Abstract
Transcription factor (TF) IIIB, which directs RNA polymerase (pol) III to its promoters, is made up of three components: the TATA box-binding protein, the TFIIB-related Brf, and the pol III-specific B′′. Certain mutations in Saccharomyces cerevisiae Brf and B′′ retain TFIIIB transcription factor activity with supercoiled DNA but are inactive with linear duplex DNA. Further analysis shows that these inactive TFIIIB–DNA complexes bind pol III and position it appropriately over the transcriptional start site but do not form DNA strand-separated open promoter complexes. It is proposed that the normal function of TFIIIB combines pol III recruitment with an active role in a subsequent step of transcriptional initiation leading to promoter opening.
Yeast RNA polymerase (pol) III is brought to its promoters by its central transcription factor (TF) IIIB, which is composed of three subunits: Brf, its TFIIB-related and archaeal TFB-related component; TATA box-binding protein (TBP), the ubiquitous component of all eukaryotic nuclear transcription; and B′′, a pol III-specific subunit. All three subunits are required for all transcription by yeast pol III. TFIIIB can bind autonomously to certain pol III promoters through a direct interaction of TBP with a strong TATA box. When such a TATA box is lacking, or when DNA is packaged into chromatin, TFIIIB is brought to the promoter by TFIIIC, its complex, bulky DNA-binding assembly factor (1–9). Once pol III has been recruited to the promoter by TFIIIB, it spontaneously and thermoreversibly generates extensive DNA strand separation (the transcription bubble) around the transcription start site in linear as well as in negatively supercoiled DNA (10).
Although it is clearly established that TFIIIB plays the central role in bringing RNA polymerase III to the immediate vicinity of the promoter, the possibility that it also can intervene in subsequent steps of transcriptional initiation has not been explored. The experiments that are described below provide evidence that this is indeed what happens. We show that, when TFIIIB is assembled with certain mutant Brf or B′′ subunits, it retains activity for directing transcription of negatively supercoiled DNA or of linear DNA that has been made especially flexible at the TATA box but is inactive for transcription of normal linear duplex DNA. Further analysis shows that pol III is recruited to the inactive TFIIIB-linear DNA complex and is brought into contact with DNA in the vicinity of the transcriptional start site but fails to form a transcription bubble and consequently also fails to make complete or abortive transcripts.
We suggest that the complete TFIIIB–DNA complex participates in transcriptional initiation by guiding already recruited pol III through subsequent steps of promoter opening and that certain deficient TFIIIB–DNA complexes fix the already recruited and promoter-proximal polymerase in a conformation that is unable to proceed along the pathway to promoter opening. The observation that pol III can be bound to a promoter in a transcriptionally closed state suggests an interesting parallel with certain mechanisms of transcriptional regulation in the bacteria; this is the subject of further commentary.
METHODS
Plasmids pU6LboxB, pU6RboxB, and pA29GRboxB containing modified SNR6 gene constructs have been described (11). A linear 366-bp transcription template based on pU6LboxB was generated by PCR amplification with primers bearing 5′ ends located 211 bp upstream and 155 bp downstream of the natural SNR6 transcription start site and was purified as described (12). DNA from pU6RboxB with hydroxymethyluracil in place of T at both ends of the SNR6 TATA box (Fig. 1a) was constructed as described (13) and was provided generously by A. Grove (University of California, San Diego). DNA probes for electrophoretic mobility-shift assay (EMSA), KMnO4, and DNase I footprinting were generated by PCR of plasmids pU6LboxB and pA29GRboxB with the 5′ end of the labeled primer located 87 bp upstream and the 5′ end of the unlabeled primer 45 bp downstream of the 5′ end of the SNR6 transcript. Probes for photochemical cross-linking were generated as described (14) (partial sequence in Fig. 3b).
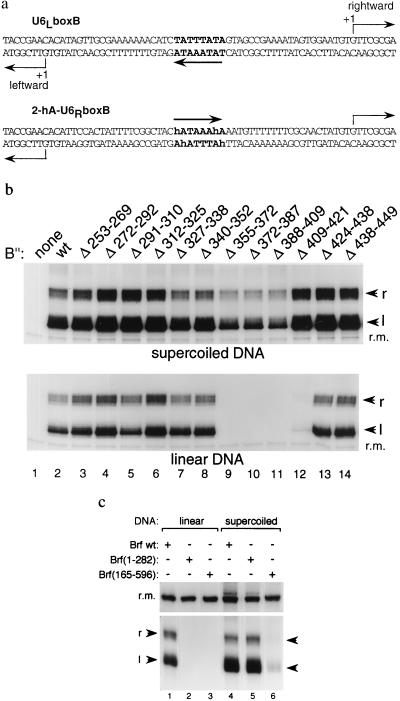
Figure 1.
B′′ and Brf deletion proteins that function for TFIIIC-independent transcription of supercoiled DNA by pol III but are inert for transcription of linear DNA. (a) Sequence at the SNR6 TATA box and divergent transcriptional initiation sites (marked with arrows) of U6LboxB and of the 2-hA variant of U6RboxB. (b) Transcription with full length (wt) and internal deletion B′′ (designated at the top) on supercoiled pU6LboxB (Top) and on a 366-bp linear U6LboxB fragment (Bottom). Rightward (r) and leftward (l) transcripts and a sample recovery marker (r.m.) are identified at the side. Full length Brf and TBP were present in all reaction mixtures. (c) Comparison of the ability of full length Brf (wt), Brf(1–282) and Brf(165–596) to function for transcription of supercoiled and linear DNA. Full length B′′ and TBP were present in all reaction mixtures.
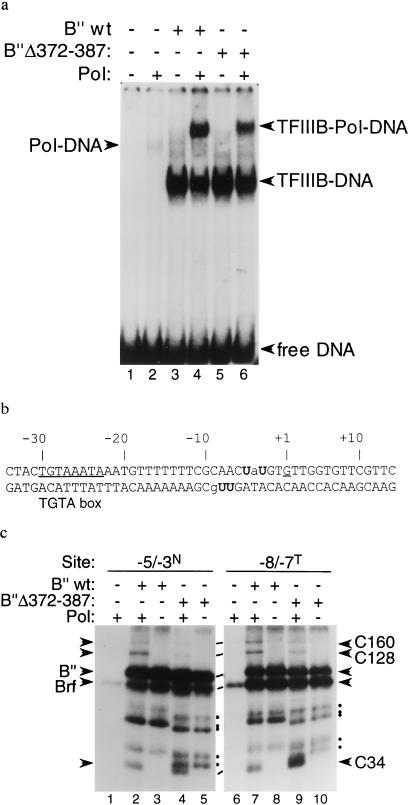
Figure 3.
Transcriptionally inactive TFIIIB–DNA complexes retain ability to assemble pol III in proximity of the transcription start site. (a) EMSA of 135-bp U6LboxB DNA, comparing the ability of TFIIIB–DNA complexes containing full length B′′ or B′′Δ372–387 to assemble pol III. All binding reactions contained TBP and full length Brf. The presence of B′′ and/or pol III is indicated above each lane. TFIIIB–DNA, TFIIIB-pol III-DNA, and nonspecific pol III–DNA complexes are identified at the sides. (b) DNA probes for site-specific photochemical cross-linking. Locations of ABdUMP (U) and adjacent 32P-labeled nucleotides (lower case) are marked. (c) Photochemical cross-linking of pol III recruited to TFIIIB–DNA complexes containing full length B′′ or B′′Δ372–387. TBPm3 and full length Brf were present in all reaction mixtures; the presence of pol III and B′′ is indicated above each lane. Cross-linked proteins are identified at the sides. B′′ fragments that cross-linked to DNA are identified at the side (•).
TFIIIC, pol III, recombinant TBP, recombinant TBPm3, recombinant full length Brf (N- and C-terminally His6-tagged), N-terminally His6-tagged Brf(1–282) and Brf(165–596) were purified and assayed as described (11, 12, 14, 15). Full length C-His6-tagged B′′, purified under nondenaturing conditions (14), was used for experiments presented in Fig. 1b and Fig. 5 and for all experiments involving Brf(165–596). Full length C-His6-tagged B′′ for the remaining experiments and the internal deletion mutants of B′′ were purified under denaturing conditions as described (5, 12). (These full length proteins are referred to in the text as “wild-type”). Quantities of pol III are specified as femtomole enzyme active for specific transcription; quantities of the other proteins were measured as described (15).
Figure 5.
KMnO4 footprinting; pol III assembled by TFIIIB[B′′Δ355–372] does not open the promoter. TFIIIB containing full length B′′ or B′′Δ355–372 was bound to a 132-bp U6LboxB-derived DNA probe with 5 (1×) or 10 (2×) fmol pol III or without pol III as indicated above each lane. For the samples in lanes 5 and 9, GTP, UTP, and CTP were added to allow formation of 7-mer nascent transcripts. Thymine residues oxidized by MnO4− are marked at the side (sequence in Fig. 1a).
Protein–DNA complexes for transcription, EMSA, footprinting, and photochemical cross-linking were formed in 20-μl volume of a previously specified reaction buffer, with 50–60 mM NaCl (15), containing 100 ng poly(dG-dC)·poly(dG-dC), 50 fmol template DNA (for transcription) or 4–10 fmol labeled DNA (for EMSA, footprinting, and photochemical cross-linking), 90–150 fmol of the specified B′′, 175–350 fmol of the specified Brf, 50–200 fmol TBP or 400 fmol TBPm3, as specified, and 5–10 fmol pol III. Multiple-round transcription was performed and analyzed as described (15). EMSA was performed as described (15), except that Brf–TBP–DNA and TFIIIB–DNA complexes were formed first, pol III was added for a further 10 min, and samples then were treated with 200 ng poly(dA-dT):poly(dA-dT) for 5 min before electrophoresis. Photochemical cross-linking was performed as described (14, 15), except that TBP–Brf–DNA or TFIIIB–DNA complexes were formed first and 100 ng sheared salmon sperm DNA then was added for 5 min followed by pol III for an additional 10 min before UV irradiation. DNA complexes for KMnO4 footprinting were formed as for EMSA, except that pol III was added for 20 min at 30°C, followed by the addition of GTP, UTP, and CTP (each to 200 μM) for 5 min, where specified. KMnO4 treatment for 1 min and processing of samples followed (10). For DNase I footprinting, TBPm3–DNA complexes were formed for 30 min, and Brf and the specified B′′ were added for an additional 30 min. Where applicable, pol III then was added for 15 min, followed by a 4-min challenge with 200 ng poly(dA-dT)·poly(dA-dT). DNase I digestion, nondenaturing gel electrophoresis, and complex isolation were performed as described (12). For quantitative analysis of footprinting (Fig. 4), the radioactivity recovered from gel slices containing free DNA, TFIIIB–DNA complexes, and pol III–TFIIIB–DNA complexes was equalized for application to the sequencing gel. A background correction for upward-smearing TFIIIB–DNA complexes was applied for the footprints of the pol III–TFIIIB–DNA complexes.
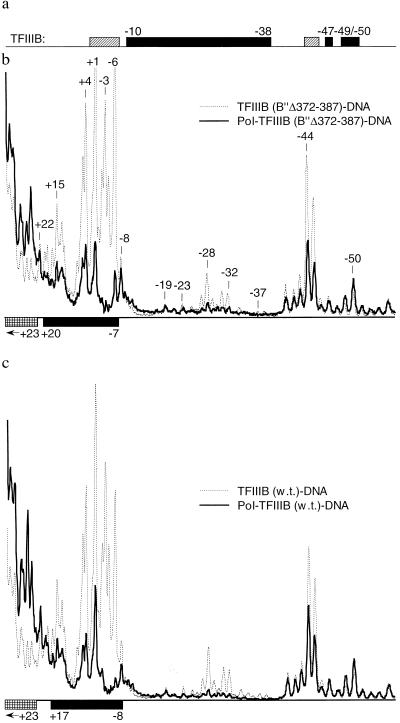
Figure 4.
Closely similar DNase I footprints of pol III in transcriptionally competent and inactive promoter complexes. TFIIIB, containing TBPm3 and full length B′′ or B′′Δ372–387, and pol III were bound to a 132-bp DNA probe containing the TGTA variant of the SNR6 promoter. Two-dimensional DNase I protection analysis (see Methods) yielded the aligned and normalized phosphorimage profiles of DNase I cleavage that are displayed. (Part of the bp −51 to −61 segment that was used for normalization is not shown.) (a) Top line: Footprints of the TFIIIB[B′′Δ372–387]-DNA and TFIIIB-DNA complexes showing regions of reduced (█) and enhanced (▨) cleavage caused by TFIIIB. (b) Comparison of the DNase I cleavage profiles of the pol III–TFIIIB–DNA complex () and TFIIIB–DNA complex (⋯ ·) formed with B′′Δ372–387. Protection () and enhanced cleavage ( ) attributable to pol III binding are marked below the panel. (c) Comparison as in b but for complexes assembled with full length B′′.
RESULTS
Recent mutational analyses of the functions of the B′′ and Brf components of TFIIIB have generated a collection of deletion variants of both proteins that retain the ability to assemble TFIIIB–DNA complexes and to direct TFIIIC-independent transcription by pol III (12, 14, 15). The transcription templates that have been used for these experiments are derivatives of the U6 snRNA gene (SNR6) (16) with strong TATA boxes that bind TFIIIB through direct interaction with TBP (6, 17–19) In the absence of TFIIIC, TFIIIB binds in either orientation to this TATA box and can direct leftward as well as rightward transcription (Fig. 1a). The orientational degeneracy can be broken by changing AT to GC at the second base pair of the TATA box (i.e., TATA→TGTA) and substituting wild-type TBP with a mutant, TBPm3 (20) that recognizes TGTA and TATA boxes (11).
Fig. 1b Upper shows that transcriptional activity is retained by B′′ derivatives with 12- to 22-aa deletions scanning through its central ≈220-aa segment when the transcribed DNA (pU6LboxB) is negatively supercoiled, as noted (12). Two externally truncated Brf proteins also retain transcriptional activity with this supercoiled DNA (Fig. 1c, lanes 4–6). Brf(1–282), which is deleted for a C-proximal segment that provides the principal interactions of Brf with TBP and with B′′, only forms relatively unstable TFIIIB–DNA complexes but is transcriptionally highly active (Fig. 1c, compare lanes 4 and 5) (15), and we presume that it harbors the principal polymerase recruitment function of TFIIIB (21, 22). Deletion of the N-proximal putative zinc finger and the first (N-proximal) TFIIB-related pseudo repeat segment of Brf yields another protein, Brf(165–596), that retains substantial ability to generate the leftward transcripts specified by the U6LboxB TATA box (Fig. 1c, lane 6) and forms TFIIIB–DNA complexes through its retained C-proximal segment (refs. 14 and 15; data not shown).
A surprising result materialized when the same experiments were repeated with linear DNA. With wild-type TFIIIB, linear and supercoiled DNA consistently generated approximately the same yields of transcripts (Fig. 1b Upper and Lower, lane 2; Fig. 1c, lanes 1 and 4). B′′ with any one of four small contiguous deletions (Fig. 1b) generated TFIIIB–DNA complexes that were now either entirely inactive (Δ355–372, Δ372–387, and Δ388–409) or highly defective (Δ409–421) (Fig. 1b Lower, lanes 9–12). TFIIIB–DNA complexes assembled with Brf(1–282) and Brf(165–596) were also transcriptionally inactive with linear DNA (Fig. 1c, lanes 2 and 3). Transcriptional inactivity persisted when assayed at different temperatures and at varying ionic strengths and also was not ameliorated by DNA-bound TFIIIC. Nevertheless, the four defective B′′ deletion proteins form stable TFIIIB–DNA complexes with linear DNA that are resistant to dissociation by heparin, as is the wild-type TFIIIB–DNA complex (12). The TFIIIB–DNA complex assembled with Brf(165–594) is less stable but readily detectable (ref. 14; data not shown).
The above transcription defects can be regarded as problems of DNA structure as well as protein structure. On the protein side, combinations of the small B′′ deletions Δ355–372, Δ372–387, or Δ388–409 (Fig. 1b) with Brf(165–596) are known to retain the ability to form TFIIIB–DNA complexes but to lose the ability to direct TFIIIC-independent transcription of supercoiled U6LboxB DNA (14). On the DNA side, we found that the transcription defect of linear U6LboxB DNA was suppressed by site-specifically increasing flexibility at the TATA box (Fig. 2). This was achieved by a simple (if somewhat arcane) change: substituting T with 5-hydroxymethyluracil(h) to generate h-A steps at the sites of sharp DNA bending by TBP (23–25). Changing the SNR6 TATA box from
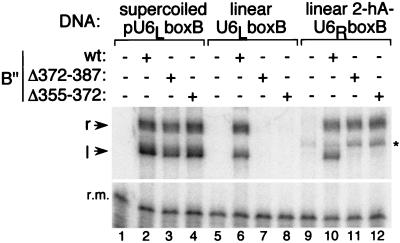
Figure 2.
Increasing flexibility at the SNR6 TATA box restores transcription of linear DNA. TFIIIB–DNA complexes containing full length B′′ (wt), B′′Δ372–387 or B′′Δ355–372 were formed on supercoiled pU6LboxB, on 366 bp U6LboxB-DNA, or on the equivalent 354-bp, hydroxymethyluracil-containing 2-hA variant of U6RboxB, as designated at the top of the figure (sequences in Fig. 1a). The asterisk marks a transcript that is generated by pol III alone and has not been characterized further.
TATAAATAhATAAAhA
to(Fig. 1a)
ATATTTATAhATTTAh
increases the stability of its TBP complex ≈100-fold (13). (See refs. 26–28 for the background of these experiments.)
The effect of these h-for-T substitutions on transcription with TFIIIB complexes assembled with wild-type B′′, B′′Δ355–372, and B′′Δ372–387 is shown in Fig. 2. Under the conditions of the assay, the three B′′ proteins were transcriptionally equivalent with supercoiled DNA, and the two deletion proteins were transcriptionally inactive with all-T linear DNA, as already specified (Fig. 2, lanes 1–8). The h-for-T substitutions of construct 2-hA-U6RboxB rescued rightward transcription with the two B′′ deletion proteins (Fig. 2, lanes 10–12). A similar experiment with Brf(165–596) yielded an identical outcome (data not shown). (It is surprising that only rightward transcription was restored for B′′Δ372–387 and B′′Δ355–372-containing transcription complexes. We think it unlikely that these B′′ deletions eliminate the leftward orientation of TFIIIB at the TATA box. More probably, only one of the TFIIIB orientations is restored to functionality for transcription by changing T-A steps to h-A steps, implying that h-A steps change the structure of the TFIIIB–DNA complex.)
We conclude that preformed structure (generated by supercoiling) and internal flexibility of DNA (as in the 2-hA construct) can affect not only the assembly (ref. 13; A. Grove, personal communication) but also the post-assembly functions of the TFIIIB–DNA complex. We propose that this involvement arises from a requirement that the parts of the TFIIIB–DNA complex be brought into a specific alignment, as discussed further below.
At what step of transcriptional initiation do these conditionally inactive TFIIIB–DNA complexes fail? The experiments that follow address this question by examining pol III recruitment by the TFIIIB–DNA complex, promoter opening, and abortive initiation of transcription. EMSAs and site-specific protein–DNA photochemical cross-linking were used to assess pol III assembly (Fig. 3). EMSA demands a pol III–TFIIIB–DNA complex that is relatively stable for the (>3 h) duration of electrophoresis required to resolve large protein–DNA complexes. Single-hit protein–DNA cross-linking measures the occupancy of pol III on unperturbed TFIIIB–DNA complexes at the time of UV-irradiation. [Only the latter technique readily detects formation of the transcriptionally highly active but unstable TFIIIB–DNA complex formed with Brf(1–282) (15).]
The ability of TFIIIB–DNA complexes formed with B′′Δ372–387 (designated TFIIIB[B′′Δ372–387]-DNA) to assemble pol III was demonstrated readily by EMSA (Fig. 3a, compare lane 6 with lanes 2 and 5), even under conditions devised to reduce nonspecific binding of pol III to DNA (Fig. 3a, lane 2, and data not shown). TFIIIB[B′′Δ355–372] was also competent in recruiting pol III (data not shown); both B′′ deletion proteins generated 30–60% of the pol III–TFIIIB–linear DNA complexes formed with wild-type B′′ (Fig. 3a, compare lanes 4 and 6, and data not shown). The less stable TFIIIB–DNA complexes formed with Brf(165–596) did not retain pol III during electrophoresis (data not shown).
Three pol III subunits, C160, C128, and the Brf-interacting C34, are accessible to the major groove-protruding photoreactive side chain of 5-[N′-(p-azidobenzoyl)-3-aminoallyl]dUMP (ABdUMP) placed upstream of the start site of transcription of the SUP4 tRNA gene (29). Site-specific DNA-protein photochemical cross-linking was used to show that TFIIIB[Brf(165–596)], TFIIIB[B′′Δ372–387] and TFIIIB[B′′Δ355–372] assemble pol III. Results for TFIIIB[B′′Δ372–387] with photoreactive probes placing ABdUMP respectively at bp −5 and −3 on the nontranscribed strand (−5/−3N; Fig. 3c, lanes 1–5) and at bp −8 and −7 on the transcribed strand (−8/−7T; Fig. 3c, lanes 6–10) are shown. The DNA contained a TGTA box (Fig. 3b), and TBPm3 (20) was used to favor a unique orientation of pol III. A low background of nonspecific pol III binding generated only very weak cross-linking of its C160, 128, 82, 53, 40 (and/or 37), and 34 subunits (Fig. 3c, lanes 1 and 6, detectable in the original autoradiogram). Addition of either intact B′′ or B′′Δ372–387 generated efficient cross-linking of the pol III C34 subunit (Fig. 3c, compare lane 2 with lane 4 and lane 7 with lane 9). The preparations of wild-type B′′ and B′′Δ372–387 contained cross-linkable fragments (Fig. 3c, lanes 3, 5, 8, and 10; designated at the sides of each panel with a dot), which did not interfere with assessing cross-linking of the pol III C34 subunit. Cross-linking of pol III C160 and C128 consistently was reduced somewhat (relative to C34 cross-linking) for TFIIIB[B′′Δ372–387] (Fig. 3c, lanes 4 and 9) compared with wild-type TFIIIB (Fig. 3c, lanes 2 and 7). Apart from this subtle effect, there were no distinctive differences between pol III placement by wild-type TFIIIB and TFIIIB[B′′Δ372–387]. The same result was seen for a DNA probe with ABdUMP (on the transcribed strand) at bp −4 and with TFIIIB[B′′Δ355–372] on all three DNA probes (data not shown).
Pol III binding to the TFIIIB[Brf(165–596)]–DNA complex also was examined, with DNA containing ABdUMP (on the nontranscribed strand) at bp −13 and −12. The C34, C128, and C160 pol III subunits were cross-linked but at lower efficiency than for intact Brf, presumably reflecting the lower affinity of pol III for this TFIIIB–DNA complex. Cross-linking of C160 and C128 was again diminished relative to C34. The C31 pol III subunit, which cross-linked weakly at this site when recruited by wild-type TFIIIB, did not cross-link when brought to DNA by TFIIIB[Brf(165–596)] (data not shown).
The assembly of pol III into a transcriptionally inactive but stable complex by TFIIIB[B′′Δ372–387] (Fig. 3a) made it possible to examine pol III placement by two-dimensional DNase I footprinting (Fig. 4). Wild-type TFIIIB and TFIIIB[B′′Δ372–387] generated nearly identical patterns of protection over the TGTA box (summarized in Fig. 4a) extending from bp −10 to approximately bp −39/−38, with additional protection at bp −47, −49, and −50 and with enhanced DNase I cleavage between bp +1 and −6 as well as at bp −44 and −45; relative levels of enhanced cleavage at bp −6 differed between the two TFIIIB–DNA complexes. Addition of pol III to both TFIIIB–DNA complexes generated nearly identical extensions of the footprint: bp −7 to +20 on the TFIIIB[B′′Δ372–387]–DNA complex (Fig. 4b) and approximately bp −8 to +17 on the wild-type TFIIIB–DNA complex (Fig. 4c); both pol III complexes enhanced DNase I cleavage downstream of bp +23 and reduced the enhanced cleavage generated by the TFIIIB–DNA complex at bp −44 and −45.
The preceding experiments clearly show that TFIIIB[B′′Δ372–387] stably assembles pol III over the start site of transcription in an unproductive complex. [Other experiments (data not shown) confirmed the persistence of transcriptional inactivity of the defective B′′ and Brf deletion proteins in assays by using a 366-bp transcription template version of the TGTA box-containing footprinting probe in conjunction with TBPm3.] We examined DNA melting at the transcriptional start in these inert pol III–TFIIIB–DNA complexes by probing for unpaired T residues with KMnO4 (10, 30). A single DNA probe derived from pU6LboxB allowed the transcribed strand (for rightward transcription) and the nontranscribed strand (for leftward transcription) to be examined simultaneously because of the bi-directional nature of the SNR6 TATA box (Fig. 1a). Addition of pol III to the wild-type TFIIIB complexes exposed T residues between bp −8 and +3 on the nontranscribed strand of the rightward-reading transcript and between bp −8 and −4 (with trace reactivity, of uncertain significance, at +8) on the transcribed strand of the leftward-reading transcript (Fig. 5, compare lanes 4 and 2; sequence in Fig. 1a; the background present in all lanes was independent of KMnO4 addition). Addition of GTP, UTP, and CTP, allowing formation of 7-mer nascent transcripts, enhanced T-reactivity at these same sites and shifted the distribution of reactivities in the direction of transcription (Fig. 5, lane 5; cf. ref. 10). Pol III-mediated melting was not seen when B′′Δ355–372 replaced wild-type B′′ in the TFIIIB–DNA complex (Fig. 5, compare lanes 8 and 9 with lane 6; other data not shown). Transcriptionally inactive pol III recruited by TFIIIB containing B′′Δ372–387, B′′Δ388–409, or Brf(165–596) also did not melt DNA at the transcriptional start sites, whereas melting was observed with transcriptionally functional TFIIIB[B′′Δ253–269] and TFIIIB[B′′Δ272–291] (data not shown). The absence of melting at the start site of transcription by pol III assembled on TFIIIB–DNA complexes containing Brf(165–596) or B′′Δ372–387 also was reflected in an absence of TFIIIB-dependent abortive initiation products when all four ribonucleotides were present or when reiterative synthesis of GpUpU was primed with the dinucleotide GpU and labeled UTP (data not shown).
DISCUSSION
The transcription–initiation factor TFIIIB participates more extensively in initiation of transcription than by merely bringing its conjugate polymerase to the vicinity of the transcriptional start site. If recruitment of polymerase to the promoter were the sole function of TFIIIB, then any single polymerase contact of sufficient affinity, such as the already identified Brf interaction with the 34-kDa subunit of pol III (21, 22) should suffice for transcriptional activation. This is not what we have observed. In fact, it has proven difficult to detect direct pol III recruitment by the Brf–TBP–DNA complex even with the sensitivity provided by photochemical cross-linking. Deletions in B′′ and also in Brf generate TFIIIB defects that are inapparent when the transcribed DNA is negatively supercoiled but are exposed when DNA is linearized (Fig. 1); these linear DNA-restricted defects can be suppressed by site-specifically increasing the flexibility of DNA within the TBP–DNA complex (Fig. 2); conversely, transcription activity for supercoiled DNA is lost when these B′′ and Brf deletion proteins are used in combination (14). This interdependence of failure and restoration implies that DNA and the TFIIIB proteins in contact with it function as an assembly, and that multisite, stereospecific interactions with pol III take place on the surface of this assembly. The required register of these interaction sites is brought about through protein–protein and protein–DNA interactions within the TFIIIB–DNA complex.
The three transcription-defective TFIIIB–DNA assemblies that have been examined are quantitatively somewhat deficient in recruiting pol III, but their ability to bring pol III to the promoter is readily detected (Figs. 3 and 4, and data not shown). Indeed, photochemical cross-linking and DNaseI footprinting only reveal subtle differences between the pol III-DNA alignments of those promoter complexes that eventually yield transcripts and those that are inactive. That polymerase can be brought into extensive contacts with promoter DNA and still fail to initiate transcription implies two explanations that are not necessarily mutually exclusive: (i) complete TFIIIB is an active participant in promoter opening by pol III; and/or (ii) defective TFIIIB restricts pol III to a configuration that blocks further progress toward transcriptional initiation. [Because pol III can autonomously and precisely initiate transcription on the duplex end of linear DNA bordered by a short 3′ overhang (31), the second of these alternative interpretations cannot be discounted.]
The proposal that multisite TFIIIB-pol III interactions determine the post-recruitment participation of TFIIIB in transcriptional initiation can be made more concrete in terms of a simple analogy. Consider three contact sites: P, Q, and R on the TFIIIB–DNA complex and their ligands p, q, r, and r′ on pol III. Suppose that the P–p interaction suffices for pol III recruitment, with the Q–q interaction providing reinforcement if P and Q are properly aligned. Suppose, also, that the R–r and R–r′ interactions come into play at a post-recruitment step of transcriptional initiation, R–r interaction facilitating a pol III isomerization that is a prerequisite to promoter opening, and/or R–r′ blocking that isomerization. Transcription-defective TFIIIB–DNA complexes could be thought of as retaining the P–p interaction with pol III but lacking the proper alignment of P, Q, and R; lacking the Q–q interaction, they recruit less effectively, and lacking the proper R placement, they either do not facilitate promoter opening (through the R–r interaction) or may actually block it (through the alternative R–r′ interaction).
Our observations, and the preceding explanation, suggest direct parallels with well analyzed mechanisms of transcriptional initiation in bacteria. Whereas Escherichia coli RNA polymerase holoenzyme (E.σ70) is autonomous for DNA binding and promoter opening at its strongest promoters, it must be recruited to other promoters by one or more transcriptional activators. The most clear-cut example of “pure” activation by recruitment is provided by an elegantly devised system (32) in which activation of transcription by a ω-Gal11p fusion protein is shown to depend strictly on a protein–protein interaction with a Gal4-λcI fusion protein that binds to a λ operator. (ω is a dispensable small subunit of RNA polymerase; segments of Gal11 and Gal4 provide the linking protein–protein interaction; the Gal11p-interacting segment of Gal4 is fused to the phage λcI protein which, in turn, binds as a dimer to its appropriately located operator DNA site.) There are also well analyzed natural promoters at which activators of transcription generate essentially pure recruitment (reviewed in refs. 33 and 34).
At other promoters, RNA polymerase requires a transcriptional activator to assist in some post-recruitment step of transcriptional initiation. The clearest example of this class of activators is provided by the σ54-RNA polymerase holoenzyme (E.σ54), which avidly forms closed promoter complexes at certain promoters but absolutely requires an activator for transcriptional initiation. The σ54 subunit may directly dictate this activator control by locking the polymerase into an inactive state in which it is unable to initiate transcription even with artificially strand-separated (transcription bubble) construct promoters (35–37).
It appears that the TFIIIB-pol III interaction combines aspects of both kinds of bacterial activator-polymerase relationships. TFIIIB is clearly and obviously a recruiter of its conjugate polymerase. But the evidence presented here shows that recruitment is not necessarily sufficient for activation and strongly implies that TFIIIB also participates at post-recruitment steps of transcriptional initiation.
Recent observations on the pol II cofactor PC4 suggest that the preceding proposal about the reaction pathway to transcriptional initiation by pol III may apply more generally to the nuclear RNA polymerases. In a minimal system consisting of TBP, TFIIB, TFIIF, and pol II, PC4 is a potent inhibitor of transcription but does not prevent recruitment of pol II-TFIIF to the TBP–TFIIB-promoter complex (38). If it could be shown that pol II is placed appropriately in these inactive transcription complexes to open the promoter (compare Figs. 3 and 4) but does not do so (compare Fig. 5), that should represent a close counterpart to the experimental evidence presented here in regard to RNA polymerase III.
Acknowledgments
We thank A. Grove for helpful discussions and provision of precious material and C. A. P. Joazeiro for a critical reading of the text. Our research was supported by a grant from the National Institute for General Medical Sciences.
ABBREVIATIONS
- pol
RNA polymerase
- TF
transcription factor
- EMSA
electrophoretic mobility-shift assay
- TBP
TAT box-binding protein
- ABdUMP
5-[N′-(p-azidobenzoyl)-3-aminoallyl]dUMP
References
- 1.Kassavetis G A, Braun B R, Nguyen L H, Geiduschek E P. Cell. 1990;60:235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- 2.Buratowski S, Zhou H. Cell. 1992;71:221–230. doi: 10.1016/0092-8674(92)90351-c. [DOI] [PubMed] [Google Scholar]
- 3.Colbert T, Hahn S. Genes Dev. 1992;6:1940–1949. doi: 10.1101/gad.6.10.1940. [DOI] [PubMed] [Google Scholar]
- 4.Kassavetis G A, Joazeiro C A, Pisano M, Geiduschek E P, Colbert T, Hahn S, Blanco J A. Cell. 1992;71:1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- 5.Kassavetis G A, Nguyen S T, Kobayashi R, Kumar A, Geiduschek E P, Pisano M. Proc Natl Acad Sci USA. 1995;92:9786–9790. doi: 10.1073/pnas.92.21.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margottin F, Dujardin G, Gerard M, Egly J M, Huet J, Sentenac A. Science. 1991;251:424–426. doi: 10.1126/science.1989075. [DOI] [PubMed] [Google Scholar]
- 7.Roberts S, Miller S J, Lane W S, Lee S, Hahn S. J Biol Chem. 1996;271:14903–14909. doi: 10.1074/jbc.271.25.14903. [DOI] [PubMed] [Google Scholar]
- 8.Rüth J, Conesa C, Dieci G, Lefèbvre O, Dusterhoft A, Ottonello S, Sentenac A. EMBO J. 1996;15:1941–1949. [PMC free article] [PubMed] [Google Scholar]
- 9.White R J. RNA Polymerase III Transcription. 2nd Ed. Springer; 1998. , in press. [Google Scholar]
- 10.Kassavetis G A, Blanco J A, Johnson T E, Geiduschek E P. J Mol Biol. 1992;226:47–58. doi: 10.1016/0022-2836(92)90123-2. [DOI] [PubMed] [Google Scholar]
- 11.Whitehall S K, Kassavetis G A, Geiduschek E P. Genes Dev. 1995;9:2974–2985. doi: 10.1101/gad.9.23.2974. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Kassavetis G A, Geiduschek E P, Hambalko M, Brent C J. Mol Cell Biol. 1997;17:1868–1880. doi: 10.1128/mcb.17.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grove, A., Galeone, A., Yu, E., Mayol, L. & Geiduschek, E. P. (1998) J. Mol. Biol., in press. [DOI] [PubMed]
- 14.Kassavetis G A, Bardeleben C, Kumar A, Ramirez E, Geiduschek E P. Mol Cell Biol. 1997;17:5299–5306. doi: 10.1128/mcb.17.9.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassavetis, G. A., Kumar, A., Ramirez, E. & Geiduschek, E. P. (1998) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 16.Brow D A, Guthrie C. Genes Dev. 1990;4:1345–1356. doi: 10.1101/gad.4.8.1345. [DOI] [PubMed] [Google Scholar]
- 17.Burnol A F, Margottin F, Schultz P, Marsolier M C, Oudet P, Sentenac A. J Mol Biol. 1993;233:644–658. doi: 10.1006/jmbi.1993.1542. [DOI] [PubMed] [Google Scholar]
- 18.Eschenlauer J B, Kaiser M W, Gerlach V L, Brow D A. Mol Cell Biol. 1993;13:3015–3026. doi: 10.1128/mcb.13.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joazeiro C A, Kassavetis G A, Geiduschek E P. Mol Cell Biol. 1994;14:2798–2808. doi: 10.1128/mcb.14.4.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strubin M, Struhl K. Cell. 1992;68:721–730. doi: 10.1016/0092-8674(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 21.Werner M, Chaussivert N, Willis I M, Sentenac A. J Biol Chem. 1993;268:20721–20724. [PubMed] [Google Scholar]
- 22.Khoo B, Brophy B, Jackson S P. Genes Dev. 1994;8:2879–2890. doi: 10.1101/gad.8.23.2879. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Geiger J H, Hahn S, Sigler P B. Nature (London) 1993;365:512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- 24.Kim J L, Nikolov D B, Burley S K. Nature (London) 1993;365:520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- 25.Juo Z S, Chiu T K, Leiberman P M, Baikalov I, Berk A J, Dickerson R E. J Mol Biol. 1996;261:239–254. doi: 10.1006/jmbi.1996.0456. [DOI] [PubMed] [Google Scholar]
- 26.Grove A, Galeone A, Mayol L, Geiduschek E P. J Mol Biol. 1996;260:196–206. doi: 10.1006/jmbi.1996.0392. [DOI] [PubMed] [Google Scholar]
- 27.Grove A, Geiduschek E P. In: Techniques in Protein Chemistry. Marshak D, editor. Vol. 8. San Diego: Academic; 1997. pp. 585–592. [Google Scholar]
- 28.Grove A, Figueiredo M, Galeone A, Mayol L, Geiduschek E P. J Biol Chem. 1997;272:13084–13087. doi: 10.1074/jbc.272.20.13084. [DOI] [PubMed] [Google Scholar]
- 29.Bartholomew B, Durkovich D, Kassavetis G A, Geiduschek E P. Mol Cell Biol. 1993;13:942–952. doi: 10.1128/mcb.13.2.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasse-Dwight S, Gralla J D. J Biol Chem. 1989;264:8074–8081. [PubMed] [Google Scholar]
- 31.Bardeleben C, Kassavetis G A, Geiduschek E P. J Mol Biol. 1994;235:1193–1205. doi: 10.1006/jmbi.1994.1073. [DOI] [PubMed] [Google Scholar]
- 32.Dove S L, Hochschild A. Genes Dev. 1998;12:745–754. doi: 10.1101/gad.12.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busby S, Ebright R H. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 34.Hochschild A, Dove S L. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang J T, Syed A, Hsieh M, Gralla J D. Science. 1995;270:992–994. doi: 10.1126/science.270.5238.992. [DOI] [PubMed] [Google Scholar]
- 36.Wedel A, Kustu S. Genes Dev. 1995;9:2042–2052. doi: 10.1101/gad.9.16.2042. [DOI] [PubMed] [Google Scholar]
- 37.Wyman C, Rombel I, North A K, Bustamante C, Kustu S. Science. 1997;275:1658–1661. doi: 10.1126/science.275.5306.1658. [DOI] [PubMed] [Google Scholar]
- 38.Malik S, Guermah M, Roeder R G. Proc Natl Acad Sci USA. 1998;95:2192–2197. doi: 10.1073/pnas.95.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]