Abstract
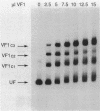
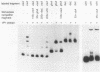
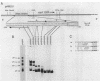
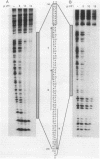
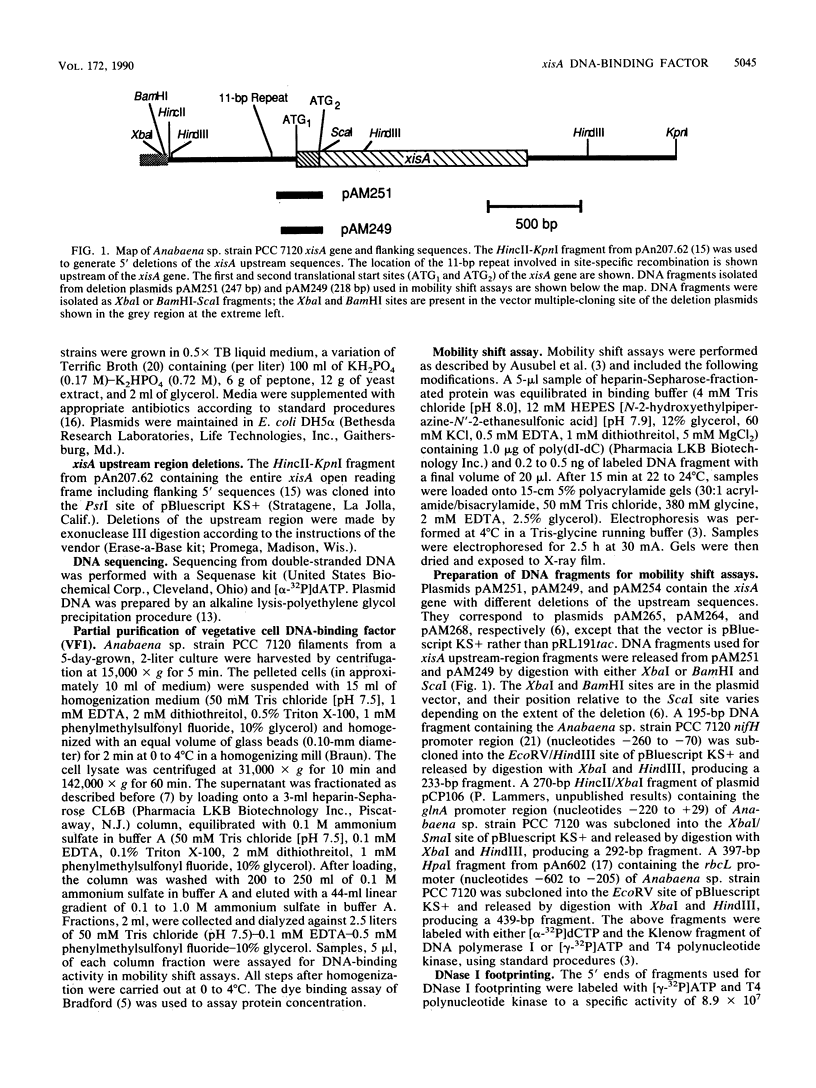
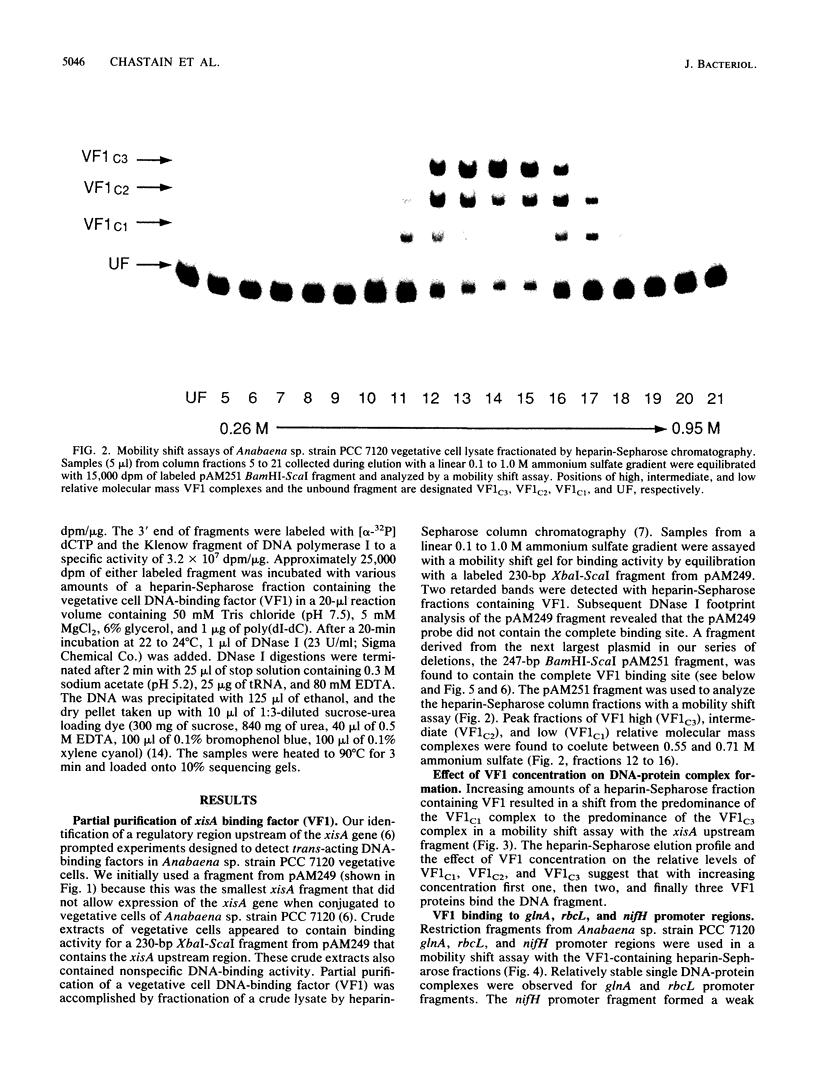
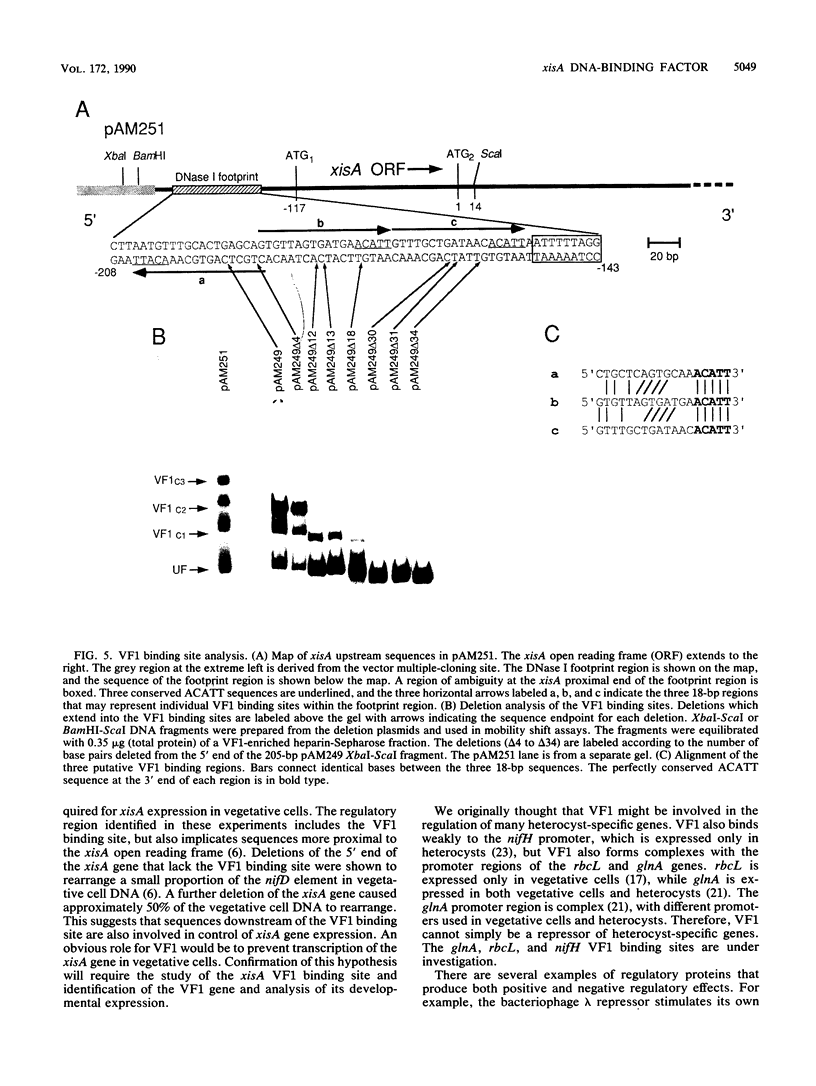
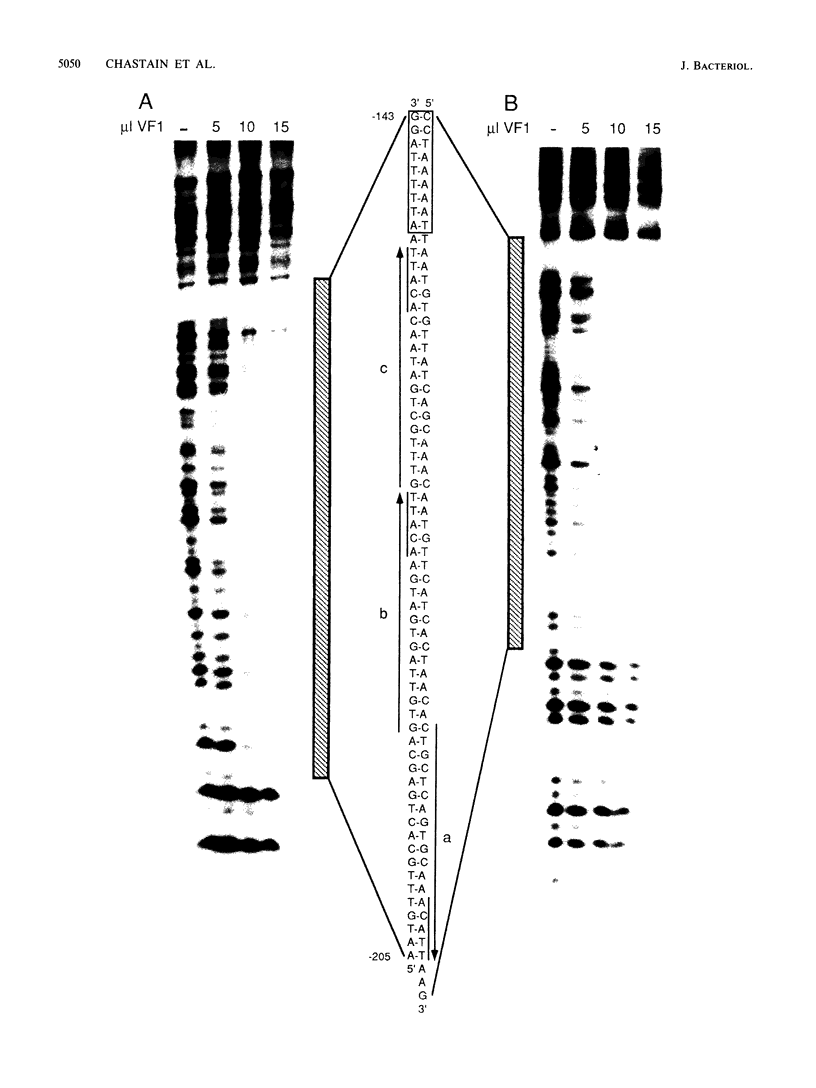
A DNA-binding factor (VF1) partially purified from Anabaena sp. strain PCC 7120 vegetative cell extracts by heparin-Sepharose chromatography was found to have affinity for the xisA upstream region. The xisA gene is required for excision of an 11-kilobase element from the nifD gene during heterocyst differentiation. Previous studies of the xisA upstream sequences demonstrated that deletion of this region is required for the expression of xisA from heterologous promoters in vegetative cells. Mobility shift assays with a labeled 250-base-pair fragment containing the binding sites revealed three distinct DNA-protein complexes. Competition experiments showed that VF1 also bound to the upstream sequences of the rbcL and glnA genes, but the rbcL and glnA fragments showed only single complexes in mobility shift assays. The upstream region of the nifH gene formed a weak complex with VF1. DNase footprinting and deletion analysis of the xisA binding site mapped the binding to a 66-base-pair region containing three repeats of the consensus recognition sequence ACATT.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H. Determination of the molecular weight of DNA-bound protein(s) responsible for gel electrophoretic mobility shift of linear DNA fragments examplified with purified viral myb protein. Nucleic Acids Res. 1988 Jun 24;16(12):5241–5248. doi: 10.1093/nar/16.12.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brusca J. S., Chastain C. J., Golden J. W. Expression of the Anabaena sp. strain PCC 7120 xisA gene from a heterologous promoter results in excision of the nifD element. J Bacteriol. 1990 Jul;172(7):3925–3931. doi: 10.1128/jb.172.7.3925-3931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Golden J. W., Haselkorn R. Identification and sequence of a gene required for a developmentally regulated DNA excision in Anabaena. Cell. 1986 Mar 28;44(6):905–911. doi: 10.1016/0092-8674(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Wasylyk B. Enhancers and transcription factors in the control of gene expression. Biochim Biophys Acta. 1988 Nov 10;951(1):17–35. doi: 10.1016/0167-4781(88)90021-8. [DOI] [PubMed] [Google Scholar]