Abstract
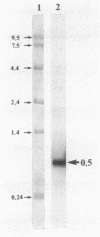
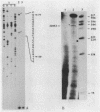
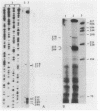
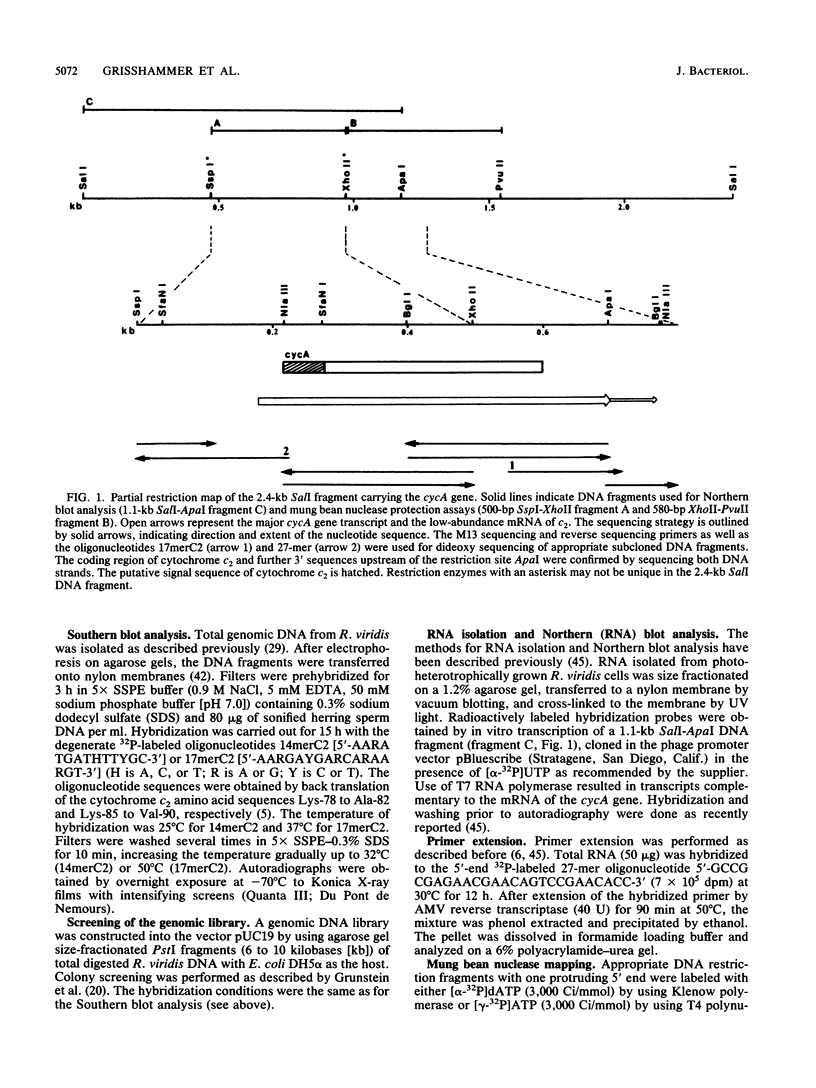
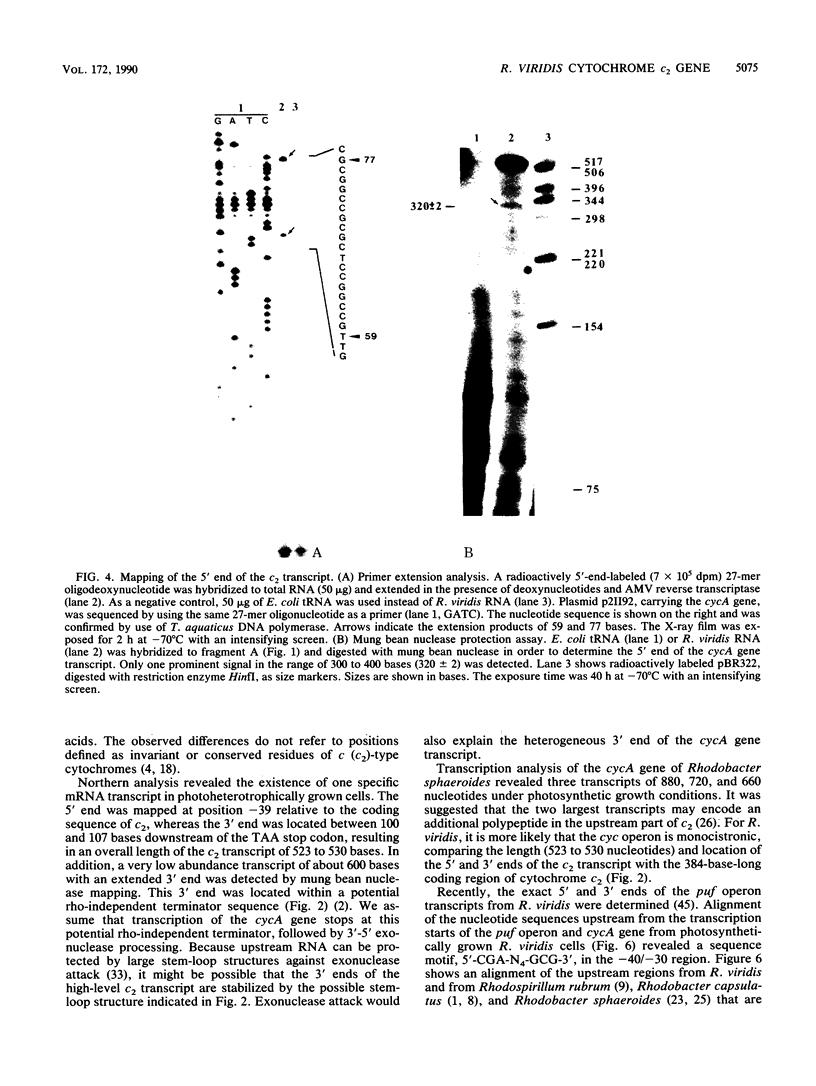
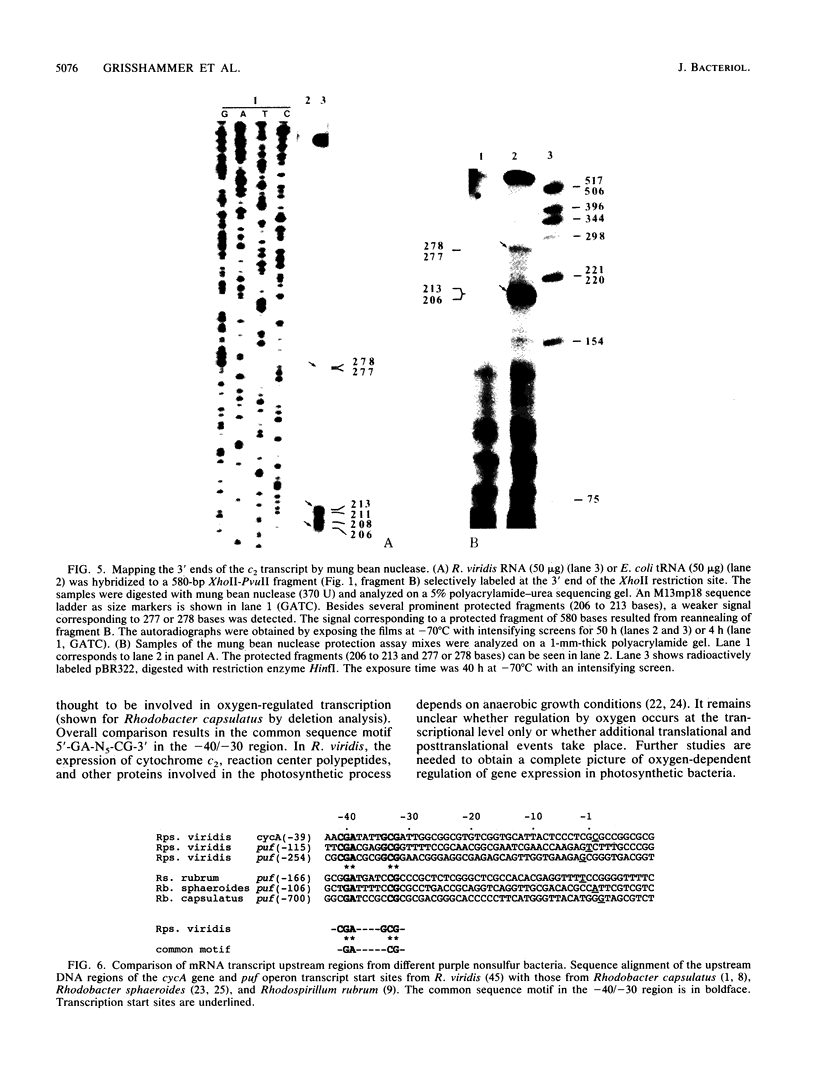
The cytochrome c2 gene (cycA) of the purple nonsulfur bacterium Rhodopseudomonas viridis was isolated from a genomic library by using two degenerate oligonucleotides containing all possible DNA sequences predicted from the published amino acid sequence of this protein (Ambler et al., Proc. Natl. Acad. Sci. USA 73:472-475, 1976). Cloning and sequence analysis of the cytochrome c2 gene indicated the presence of a typical procaryotic 20-residue signal peptide, suggesting that this periplasmic protein in synthesized in vivo as a precursor. In addition, four amino acids were found to be different by comparing the published sequence of the mature protein with that deduced from the isolated cycA gene (Lys-14----Leu, Ser-46----Ala, Ile-84----Val, Leu-97----Ile). Northern (RNA) blot analysis and fine mapping of the 5' and 3' ends of the cycA gene transcript from photoheterotrophically grown R. viridis cells revealed one abundant transcript of 523 to 530 nucleotides in length, with the transcription start site at position -39 relative to the coding region of cytochrome c2. A low-abundance transcript with an extended 3' end (about 600 bases in length) is thought to be processed by exonucleases, resulting in the slightly shorter main transcript.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Allen J. P. Crystallization and preliminary X-ray diffraction analysis of cytochrome c2 from Rhodobacter sphaeroides. J Mol Biol. 1988 Nov 20;204(2):495–496. doi: 10.1016/0022-2836(88)90592-x. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Hermoso J., Meyer T. E., Bartsch R. G., Kamen M. D. Cytochrome c2 sequence variation among the recognised species of purple nonsulphur photosynthetic bacteria. Nature. 1979 Apr 12;278(5705):659–660. doi: 10.1038/278659a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Kamen M. D. Primary structure determination of two cytochromes c2: close similarity to functionally unrelated mitochondrial cytochrome C. Proc Natl Acad Sci U S A. 1976 Feb;73(2):472–475. doi: 10.1073/pnas.73.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Bélanger G., Gingras G. Structure and expression of the puf operon messenger RNA in rhodospirillum rubrum. J Biol Chem. 1988 Jun 5;263(16):7639–7645. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G., Giesbrecht P. Rhodopseudomonas viridis, nov. spec., ein neu isoliertes, obligat phototrophes Bakterium. Arch Mikrobiol. 1966 Mar 31;53(3):255–262. [PubMed] [Google Scholar]
- Dus K., Sletten K., Kamen M. D. Cytochrome c2 of Rhodospirillum rubrum. II. Complete amino acid sequence and phylogenetic relationships. J Biol Chem. 1968 Oct 25;243(20):5507–5518. [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Meyer T. E., Cusanovich M. A., Daldal F., Rayment I. Crystallization and preliminary analysis of crystals of cytochrome c2 from Rhodopseudomonas capsulata. J Mol Biol. 1987 May 5;195(1):229–231. doi: 10.1016/0022-2836(87)90341-x. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Donohue T. J., Havelka W. A., Kaplan S. DNA sequence and in vitro expression of the B875 light-harvesting polypeptides of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):742–750. doi: 10.1128/jb.169.2.742-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F. S., Oesterhelt D. Microaerophilic growth and induction of the photosynthetic reaction center in Rhodopseudomonas viridis. J Bacteriol. 1989 May;171(5):2827–2834. doi: 10.1128/jb.171.5.2827-2834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., DeHoff B. S., Donohue T. J., Gumport R. I., Kaplan S. Transcriptional analysis of puf operon expression in Rhodobacter sphaeroides 2.4.1 and an intercistronic transcription terminator mutant. J Biol Chem. 1989 Nov 15;264(32):19354–19365. [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Saeda M., Masaki K., Kasai N., Miki M., Hayashi K. Crystallization and preliminary X-ray diffraction study of ferrocytochrome c2 from Rhodopseudomonas viridis. J Mol Biol. 1986 Oct 5;191(3):579–580. doi: 10.1016/0022-2836(86)90152-x. [DOI] [PubMed] [Google Scholar]
- Newbury S. F., Smith N. H., Robinson E. C., Hiles I. D., Higgins C. F. Stabilization of translationally active mRNA by prokaryotic REP sequences. Cell. 1987 Jan 30;48(2):297–310. doi: 10.1016/0092-8674(87)90433-8. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Freer S. T., Xuong N. H., Alden R. A., Kraut J. The structure of oxidized cytochrome c 2 of Rhodospirillum rubrum. J Biol Chem. 1973 Jun 10;248(11):3910–3921. doi: 10.2210/pdb1c2c/pdb. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Kraut J., Kamen M. D. Structural bases for function in cytochromes c. An interpretation of comparative x-ray and biochemical data. J Biol Chem. 1973 Nov 25;248(22):7701–7716. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self S. J., Hunter C. N., Leatherbarrow R. J. Molecular cloning, sequencing and expression of cytochrome c2 from Rhodospirillum rubrum. Biochem J. 1990 Jan 15;265(2):599–604. doi: 10.1042/bj2650599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer K. A., Lottspeich F., Gruenberg H., Lang F., Oesterhelt D., Michel H. Amino acid sequence of the cytochrome subunit of the photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1987 Aug;6(8):2197–2202. doi: 10.1002/j.1460-2075.1987.tb02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C., Dunger I., Michel H. Structure and transcription of the genes encoding the B1015 light-harvesting complex beta and alpha subunits and the photosynthetic reaction center L, M, and cytochrome c subunits from Rhodopseudomonas viridis. J Bacteriol. 1990 Jun;172(6):2877–2887. doi: 10.1128/jb.172.6.2877-2887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]