metastases following resection only vs cryotherapy with or without resection for colorectal liver metastases, calculated by Kaplan-Meier analysis.
Table IV. Multivariate analysis of the clinical factors that affected pulmonary disease-free interval after surgical treatment for colorectal liver metastases (n=298).
| Variables | b | SE | Sig | Exp(B) |
|---|---|---|---|---|
| Operation type | – | – | – | – |
| Resection vs cryotherapy±resection | −0.378 | 0.180 | 0.036 | 0.685 |
| Preoperative CEA level (ng/ml) | – | – | 0.005 | – |
| > 100.0 vs unknown | 0.308 | 0.188 | 0.101 | 1.360 |
| > 100.0 vs <5.0 | −0.628 | 0.177 | 0.000 | 0.534 |
| > 100.0 vs 5.01–100.0 | 0.035 | 0.134 | 0.795 | 1.036 |
b, regression coefficient; SE, standard error; Sig, significance; Exp(B), −eB; CEA, carcinoembryonic antigen.
Figure 2. .
Pulmonary disease-free interval after hepatic resection (solid line, n=160) and cryotherapy with or without resection (dashed line, n=138) for colorectal liver metastases (p=0.049).
Discussion
Cryotherapy alone or in combination with resection has been increasingly recognized as an effective local treatment modality for non-resectable colorectal liver metastases. It has increased the scope of patients to receive a potentially curative therapy, achieving a median survival of more than 2 years in most published series and is associated with 5-year survival in approximately one-fifth of patients 5. The most common site of extra-abdominal metastases from colorectal carcinoma is the lung and the incidence of this following hepatic resection vs cryotherapy has not been reported previously. This paper clearly shows two most important differences regarding pulmonary metastases between patients treated with resection only and cryotherapy with or without resection. Firstly, among the 10 clinical variables (Table I), cryotherapy with or without resection demonstrates the greatest correlation with pulmonary metastases. Secondly cryotherapy with or without resection is independently associated with shorter pulmonary disease-free interval.
The resection group is associated with fewer, but larger tumour nodules and more metachronous disease than the cryotherapy group. We acknowledge that even though these variables were not found to be significant in either the univariate or the multivariate analyses for the risk of developing pulmonary metastases (Tables II and IV), it is still possible that these discrepancies were due to a difference in the known prognostic features of the two groups.
We think that technique-related failure should be considered. For any haematogenous or lymphatic metastasis to occur, firstly tumour cells have to be released into the circulation or interstitial tissues, respectively. During hepatic cryotherapy, introducing a large cryo-probe into tumours might physically dislodge tumour cells into the systemic circulation. Bleeding from the probe introduction point is often seen and a communication of dislodged tumour cells and the vascular space is clearly present. This is in contrast to a resection, where en bloc removal of tumour is performed. It is also thought that tumour cells are held together by intercellular adhesion molecules 21. Cryotherapy-induced cell necrosis may destroy the basement membrane and micro-vascular endothelium and may also interfere with the cell to cell adhesion, hence some viable tumour cells, especially at the periphery of the ice-ball, may release themselves and enter the circulation. Once tumour cells enter the systemic circulation, they are most likely to be entrapped in the microvasculature of the lung and may be related to why the lung is the most frequent site of haematogenous metastasis.
However, subsequent endothelial adhesion, invasion and basement membrane penetration have to occur, in order for pulmonary metastasis to develop 22. We know that during an inflammatory response, cytokines serve important roles in chemotaxis and adhesion between leukocytes and endothelium. Various cytokines are produced by leukocytes and lymphocytes and endothelial cells, such as tumour necrosis factor (TNF), interleukin (IL)-1 and interferon-gamma (IFN-gamma). These cytokines up-regulate the expression of various adhesion molecules, such as the endothelial leukocyte adhesion molecule-1 (ELAM-1), vascular cell adhesion molecule-1 (VCAM-1) or the intercellular adhesion molecule-1 (ICAM-1) 23, which in turn facilitate white cells to adhere to the activated endothelial cells, migrate through the vessel wall, and penetrate areas of infection or tissue damage. Tumour cell interaction with endothelium and subendothelial matrix constitutes a crucial factor in metastasis, and the adhesion of tumour cells to the endothelium might share the same mechanism as that in inflammation 24,25. Many studies have demonstrated that cytokines increase the adhesion of tumour cells in vitro26,27,28,29. Dejana et al. 28 showed that IL-1 promotes tumour cell adhesion to cultured human endothelial cells and Giavazzi et al. 30 demonstrated that IL-1 enhanced human melanoma metastasis in nude mice. Yanase et al. 31 concluded that TNF, IL-1 and particularly IL-6 augmented expression of VCAM-1 on endothelium, _which resulted in enhancement of adhesion between endothelium and renal cell carcinoma cells.
It is known that cryotherapy induces cytokine release, which is implicated in acute lung inflammation and the cryo-shock phenomenon 5. Seifert et al. 32 demonstrated that cryotherapy resulted in increasing TNF-α and IL-6 release in both human and a rat model. The exact mechanism(s) of increased cytokine release is less clear and it may be related to the thawing phase of cryotherapy, which results in plasma membrane disruption and dispersion of intact cellular structures into the systemic circulation, which in turn induce an inflammatory response 33. It is plausible to suggest that cryotherapy-induced cytokine release may up-regulate endothelial adhesion molecules as well, which in turn facilitate tumour cell attachment to the pulmonary endothelium.
In fact, both tumour-suppressing and tumour-enhancing effects were documented after cryotherapy in several animal models without specific data depicting the underlying mechanism. Shibata et al. 34 concluded that cryotherapy enhanced the growth of tumour metastases compared with surgical excision in rats. Joosten et al. 35 reported that cryotherapy of tumour implantation in mice resulted in inhibition of secondary and metastatic tumour growth in the lung, as compared with resection, and suggested that a cytokine response induced by cryoablation of tumour tissue might be attributed to this finding. El-Shakhs et al. 36 also failed to demonstrate that hepatic cryotherapy enhances tumour dissemination in a rat model. The different findings might be related to the difference in their study designs. However, in all studies, the immune-modulating effects of cryotherapy on tumour activity were suggested, as cryotherapy is associated with greater cytokine release than resection and other ablative techniques, such as radiofrequency ablation (RFA).
Indeed, RFA has not been shown to be related to large cytokine production and lung inflammation 33. It will be of great interest to see if lung metastasis rates following RFA are different from cryotherapy; however, the data in our study suggest that the inhibition of cytokine release, or the blocking of their effect, may be of broad value in cancer surgery to reduce the risk of systemic metastasis.
References
- 1.Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer 1993;71(12 Suppl):4252–66. [DOI] [PubMed] [Google Scholar]
- 2.Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–76. doi: 10.1007/s002689900381. discussion 276–7. [DOI] [PubMed] [Google Scholar]
- 3.van Ooijen B, Wiggers T, Meijer S, van der Heijde MN, Slooff MJ, van de Velde CJ, et al. Hepatic resections for colorectal metastases in The Netherlands. A multiinstitutional 10-year study. Cancer. 1992;70:28–34. doi: 10.1002/1097-0142(19920701)70:1<28::aid-cncr2820700105>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84:1136–40. [PubMed] [Google Scholar]
- 5.Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43:141–54. [PubMed] [Google Scholar]
- 6.Ravikumar TS, Steele GDJ. Hepatic cryosurgery. Surg Clin North Am. 1989;69:433–40. doi: 10.1016/s0039-6109(16)44798-5. [DOI] [PubMed] [Google Scholar]
- 7.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 8.Yan DB, Clingan P, Morris DL. Hepatic cryotherapy and regional chemotherapy with or without resection for liver metastases from colorectal carcinoma-how many are too many? Cancer. 2003;98:320–30. doi: 10.1002/cncr.11498. [DOI] [PubMed] [Google Scholar]
- 9.Finlay IG, Seifert JK, Stewart GJ, Morris DL. Resection with cryotherapy of colorectal hepatic metastases has the same survival as hepatic resection alone. Eur J Surg Oncol. 2000;26:199–202. doi: 10.1053/ejso.1999.0776. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt PH, Dwerryhouse SJ, Zhao J, Morris DL. Multiple bilobar liver metastases: cryotherapy for residual lesions after liver resection. J Surg Oncol. 1998;67:112–16. doi: 10.1002/(sici)1096-9098(199802)67:2<112::aid-jso7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Dwerryhouse SJ, Seifert JK, McCall JL, Iqball J, Ross WB, Morris DL. Hepatic resection with cryotherapy to involved or inadequate resection margin (edge freeze) for metastases from colorectal cancer. Br J Surg. 1998;85:185–7. doi: 10.1046/j.1365-2168.1998.00576.x. [DOI] [PubMed] [Google Scholar]
- 12.Seifert JK, Morris DL. Indicators of recurrence following cryotherapy for hepatic metastases from colorectal cancer. Br J Surg. 1999;86:234–40. doi: 10.1046/j.1365-2168.1999.00995.x. [DOI] [PubMed] [Google Scholar]
- 13.Seifert JK, France MP, Zhao J, Bolton EJ, Finlay I, Junginger T, et al. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor α in a rat model. World J Surg. 2002;26:1333–41. doi: 10.1007/s00268-002-6139-5. [DOI] [PubMed] [Google Scholar]
- 14.Seifert JK, Morris DL. Pretreatment echogenicity of colorectal liver metastases predicts survival after hepatic cryotherapy. Dis Colon Rectum. 1999;42:43–9. doi: 10.1007/BF02235181. [DOI] [PubMed] [Google Scholar]
- 15.Jourdan JL, Morris DL. Cryotherapy for unresectable liver cancers. Asian J Surg. 2000;23:16–21. [Google Scholar]
- 16.Hewitt PM, Dwerryhouse SJ, Zhao J, Morris DL. Multiple bilobar liver metastases: cryotherapy for residual lesions after liver resection. J Surg Oncol. 1998;67:112–16. doi: 10.1002/(sici)1096-9098(199802)67:2<112::aid-jso7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Ross WB, Horton M, Bertolino P, Morris DL. Cryotherapy of liver tumours-a practical guide. HPB Surg. 1995;8:167–73. doi: 10.1155/1995/93283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCall JL, Jorgensen JO, Morris DL. Hepatic artery chemotherapy for colorectal liver metastases. Aust N Z J Surg. 1995;65:383–9. doi: 10.1111/j.1445-2197.1995.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 19.Morris DL, Horton MD, Dilley AV, Warlters A, Clingan PR. Treatment of hepatic metastases by cryotherapy and regional cytotoxic perfusion. Gut. 1993;34:1156–7. doi: 10.1136/gut.34.9.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soon PS, Glenn D, Jorgensen J, Morris DL. Fluorodeoxy-uridine causes bilomas after hepatic cryotherapy. J Surg Oncol. 1998;69:45–50. doi: 10.1002/(sici)1096-9098(199809)69:1<45::aid-jso9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Weiss L, Orr FW, Honn KV. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Mestastasis. 1989;7:127–67. doi: 10.1007/BF01787020. [DOI] [PubMed] [Google Scholar]
- 22.Hellman K. Orr FW, Buchanan MR, Weiss L. CRC; Boca Raton: 1991. Entry of cancer cells into the circulation, Microcirculation in cancer metastasis; pp. 67–79. [Google Scholar]
- 23.Shimizu Y, Newman W, Gopal TV, Horgan KJ, Graber N, Beall LD, et al. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1 and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991;113:1203. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss L, Orr FW, Honn KV. Interactions of cancer cells with the microvasculature during metastasis. FASEB J. 1988;2:12. doi: 10.1096/fasebj.2.1.3275560. [DOI] [PubMed] [Google Scholar]
- 25.Rice GE, Gimbrone MA, Bevilacqua MP. Tumor cell-endothelial interactions: increased adhesion of human melanoma cells to activated vascular endothelium. Am J Pathol. 1998;133:204. [PMC free article] [PubMed] [Google Scholar]
- 26.Rice GE, Bevilacqua MP. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 27.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, et al. Contribution of carbohydrate antigens siayl Lewis A and siayl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–61. [PubMed] [Google Scholar]
- 28.Dejana E, Bertocchi F, Bortolami MC, Regonesi A, Tonta A, Breviario F, et al. Interleukin 1 promotes tumor cell adhesion to culture human endothelial cells. J Clin Invest. 1988;82:1466–70. doi: 10.1172/JCI113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KH, Lawly TJ, Xu Y, Swerlick RA. VCAM-1, ELAM-1 and ICAM-1 independent adhesion of melanoma cells to cultured human dermal microvascular endothelial cells. J Invest Dermatol. 1992;98:79. doi: 10.1111/1523-1747.ep12495643. [DOI] [PubMed] [Google Scholar]
- 30.Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, et al. Interleukin 1-induced augmentatation of experimental metastasis from a human melanoma in nude mice. Cancer Res. 1990;50:4771–5. [PubMed] [Google Scholar]
- 31.Yanase M, Tsukamoto T, Kumamoto Y. Investigative urology: cytokines modulate in vitro invasiveness of renal cell carcinoma cells through action on the process of cell attachment to endothelial cells. J Urol. 1995;153:844–8. [PubMed] [Google Scholar]
- 32.Seifert JK, Malcolm PF, Zhao J, Bolton EJ, Finlay I, Junginger T, et al. Large volume hepatic freezing: associated with significant release of the cytokines interleukin-6 and tumor necrosis factor in a rat model. World J Surg. 2002;26:1333–41. doi: 10.1007/s00268-002-6139-5. [DOI] [PubMed] [Google Scholar]
- 33.Chapman WC, Debelak JP, Wright Pinson C, Washington MK, Atkinson JB, Venkatakrishnan A, et al. Hepatic cryo-ablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg. 2000;321:752–61. doi: 10.1097/00000658-200005000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata T, Yanashita T, Suzuki K, Takeichi N, Micallef M, Hasakawa M, et al. Enhancement of experimental pulmonary metastasis and inhibition of subcutaneously transplanted tumor growth following cryosurgery. Anticancer Res. 1998;18:4443–8. [PubMed] [Google Scholar]
- 35.Joosten JJA, Muijen GNP, Wobbes T, Reuers TJM. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;41:49–58. doi: 10.1006/cryo.2001.2302. [DOI] [PubMed] [Google Scholar]
- 36.El-Shakhs SA, Shimi SA, Cuschieri A. Effective hepatic cryoablation: does it enhance tumor dissemination? World J Surg. 1999;23:306–10. doi: 10.1007/pl00013192. [DOI] [PubMed] [Google Scholar]