Abstract
When mice were injected intracerebrally with doses of Bordetella pertussis vaccine greater than 5 ImD 50 and challenged intracerebrally 14 days later with virulent B. pertussis there was an immediate reduction in the numbers of organisms. An analysis of this in vivo bactericidal effect has shown that large doses of an unrelated vaccine, Salmonella typhosa, equivalent in cell mass to about 50 ImD 50 of B. pertussis vaccine can achieve this effect, so for such doses the effect must be partly non-specific. This action is not maintained and so is not ultimately protective. Local immunoglobulin was also demonstrable 14 days after 300 ImD 50 of B. pertussis vaccine but following smaller doses of 10-20 ImD 50 it could not be found until after the mice had been infected and the blood-brain barrier impaired. A similar immediate reduction in the numbers of infecting organisms inoculated 1 day after vaccination has been shown to follow very small, non-protective doses of vaccines unrelated to B. pertussis and to be achieved with lipopolysaccharide and endotoxin isolated from B. pertussis. Brains were not sterilized and only in mice receiving protective B. pertussis vaccine was the lowering of infection maintained beyond 2 days and the brains eventually sterilized. The antibody passively protecting mice against intracerebral infection was found in the 19S and 11 S globulin fractions of the serum of once-vaccinated mice and in the 11 S and 7 S fractions of the serum of rabbits and ascitic fluid of mice receiving repeated doses of vaccine. The IgM probably eliminated infections by immediate sterilization but had to be present locally to do so since it was unable to pass from the circulation into the brain, and was therefore inactive when injected intraperitoneally. The IgA and IgG were not so restricted and both the 11 S and 7 S globulins were capable of exerting an immediate suppressive effect on infecting organisms. The 7 S globulin was also capable of a maintained or delayed suppressive effect. Lymphocytes from fully protected once-vaccinated mice, transferred 2-3 weeks after intraperitoneal vaccination, were able to confer some protection when injected intraperitoneally or intracerebrally into recipient mice infected 2 weeks after transfer. Homologous, non-concentrated antiserum from once-vaccinated mice, injected intraperitoneally 1 hr. before infection sometimes augmented the transferred immunity, whereas alone it was inactive.
Full text
PDF





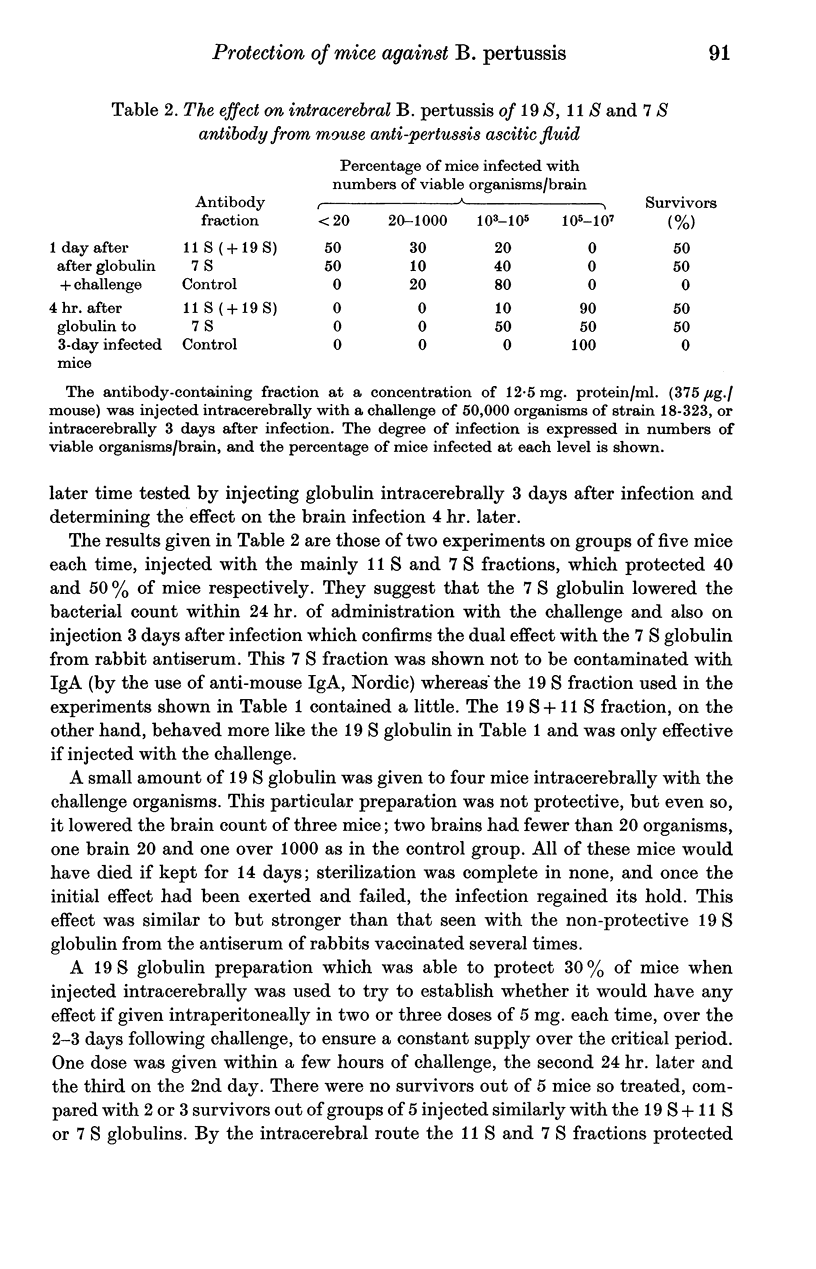












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., MUNOZ J. Mouse antibody. I. Characterization and properties of antibody in mouse peritoneal fluid. J Immunol. 1961 Oct;87:426–432. [PubMed] [Google Scholar]
- ANDERSEN E. K. Demonstration of promunity in the early immunity of pertussis vaccinated mice. Acta Pathol Microbiol Scand. 1957;40(3):227–234. [PubMed] [Google Scholar]
- Ackers J. P., Dolby J. M. The antigen of Bordetella pertussis that induces bactericidal antibody and its relationship to protection of mice. J Gen Microbiol. 1972 Apr;70(2):371–382. doi: 10.1099/00221287-70-2-371. [DOI] [PubMed] [Google Scholar]
- Actor P., Pitkin D. Immunity to Vibrio cholerae in the mouse. II. Effect of a cell-adherent immune factor. Infect Immun. 1973 Jan;7(1):35–38. doi: 10.1128/iai.7.1.35-38.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams G. J., Hopewell J. W. Enhancement of intracerebral infection of mice with Bordetella pertussis. J Med Microbiol. 1970 Feb;3(1):15–27. doi: 10.1099/00222615-3-1-15. [DOI] [PubMed] [Google Scholar]
- BERENBAUM M. C., UNGAR J., STEVENS W. K. Intracranial infection of mice with Bordetella pertussis. J Gen Microbiol. 1960 Apr;22:313–322. doi: 10.1099/00221287-22-2-313. [DOI] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLBY J. M., STANDFAST A. F. The intracerebral infection of mice with Bordetella pertussis. J Hyg (Lond) 1961 Jun;59:205–216. doi: 10.1017/s0022172400038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolby J. M., Dolby D. E. The antibody activities of 19S and 7S fractions from rabbit antisera to Bordetella pertussis. Immunology. 1969 Jun;16(6):737–747. [PMC free article] [PubMed] [Google Scholar]
- Dolby J. M. Passive protection of mice against intracerebral infections with Bordetella pertussis. J Hyg (Lond) 1972 Dec;70(4):707–718. doi: 10.1017/s0022172400022555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolby J. M. The antibacterial effect of Bordetella pertussis antisera. Immunology. 1965 May;8(5):484–498. [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. Interference immunity produced by pertussis vaccine to pertussis infection in mice. Br J Exp Pathol. 1954 Dec;35(6):603–608. [PMC free article] [PubMed] [Google Scholar]
- HIRSCHFELD J. Individual precipitation patterns of normal rabbit sera. A preliminary report. Acta Pathol Microbiol Scand. 1959;46:229–238. doi: 10.1111/j.1699-0463.1959.tb00334.x. [DOI] [PubMed] [Google Scholar]
- HOLT L. B., SPASOJEVIC V., DOLBY J. M., STANDFAST A. F. Immunity in mice to an intracerebral challenge of Bordetella pertussis. J Hyg (Lond) 1961 Sep;59:373–378. doi: 10.1017/s0022172400039036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L. B. The pathology and immunology of Bordetella pertussis infection. J Med Microbiol. 1972 Nov;5(4):407–424. doi: 10.1099/00222615-5-4-407. [DOI] [PubMed] [Google Scholar]
- Iida T., Tajima M. Stimulation of non-specific resistance by heterologous endotoxins and experimental immunity to Bordetella pertussis in mice. Immunology. 1971 Aug;21(2):313–322. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä O., Mitchison N. A. The role of cell number and source in adoptive immunity. Immunology. 1965 Jun;8(6):539–548. [PMC free article] [PubMed] [Google Scholar]
- Nagano Y., Maehara N. Enhancement of endotoxin-induced virus-inhibiting factor or interferon production by pretreatment of the rabbit with Newcastle disease virus. Jpn J Microbiol. 1972 Sep;16(5):397–402. doi: 10.1111/j.1348-0421.1972.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Rabin B. S., Rose N. R. Antibody formation by adoptively transferred mouse peripheral blood leucocytes. Immunology. 1970 Feb;18(2):259–267. [PMC free article] [PubMed] [Google Scholar]
- STANDFAST A. F. A report on the laboratory assays carried out at the Lister Institute of Preventive Medicine on the typhoid vaccines used in the field study in Yugoslavia. Bull World Health Organ. 1960;23:37–45. [PMC free article] [PubMed] [Google Scholar]
- STANDFAST A. F. The comparison between field trials and mouse protection tests against intranasal and intracerebral challenges with Bordetella pertussis. Immunology. 1958 Apr;1(2):135–143. [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfast A. F., Dolby J. M. The influence of the route of immunization on the protection of mice infected intracerebrally with Bordetella pertussis. J Hyg (Lond) 1972 Sep;70(3):487–501. doi: 10.1017/s0022172400063075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Jakus C. M. Intracerebral mouse protection test for pertussis vaccine. I. Apparent absence of humoral protective antibody under the usual test conditions. Can J Microbiol. 1968 Sep;14(9):989–997. doi: 10.1139/m68-164. [DOI] [PubMed] [Google Scholar]
- Zatz M. M., Gingrich R., Lance E. M. The effect of histocompatibility antigens on lymphocyte migration in the mouse. Immunology. 1972 Nov;23(5):665–675. [PMC free article] [PubMed] [Google Scholar]



