Abstract
Objective. The aim of this study is to report our experience using self-expandable covered metallic stents (Wallstent) to treat different types of biliary strictures after orthotopic liver transplantation (OLT). Patients and methods. Between January 1999 and July 2004, 222 OLTs were performed with choledocho-choledochostomy (CC) bile duct reconstruction. An anastomotic biliary stricture was diagnosed and treated by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous procedures in 100 patients (45%). The group of 21 patients (mean age 57.0±5.6 years) that were eventually treated with a biliary Wallstent was studied retrospectively. Results. Significant persistent proximal or anastomotic strictures were diagnosed in 4 and 17 patients, respectively. A Wallstent was inserted by ERCP or through a percutaneous route in 18 and 3 patients, respectively. The mean interval between diagnosis and Wallstent insertion was 179.7±292.8 (0–1113) days. The mean total number of procedures required per patient was 7.4±5.5. The mean stent primary patency duration was 10.8±7.8 (0.9–25.1) months with a 24-month primary patency rate of 26% at a mean follow-up time of 37.8±17.2 months. A hepatico-jejunostomy was performed in five patients (24%). Two patients (10%) underwent retransplantation for diffuse ischemic cholangitis or chronic rejection. The overall complication rate was 4%. Conclusion. Treatment of post-transplant biliary stenosis using a Wallstent is a valuable option for delaying or avoiding surgery in up to 70% of patients. Proximal stenosis can be treated in the same manner in selected patients with major comorbidities.
Keywords: Liver transplantation, biliary strictures, self-expandable metallic stent, endoscopic retrograde cholangiopancreatography
Introduction
Bile duct anastomotic stricture is a common problem after orthotopic liver transplantation (OLT) with a reported incidence of 15–20% 1. The two usual types of bile duct reconstruction performed at the time of OLT are choledocho-choledochostomy (CC) and hepatico-jejunostomy (HJ). Technical factors such as duct size, size discrepancy, retransplantation and diagnosis of liver disease such as sclerosing cholangitis will influence the selection of CC versus HJ. Biliary complications have always been a significant cause of post-transplantation morbidity 2.
Endoscopic or trans-hepatic balloon dilatation and stent insertion is a well recognized therapeutic option for treatment of bile duct strictures 1. However, the use of self-expandable metallic stents to treat benign conditions is not recommended.
The aim of this study was to report our experience using Wallstents to treat different types of biliary strictures occurring after OLT.
Patients and methods
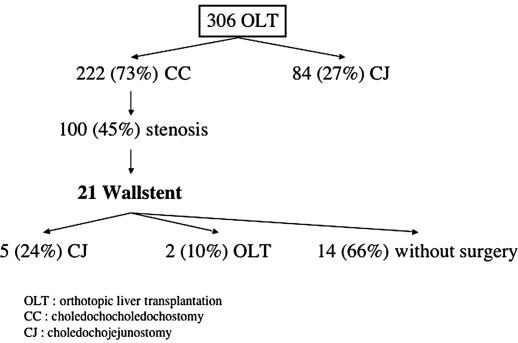
Between January 1999 and July 2004, 306 cadaveric OLT were performed in 279 patients at our institution. Among these, 222 (73%) had a CC and 84 had an HJ. The HJ patients are excluded from the analysis. Various postoperative biliary anomalies including early postoperative inflammatory stenosis secondary to edema, anastomotic stricture and ischemic cholangiopathy were diagnosed in 100 CC patients (45%). This retrospective study reports all CC patients (n=21) with intrahepatic, proximal or anastomotic stenosis treated by Wallstent.
Elevated liver function tests, angiocholitis or abnormal radiological findings such as dilatation of the proximal bile duct or biloma prompted imaging of the biliary tree by endoscopic retrograde cholangiopancreatography (ERCP). If a stricture was diagnosed, endoscopic sphincterotomy, balloon dilatation and plastic stent insertion was performed. Patients were then rescheduled every 6 weeks for dilatation and stent insertion until complete resolution of the stricture. The same operator (A.R.) performed all the endoscopic procedures. The percutaneous trans-hepatic route was used only when the stricture could not be canulated at the time of ERCP.
Self-expandable metallic stent (Wallstent) insertion was selected for patients with: (i) a persisting stricture despite a minimum of three adequate dilatations with an 8 mm balloon; (ii) a stricture occurring more than 6 months post-OLT with obvious immediate absence of response to dilatation. All patients were treated with a self-expandable biliary metallic Wallstent™ endoprothesis with Permalume™ covering and Unistep™ plus delivery system from Boston Scientific USA (the simple term Wallstent will be used throughout the text).
Primary patency time was defined by the time interval between Wallstent insertion and the first episode of clinical obstruction requiring treatment. Obstruction was defined by clinical jaundice or angiocholitis and abnormal liver function tests showing cholestasis. The obstruction was always confirmed by ERCP or percutaneous trans-hepatic cholangiogram. Patients without symptoms and with normal liver function tests were considered as having a patent stent. Secondary patency time was defined by the total duration of stent patency even though re-intervention might have been required for removal of biliary sludge but without requiring a new Wallstent or plastic stent insertion.
Demographic and clinical data were collected using systematic review of patients’ files and interrogation of a computerized database. Statistics were calculated using Statview version 5.0 for MacIntosh.
Results
Ten men and 11 women with a mean age of 57±5.6 years (mean±SD) were studied. The patients’ characteristics are summarized in Table I. Indications for transplantation included alcoholic cirrhosis (three patients), chronic hepatitis B (one patient) or C (three patients), primary biliary cirrhosis (two patients), non-alcoholic steato-hepatitis (two patients), α1-antitrypsin deficiency (one patient), giant cell hepatitis (one patient), cryptogenic cirrhosis (five patients), idiopathic fulminant hepatic failure (one patient) and hepatocellular carcinoma (two patients).
Table I. Patients’ characteristics.
| Parameter | Value |
|---|---|
| Weight (kg) | 72.2±12.7 |
| Height (cm) | 166±7 |
| Body mass index (BMI) | 26.3±4.3 |
| Child Pugh score (B–C) | 11±1.5 (9–12) |
| MELD | 19.6±10.5 |
| Donor age (year) (min–max) | 45.5±16.2 (17–70) |
| Donor CMV +/ − | 13/8 |
The operative time and cold and warm ischemic times for the 21 studied cases were 225±60, 460±158 and 40±9 min, respectively. Blood losses were 1420±1100 ml (range 400–3500). Donor age was 45.5±16.2 years (range 17–70).
Proximal and anastomotic stenosis was diagnosed in 4 and 17 patients, respectively. A Wallstent was inserted by ERCP in 18 patients and by percutaneous approach in 3 patients. A concomitant bile leak was identified in four patients (19%) (Figure 1).
Figure 1. .
Evolution for patients with Wallstent. OLT, orthotopic liver transplantation; CC, choledocho-choledochostomy; HJ, hepatico-jejunostomy.
The mean interval between diagnosis and Wallstent insertion was 179.7±292.8 (0–1113) days. The total number of procedures required per patient was 7.4±5.5, with 3.7±3.9 procedures performed before and 2.7±3.4 after Wallstent insertion. Globally, 1.8±1 Wallstents were inserted per patient with nine patients needing a second Wallstent. Eight patients required endoscopic cleaning of biliary sludge inside the Wallstent during the course of the follow-up. Seven patients eventually underwent plastic stent insertion for inadequate drainage by Wallstent only during follow-up.
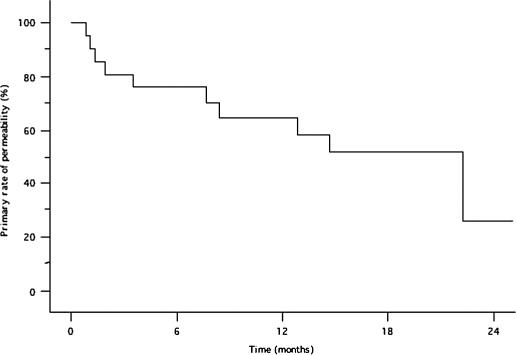
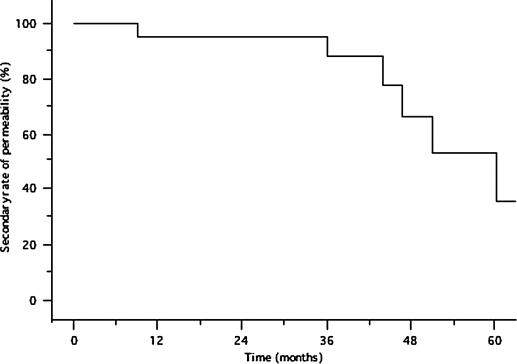
The mean primary patency duration of the Wallstent was 10.8±7.8 (0.9–25.1) months. After a mean follow-up time of 37.8±17.2 months, the 12-, 18-, and 24-month primary patency rates were 64%, 51%, and 26%, respectively (Figure 2). The yearly secondary patency rates from 1 to 5 years were: 95%, 95%, 88%, 66%, and 35%, respectively (Figure 3).
Figure 2. .
Primary patency rate of the Wallstent.
Figure 3. .
Secondary patency rate of the Wallstent.
Fourteen patients (66%) were treated solely by Wallstent without recurrence of problem. An HJ was performed in five (24%) patients because of recurrent obstruction of the Wallstent, in the context of a good general condition and complete recovery from the OLT. Two patients (10%) underwent retransplantation for diffuse ischemic cholangitis (n=1) and chronic rejection (n=1) after a mean interval of 782.1±638.2 days after diagnosis. No graft was lost secondary to the use of a Wallstent. A total of 156 procedures were realized in our studied population. Six complications (4%) of those 156 procedures were encountered in 6 patients: 3 pancreatitis, 2 cholangitis, and 1 case of aeroportia. All those complications were medically treated with a rapid recovery and a favorable outcome without prolongation of hospital stay.
When an HJ bile reconstruction was required in the course of the follow-up, no technical difficulty was encountered at the time of surgery for removal of the Wallstent. All the patients had a patent bile duct once the Wallstent was removed. The HJ bile reconstructions were easily performed without postoperative anastomotic leak or anastomotic stricture in the course of the follow-up.
Discussion
Biliary Wallstents were proven to be superior to plastic stents in the context of malignant biliary obstruction because of increased patency duration. However, in the setting of benign disease, such as bile duct stricture post liver transplantation, the use of Wallstents has been associated with poor results and is not recommended 3.
Bile duct complications are a common event after liver transplantation, with a reported incidence of 15–20% 1,4. Postoperative bile duct anastomotic strictures are a frequent cause of postoperative allograft dysfunction. Sutcliffe et al. showed that 49% of patients who had a demonstrable bile duct obstruction also had histological evidence of nonbiliary pathology 5. Multiple factors are involved in the physiopathology of the strictures including technical factors at the time of surgery, bile duct size, prolonged cold ischemia time, previous portocaval shunt, vascular compromise, acute or chronic rejection, CMV infection, ABO incompatibility, and recurrent disease such as sclerosing cholangitis. However, the nonsurgical causes are more frequently associated with multiple intrahepatic strictures 1,6.
ERCP is the first-line modality for bile duct imaging after a CC. When treatment is required, a survey of practices in the USA found that therapeutic ERCP, percutaneous and surgical approaches were selected as the initial therapeutic modality in 45%, 22%, and 29% of cases, respectively 7. Those approaches were successful in 27–91% of the cases 8. Therapeutic ERCP will allow balloon dilatation of the stricture with or without stent insertion. Stent insertion gives a better success rate than dilatation alone 8,9. However, the stent must be changed regularly, with an average lifespan of 3–4 months 9,10.
Patients presenting with a persistent stenosis despite adequate balloon dilatation and stenting will be best served by surgical revision and reconstruction using an HJ. Low bile duct stricture recurrence rate and a 4-year success rate of 88% has been obtained post HJ 11. Nonetheless, reconstruction can be difficult in patients with multiple comorbidities or previous abdominal surgery, with a higher recurrence rate 12. Timing of reconstructive bile duct surgery will also be of essence in the context of a patient recovering from a liver transplantation with all the inherent complications of the initial surgery and the potential morbidity of the upcoming reconstructive surgery.
In our population of transplanted patients, we favor ERCP as a primary mode of evaluation and treatment of all postoperative patients harboring cholestasis. If ERCP fails, diagnosis will be made by percutaneous trans-hepatic cholangiogram. Treatment will be selected based on assessment of the global clinical situation. An HJ will be selected only for the good candidates. Patients with a worse outcome post OLT or presenting with major comorbidities will be considered for Wallstent insertion with a global success rate of bile duct salvage of 70%. Despite good initial results with Wallstents, a primary patency rate of 26% at 24 months was obtained. Secondary patency rate can be improved by ERCP revision of the Wallstent, insertion of a plastic stent or use of a second Wallstent 3. However, this approach is plagued by increased cost, repeated ERCP with consequent patient discomfort and potential morbidity of the procedures. In our experience, Wallstents were not associated with any complications beside obstruction. No patient needed a second transplantation because of complications related to Wallstent insertion. ERCP and Wallstent insertion did not impact in any way on the reconstructive surgery. The Wallstents were easily removed since they were covered stents and probably also because of the woven conception. Furthermore, Wallstent insertion did not cause any change in the bile duct epithelium seen at pathology analysis. No stenosis of the HJ was encountered at follow-up and all the patients have liver function tests.
Recently, we partially changed our approach, using what we named ‘temporary’ Wallstent. We have treated four patients with short anastomotic strictures by Wallstent insertion with the intention of eventual removal. We used 8 cm long covered stents that were purposely positioned to leave a length of 1–1.5 cm protruding into the lumen of the duodenum. Those stents were removed 6 months later with excellent results. This modality is still under study.
Conclusions
Post liver transplantation bile duct anastomotic strictures can be treated successfully by therapeutic ERCP with balloon dilatation and stent insertion. In a situation of persistent stenosis, a reconstructive surgery by HJ is the standard treatment. Wallstent insertion is a valuable alternative. It allows the clinician to avoid or delay a reconstructive procedure in up to 70% of the patients. Endoscopic revision of the stent is simple with a low complication rate. Proximal stenosis may also be treated by Wallstent insertion in selected patients with major comorbidities. No technical problem was encountered during the removal of the stent at the time of surgery when HJ was needed. The use of a temporary Wallstent to dilate a post-transplant stenosis is presently under study.
Footnotes
Presented at the annual AHPBA meeting, 14–17 April 2005, Fort Lauderdale, FL, USA.
References
- 1.Ostroff JW. Post-transplant biliary problems. Gastrointest Endosc Clin North Am. 2001;11:163–83. [PubMed] [Google Scholar]
- 2.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–5. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culp WC, McCowan TC, Lieberman RP, Goertzen TC, LeVeen RF, Heffron TG. Biliary strictures in liver transplant recipients: treatment with metal stents. Radiology. 1996;199:339–46. doi: 10.1148/radiology.199.2.8668775. [DOI] [PubMed] [Google Scholar]
- 4.Rossi G, Lucianetti A, Gridelli B, Colledan M, Caccamo L, Albani AP, et al. Biliary tract complications in 224 orthotopic liver transplantations. Transplant Proc. 1994;26:3626–8. [PubMed] [Google Scholar]
- 5.Sutcliffe R, Maguire D, Mroz A, Portmann B, O'Grady J, Bowles M, et al. Bile duct strictures after adult liver transplantation: a role for biliary reconstructive surgery? Liver Transpl. 2004;10:928–34. doi: 10.1002/lt.20146. [DOI] [PubMed] [Google Scholar]
- 6.Culp WC, McCowan TC, Lieberman RP, Goertzen TC, LeVeen RF. Treatment of biliary strictures with metallic stents in liver transplant recipients. J Vasc Interv Radiol. 1996;7:457–8. doi: 10.1016/s1051-0443(96)72891-9. [DOI] [PubMed] [Google Scholar]
- 7.Vallera RA, Cotton PB, Clavien PA. Biliary reconstruction for liver transplantation and management of biliary complications: overview and survey of current practices in the United States. Liver Transpl Surg. 1995;1:143–52. doi: 10.1002/lt.500010302. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DA, Petersen BT, Poterucha JJ, Gostout CJ. Endoscopic therapy of anastomotic bile duct strictures occurring after liver transplantation. Gastrointest Endosc. 2000;51:169–74. doi: 10.1016/s0016-5107(00)70413-5. [DOI] [PubMed] [Google Scholar]
- 9.Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, et al. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–31. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 10.Rossi AF, Grosso C, Zanasi G, Gambitta P, Bini M, De Carlis L, et al. Long-term efficacy of endoscopic stenting in patients with stricture of the biliary anastomosis after orthotopic liver transplantation. Endoscopy. 1998;30:360–6. doi: 10.1055/s-2007-1001283. [DOI] [PubMed] [Google Scholar]
- 11.Pitt HA, Kaufman SL, Coleman J, White RI, Cameron JL. Benign postoperative biliary strictures. Operate or dilate? Ann Surg. 1989;210:417–25. doi: 10.1097/00000658-198910000-00001. discussion 426–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thethy S, Thomson B, Pleass H, Wigmore SJ, Madhavan K, Akyol M, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–53. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]