Abstract
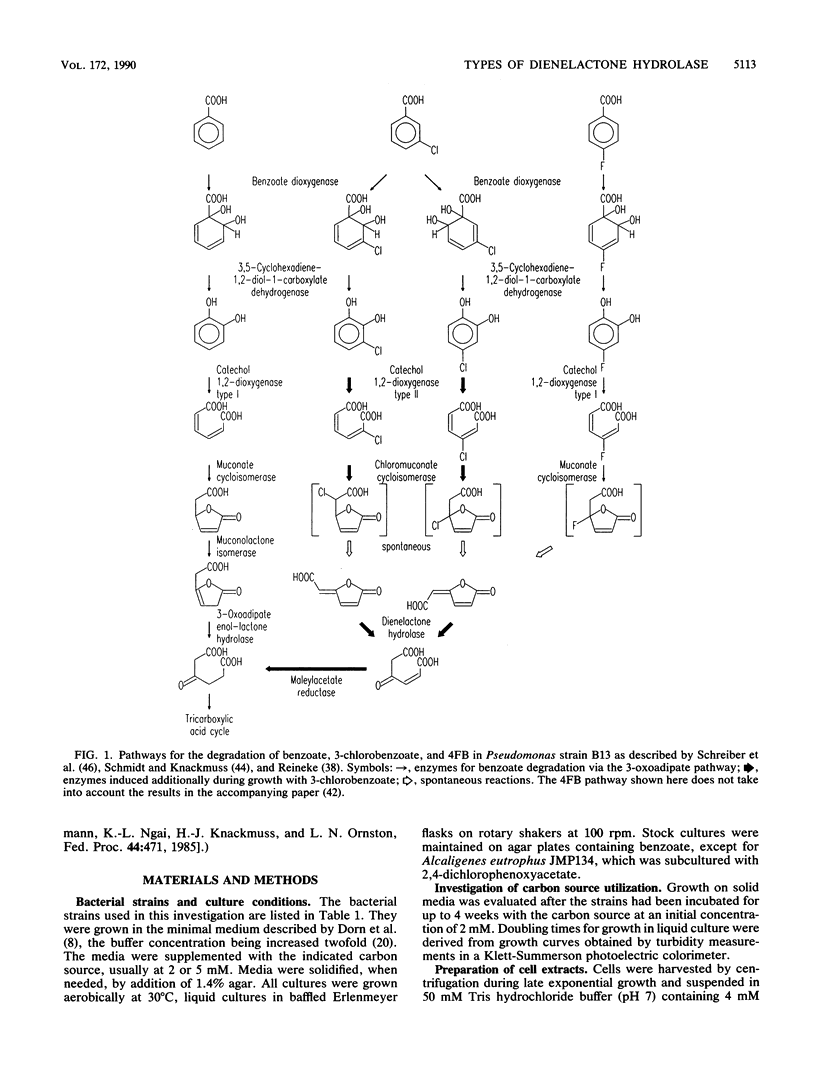
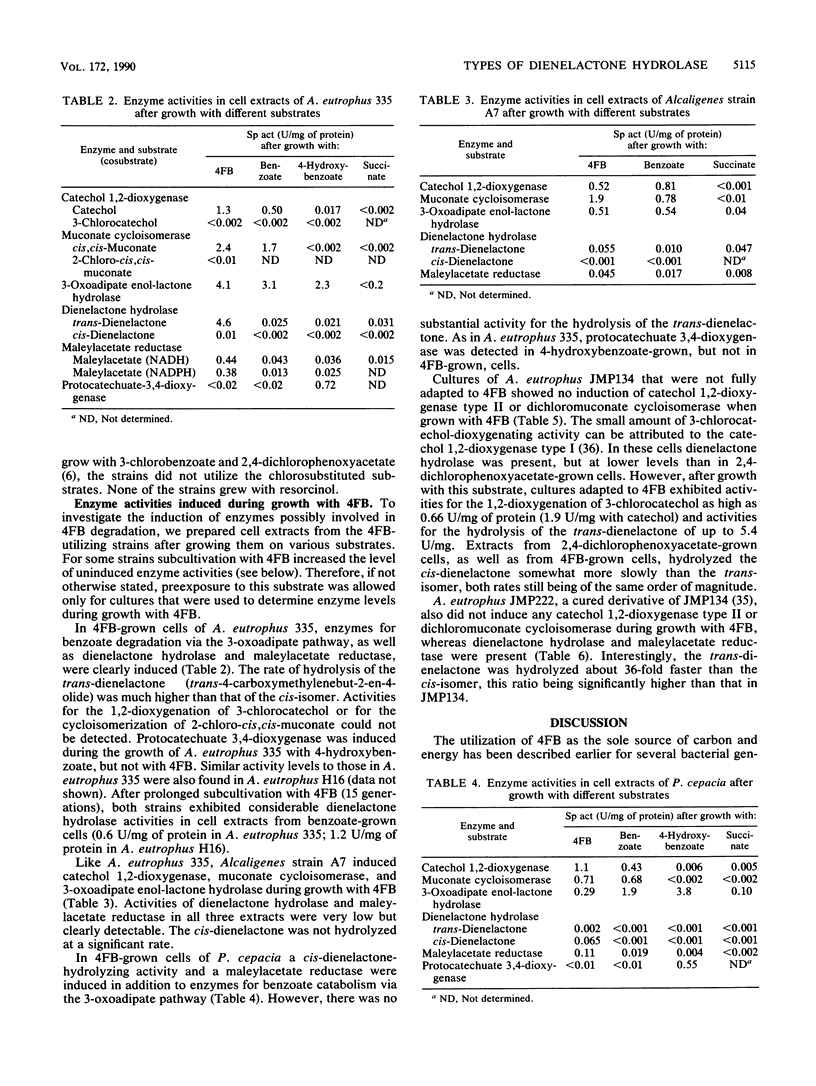
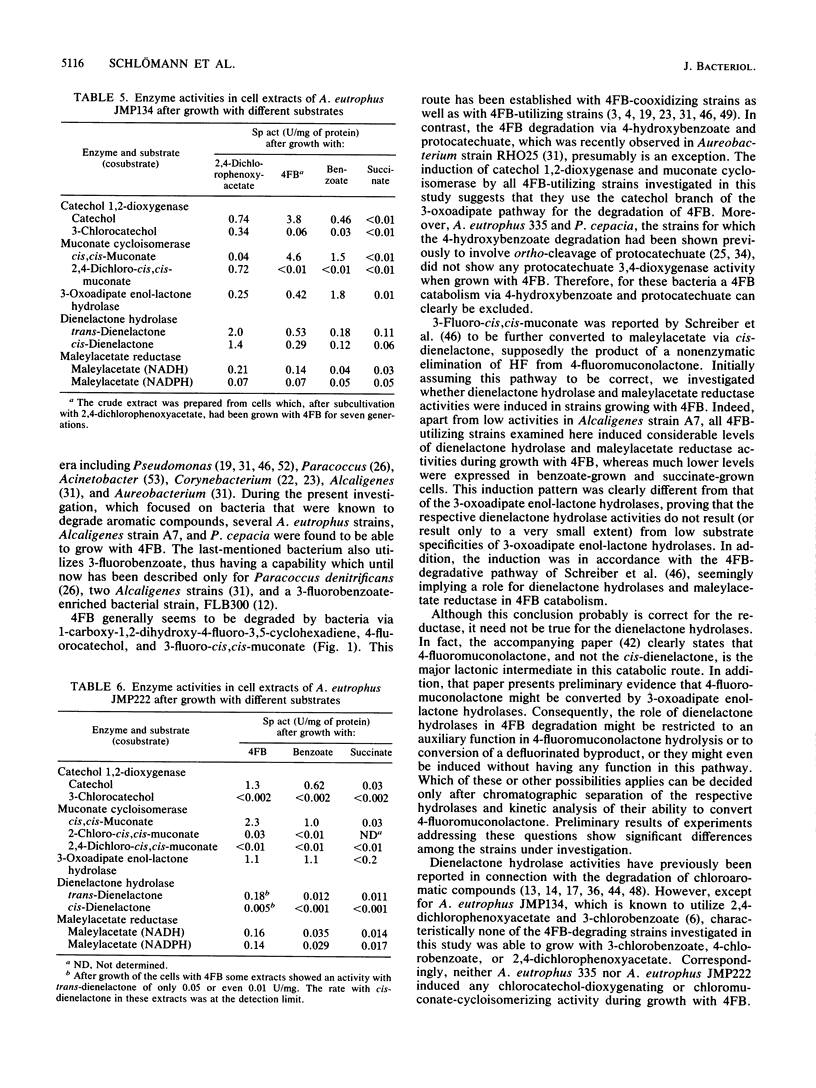
Of various benzoate-utilizing bacteria tested, Alcaligenes eutrophus 335, A. eutrophus H16, A. eutrophus JMP222, A. eutrophus JMP134, Alcaligenes strain A7, and Pseudomonas cepacia were able to grow with 4-fluorobenzoate as the sole source of carbon and energy. P. cepacia also utilizes 3-fluorobenzoate. Except for A. eutrophus JMP134, which is known to grow with 2,4-dichlorophenoxyacetate and 3-chlorobenzoate (R. H. Don and J. M. Pemberton, J. Bacteriol. 145:681-686, 1981), the strains were unable to grow at the expense of these compounds or 4-chlorobenzoate. Assays of cell extracts revealed that all strains express dienelactone hydrolase and maleylacetate reductase activities in addition to enzymes of the catechol branch of the 3-oxoadipate pathway when growing with 4-fluorobenzoate. Induction of dienelactone hydrolase and maleylacetate reductase apparently is not necessarily connected to synthesis of catechol 1,2-dioxygenase type II and chloromuconate cycloisomerase activities, which are indispensable for the degradation of chlorocatechols. Substrate specificities of the dienelactone hydrolases provisionally differentiate among three types of this activity. (i) Extracts of A. eutrophus 335, A. eutrophus H16, A. eutrophus JMP222, and Alcaligenes strain A7 convert trans-4-carboxymethylenebut-2-en-4-olide (trans-dienelactone) much faster than the cis-isomer (type I). (ii) The enzyme present in P. cepacia shows the opposite preference for the isomeric substrates (type II). (iii) Cell extracts of A. eutrophus JMP134, as well as purified dienelactone hydrolase from Pseudomonas strain B13 (E. Schmidt and H.-J. Knackmuss, Biochem. J. 192:339-347, 1980), hydrolyze both dienelactones at rates that are of the same order of magnitude (type III). This classification implies that A. eutrophus JMP134 possesses at least two different dienelactone hydrolases, one of type III encoded by the plasmid pJP4 and one of type I, which is also present in the cured strain JMP222.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Tranter E. K., Darrah J. A. The utilization of some halogenated aromatic acids by Nocardia. Oxidation and metabolism. Biochem J. 1968 Jan;106(1):211–227. doi: 10.1042/bj1060211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K. F., Callely A. G., Livingstone A., Fewson C. A. Metabolism of monofluorobenzoates by Acinetobacter calcoaceticus N.C.I.B. 8250. Formation of monofluorocatechols. Biochim Biophys Acta. 1975 Oct 9;404(2):169–179. doi: 10.1016/0304-4165(75)90323-2. [DOI] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Moss P., Fernley H. N. Bacterial metabolism of 4-chlorophenoxyacetate. Biochem J. 1971 May;122(4):509–517. doi: 10.1042/bj1220509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Meyer M., Schlegel H. G. Transfer and expression of the herbicide-degrading plasmid pJP4 in aerobic autotrophic bacteria. Arch Microbiol. 1983 Feb;134(2):92–97. doi: 10.1007/BF00407938. [DOI] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979 Jan;137(1):13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J. 1971 May;122(4):533–542. doi: 10.1042/bj1220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S. Nucleotide homology and organization of chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Mol Gen Genet. 1988 Jan;211(1):113–120. doi: 10.1007/BF00338401. [DOI] [PubMed] [Google Scholar]
- Harper D. B., Blakley E. R. The metabolism of p-fluorobenzoic acid by a Pseudomonas sp. Can J Microbiol. 1971 Aug;17(8):1015–1023. doi: 10.1139/m71-162. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Reineke W., Knackmuss H. J. Metabolism of 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate by a pseudomonad. Appl Environ Microbiol. 1979 Mar;37(3):421–428. doi: 10.1128/aem.37.3.421-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Dissimilation of aromatic compounds by Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):468–475. doi: 10.1128/jb.107.2.468-475.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhm A. E., Schlömann M., Knackmuss H. J., Pieper D. H. Purification and characterization of dichloromuconate cycloisomerase from Alcaligenes eutrophus JMP 134. Biochem J. 1990 Mar 15;266(3):877–883. [PMC free article] [PubMed] [Google Scholar]
- MALEK I., RADOCHOVA M., LYSENKO O. TAXONOMY OF THE SPECIES PSEUDOMONAS ODORANS. J Gen Microbiol. 1963 Dec;33:349–355. doi: 10.1099/00221287-33-3-349. [DOI] [PubMed] [Google Scholar]
- Malashenko Iu R., Romanovskaia V. A., Kryshtab T. P., Pogrebnoi I. P. Diauksotrofnye svoistva mikroorganizmov, assimiliruiushchikh uglevodorody C2--C4. Mikrobiologiia. 1979 Sep-Oct;48(5):798–802. [PubMed] [Google Scholar]
- Medvedev Iu V., Girfanova T. F., Gridnev V. N. Issledovanie élektroforeticheskikh svoistv populiatsii kletok Escherichia coli metodom mikroélektroforeza. Mikrobiologiia. 1987 Jan-Feb;56(1):145–149. [PubMed] [Google Scholar]
- Ngai K. L., Schlömann M., Knackmuss H. J., Ornston L. N. Dienelactone hydrolase from Pseudomonas sp. strain B13. J Bacteriol. 1987 Feb;169(2):699–703. doi: 10.1128/jb.169.2.699-703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmanns R. H., Müller R., Otto M. K., Lingens F. Evidence for a new pathway in the bacterial degradation of 4-fluorobenzoate. Appl Environ Microbiol. 1989 Oct;55(10):2499–2504. doi: 10.1128/aem.55.10.2499-2504.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- RADLER F. Untersuchungen über den Verlauf der Stoffwechselvorgänge bei Azotobacter chroococcum Beij. Arch Mikrobiol. 1955;22(4):335–367. [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Schlömann M., Fischer P., Schmidt E., Knackmuss H. J. Enzymatic formation, stability, and spontaneous reactions of 4-fluoromuconolactone, a metabolite of the bacterial degradation of 4-fluorobenzoate. J Bacteriol. 1990 Sep;172(9):5119–5129. doi: 10.1128/jb.172.9.5119-5129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Remberg G., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochem J. 1980 Oct 15;192(1):331–337. doi: 10.1042/bj1920331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A., Hellwig M., Dorn E., Reineke W., Knackmuss H. J. Critical Reactions in Fluorobenzoic Acid Degradation by Pseudomonas sp. B13. Appl Environ Microbiol. 1980 Jan;39(1):58–67. doi: 10.1128/aem.39.1.58-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwien U., Schmidt E. Improved degradation of monochlorophenols by a constructed strain. Appl Environ Microbiol. 1982 Jul;44(1):33–39. doi: 10.1128/aem.44.1.33-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpee K. W., Duxbury J. M., Alexander M. 2,4-Dichlorophenoxyacetate metabolism by Arthrobacter sp.: accumulation of a chlorobutenolide. Appl Microbiol. 1973 Sep;26(3):445–447. doi: 10.1128/am.26.3.445-447.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Tranter E. K., Cain R. B. The utilization of some halogenated aromatic acids by Nocardia. Effects on growth and enzyme induction. Biochem J. 1968 Jan;106(1):203–209. doi: 10.1042/bj1060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]



