Abstract
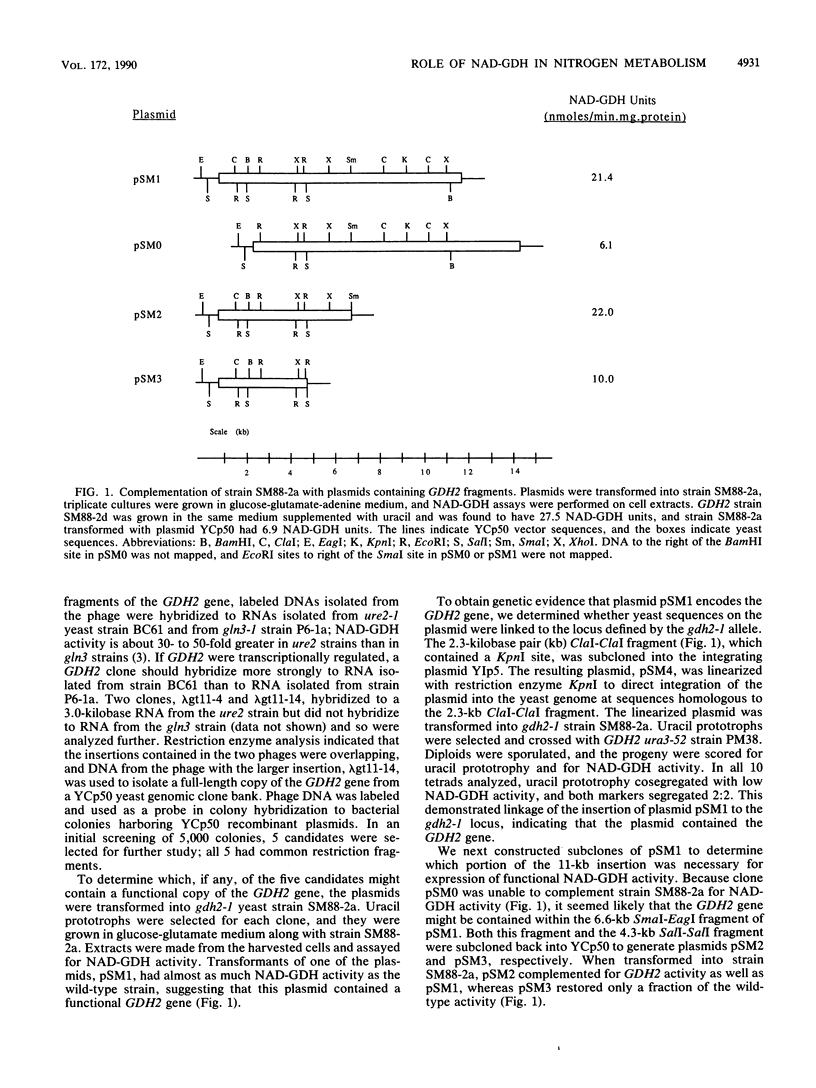
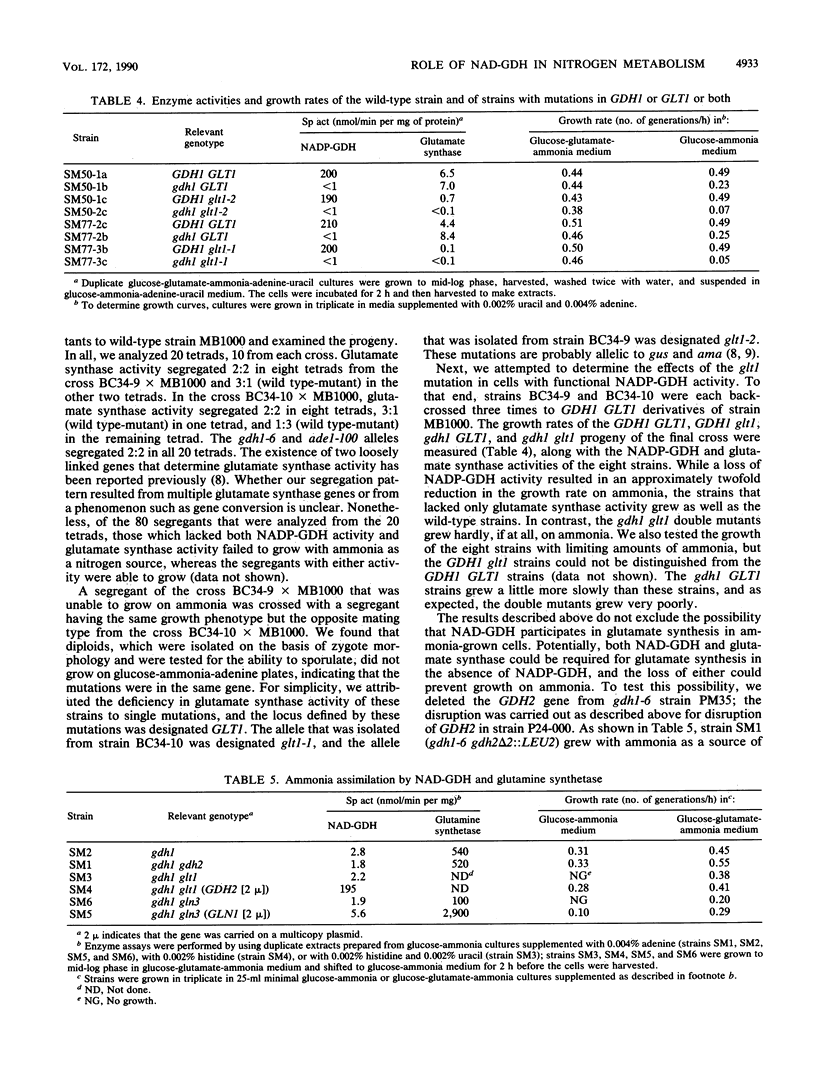
We cloned GDH2, the gene that encodes the NAD-linked glutamate dehydrogenase in the yeast Saccharomyces cerevisiae, by purifying the enzyme, making polyclonal antibodies to it, and using the antibodies to screen a lambda gt11 yeast genomic library. A yeast strain with a deletion-disruption allele of GDH2 which replaced the wild-type gene grew very poorly with glutamate as a nitrogen source, but growth improved significantly when the strain was also provided with adenine or other nitrogenous compounds whose biosynthesis requires glutamine. Our results indicate that the NAD-linked glutamate dehydrogenase catalyzes the major, but not sole, pathway for generation of ammonia from glutamate. We also isolated yeast mutants that lacked glutamate synthase activity and present evidence which shows that normally NAD-linked glutamate dehydrogenase is not involved in glutamate biosynthesis, but that if the enzyme is overexpressed, it may function reversibly in intact cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark-Adams C. D., Winston F. The SPT6 gene is essential for growth and is required for delta-mediated transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Feb;7(2):679–686. doi: 10.1128/mcb.7.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Regulation of nitrogen assimilation in Saccharomyces cerevisiae: roles of the URE2 and GLN3 genes. J Bacteriol. 1988 Feb;170(2):708–713. doi: 10.1128/jb.170.2.708-713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillien R., Aigle M., Lacroute F. Yeast mutants pleiotropically impaired in the regulation of the two glutamate dehydrogenases. Biochem Biophys Res Commun. 1973 Jul 17;53(2):367–372. doi: 10.1016/0006-291x(73)90671-2. [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Grenson M. Absence of involvement of glutamine synthetase and of NAD-linked glutamate dehydrogenase in the nitrogen catabolite repression of arginase and other enzymes in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Sep 9;60(1):150–157. doi: 10.1016/0006-291x(74)90185-5. [DOI] [PubMed] [Google Scholar]
- Dubois E., Vissers S., Grenson M., Wiame J. M. Glutamine and ammonia in nitrogen catabolite repression of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1977 Mar 21;75(2):233–239. doi: 10.1016/0006-291x(77)91033-6. [DOI] [PubMed] [Google Scholar]
- Folch J. L., Antaramián A., Rodríguez L., Bravo A., Brunner A., González A. Isolation and characterization of a Saccharomyces cerevisiae mutant with impaired glutamate synthase activity. J Bacteriol. 1989 Dec;171(12):6776–6781. doi: 10.1128/jb.171.12.6776-6781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Dubois E., Piotrowska M., Drillien R., Aigle M. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. Evidence for the gdhA locus being a structural gene for the NADP-dependent glutamate dehydrogenase. Mol Gen Genet. 1974;128(1):73–85. doi: 10.1007/BF00267295. [DOI] [PubMed] [Google Scholar]
- HIERHOLZER G., HOLZER H. REPRESSION DER SYNTHESE VON DPN-ABHAENGIGER GLUTAMINSAEUREDEHYDROGENASE IN SACCHAROMYCES CEREVISIAE DURCH AMMONIUMIONEN. Biochem Z. 1963 Dec 3;339:175–185. [PubMed] [Google Scholar]
- HOLZER H., SCHNEIDER S. Anreicherung und Trennung einer DPN-spezifischen und einer TPN-spezifischen Glutaminsäure-dehydrogenase aus Hefe. Biochem Z. 1957;329(5):361–369. [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn J. R., Pateman J. A. Mutants of Aspergillus nidulans lacking nicotinamide adenine dinucleotide-specific glutamate dehydrogenase. J Bacteriol. 1976 Jan;125(1):42–47. doi: 10.1128/jb.125.1.42-47.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrain C., Vissers S., Dubois E., Legrain M., Wiame J. M. Regulation of glutamine synthetase from Saccharomyces cerevisiae by repression, inactivation and proteolysis. Eur J Biochem. 1982 Apr;123(3):611–616. doi: 10.1111/j.1432-1033.1982.tb06576.x. [DOI] [PubMed] [Google Scholar]
- Mitchell A. P., Magasanik B. Purification and properties of glutamine synthetase from Saccharomyces cerevisiae. J Biol Chem. 1983 Jan 10;258(1):119–124. [PubMed] [Google Scholar]
- Mitchell A. P., Magasanik B. Regulation of glutamine-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2758–2766. doi: 10.1128/mcb.4.12.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F., Wiame J. M. Occurrence of a catabolic L-serine (L-threonine) deaminase in Saccharomyces cerevisiae. Eur J Biochem. 1982 Apr;123(3):571–576. doi: 10.1111/j.1432-1033.1982.tb06570.x. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Roon R. J., Even H. L., Larimore F. Glutamate synthase: properties of the reduced nicotinamide adenine dinucleotide-dependent enzyme from Saccharomyces cerevisiae. J Bacteriol. 1974 Apr;118(1):89–95. doi: 10.1128/jb.118.1.89-95.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Regulation of NAD-dependent glutamate dehydrogenase by protein kinases in Saccharomyces cerevisiae. J Biol Chem. 1984 Jan 25;259(2):1288–1293. [PubMed] [Google Scholar]
- Wiame J. M., Grenson M., Arst H. N., Jr Nitrogen catabolite repression in yeasts and filamentous fungi. Adv Microb Physiol. 1985;26:1–88. doi: 10.1016/s0065-2911(08)60394-x. [DOI] [PubMed] [Google Scholar]



