Abstract
Radiolytic reduction in frozen solutions and crystals is a useful method for generation of trapped intermediates in protein based radical reactions. In this communication we define the conditions which provide the maximum yield of one electron reduced myoglobin at 77 K using 60Co γ-irradiation in aqueous glycerol glass. The yield reached 50% after 20 kGy, was almost complete at ∼160 kGy total dose, and does not depend on the protein concentration in the range 0.01 – 5 mM.
Keywords: Gamma radiolysis, Cryoreduction yield, Heme protein
Introduction
Cryogenic radiolytic reduction of metalloenzymes is often used as a convenient tool to generate and trap the unstable intermediates of important biochemical reactions (Davydov, Magonov 1981; Davydov, Khangulov 1983; Schlichting et al. 2000; Davydov et al. 2001; Davydov et al. 2002; Denisov et al. 2002a; Denisov et al. 2005; Sligar et al. 2005). In many cases these intermediates are elusive and impossible to generate by other experimental approaches (Shintaku et al. 2005). Radiolysis is traditionally used for the generation of unstable intermediates and radicals (Ausloos 1968; Jonah 2001). However, application of pulsed radiolysis to generate transient species in solution is usually limited to fast processes and relatively low yields. In addition, for most biological systems at ambient conditions, it is difficult to estimate quantitatively the effect of X-ray or γ-irradiation on specific redox centers. Radiolysis of a complex system such as a protein solution produces a plethora of radicals, which subsequently react to form diverse end products, the latter also depending on solvent conditions and presence of other reactive components in solution (Spinks 1990; Wojnarovits 2003). Cryogenic radiolysis of frozen solutions helps to avoid some of these difficulties because the diffusion of most of radiolysis products is severely limited in the glassy matrix (Willard 1975; Lin et al. 1976; Kevan 1980). As a result cryogenic radiolysis in solid matrices provides the possibility to accumulate trapped intermediates over extended periods of time, and to improve overall yield of the intermediate (Symons 1995; Feldman 1999; Tanskanen et al. 2005).
Cryogenic radiolysis and radiolytic reduction (often termed “photoreduction” due to the radiolysis, caused by X-ray photons) are also recognized as important side reactions which alter the redox state of metalloproteins during data collection in X-ray crystallography and X-ray spectroscopy (Schlichting et al. 2000; Berglund et al. 2002; Yano et al. 2005). In addition to specific structural changes in the first coordination sphere of metal atoms, these reactions are also considered as a main source of radiation damage to protein crystals. With the broad use of high intensity beam lines on third and future generation synchrotrons, the interest to the cryogenic radiation chemistry of proteins is increasing (Murray, Garman 2002; O'Neill et al. 2002; Murray et al. 2004; Nave, Garman 2005; Garman, Owen 2006).
Here we report the results of systematic study of the yield of cryoradiolytic reduction of “met” myoglobin (ferric Fe3+ heme) as a function of irradiation dose for the broad range of the heme protein concentrations. Spectroscopic studies of metmyoglobin with different ligands reduced by cryogenic γ-radiolysis (Magonov et al. 1978; Gasyna 1979) and cryogenic photolysis (Lamb et al. 1998; Engler et al. 2000) have been reported. However, none of this prior work has studied the saturation of cryoreduction yield at high doses, and possible dependence of radiolytic reduction yield on the protein concentration. Currently, the ability to obtain the highest possible yield of unstable redox intermediates generated in cryoradiolytic reduction experiments is very important for the success of the structural and spectroscopic studies of metalloenzyme mechanisms. This is especially valuable for the X-ray crystallographic studies, where partial occupancy or multiple conformational substates may preclude one from a definite structural conclusion. Similar information on radiolytic yield would be exceedingly valuable for spectroscopic characterization of the intermediates by methods such as Mössbauer (Yoo et al. 2000), X-ray absorption (Yano et al. 2005), resonance Raman (Ibrahim et al. 2003), and optical absorption (Denisov et al. 2002b; Denisov et al. 2002c), which, unlike EPR, detect all possible oxidation states of the metal center and usually produce highly overlapped spectra if multiple species are present in the sample.
Materials and Methods
Horse skeletal metmyoglobin (Mb) was purchased from Sigma (batch M0630) and used without additional purification. All other chemicals were of analytical or spectroscopic grade and doubly ionized water was used for buffer preparation. Because one of the goals of this work was to optimize of the yield of peroxo-ferric heme enzyme complexes obtained as a result of one-electron reduction of oxygenated ferrous heme enzymes, all solutions were prepared aerobically.
Samples of different concentrations were prepared in 150 mM phosphate buffer at pH 7.5 and mixed with glycerol to the final ratio of glycerol/buffer 2:1 (w/w). This glycerol/water ratio provides an optically transparent glass over the temperature range 77 K - 300 K used in these experiments (Shibata et al. 1999). Samples of higher Mb concentration (5 – 8 mM) were prepared by direct addition of the weighted amount of mixed glycerol-water solvent (80 – 150 mg) to the selected sample of lyophilized protein (10 – 15 mg). The resulting Mb concentration was then calculated using known densities of the solvent and protein and verified by UV-VIS spectroscopy. Thin layer cells for the measurements at high concentrations were assembled using two square plates of UV-enhanced polymetacrylate (Astra Products, Baldwin, NY). For the lower concentrations the metacrylate semimicrocells from Fisher were used as described (Denisov et al. 2001; Denisov et al. 2002a). The samples of met Mb with concentrations from 0.01 mM to 0.15 mM were prepared from concentrated stock solutions in phosphate buffer by dilution with calculated amounts of glycerol and the same buffer to give the necessary final protein concentrations in 65% glycerol, 50 mM phosphate buffer at pH 7.5. Irradiation was conducted at 77 K using a 60Co source with the dose rate 220 Gy/min. During irradiation and all subsequent operations the samples were kept fully immersed in liquid nitrogen or in the optical cryostat at the indicated temperatures. Dose rate was measured using Radiachromic film FWT-60-00 (Far West Technology Inc., Goleta, CA) calibrated at NIST.
The radiolytic reduction yield (Mb(Fe3+) → Mb(Fe2+)) was estimated using optical spectroscopy. Spectra were measured at 80 K – 140 K using the home-made cryostat as previously described (Denisov et al. 2001; Denisov et al. 2002). After irradiation all samples were photobleached at 77 K using the white light from the 150 W lamp (Oriel Corp., Stratford, CT, USA) to remove the intense absorption of trapped electrons. The total concentration of myoglobin in each sample was measured by optical absorption at 293 K using known molar absorption coefficients at 409 nm (186 mM-1cm-1) and at 502 nm (10.2 mM-1cm-1) (Antonini, Brunori 1971). The concentration of reduced Mb in each sample was estimated from optical spectra measured after irradiation using the standard spectrum of this intermediate as described in the text. Spectral decomposition utilized experimental spectra of irradiated glycerol-buffer solvent and apo-Mb for background subtraction.
It is known that the spectral characteristics of cryoreduced Mb are quite distinct from those of ferrous Mb under ambient conditions (Gasyna 1979; Engler et al. 2000). This difference is due to the fact that the cryoreduced heme protein is trapped in the low-temperature glass and cannot undergo conformational relaxation following reduction. Thus, it was necessary to determine experimentally the spectrum of the cryoreduced ferrous Mb for later use as a spectral standard. For this the set of the spectra obtained with the samples of the same Mb concentrations irradiated with different doses were used. Assuming the simple transition from ferric myoglobin to ferrous myoglobin (two spectrally distinguishable species plus background) it was possible to derive quantitatively the unknown spectrum of ferrous Mb using the known spectrum of ferric Mb. The yield for each sample in these calculations was estimated from the amplitudes of the first derivative spectra at the near-UV region since the Soret bands of ferric and cryoreduced ferrous Mb are well resolved (see Fig. 1). The resulting spectrum of cryoreduced ferrous Mb has the typical features characteristic for the spectra of other low-spin ferrous proteins such as cytochrome c (Levantino et al. 2005) and Mb photoreduced at the low-temperature glass (Lamb et al. 1998; Engler et al. 2000).
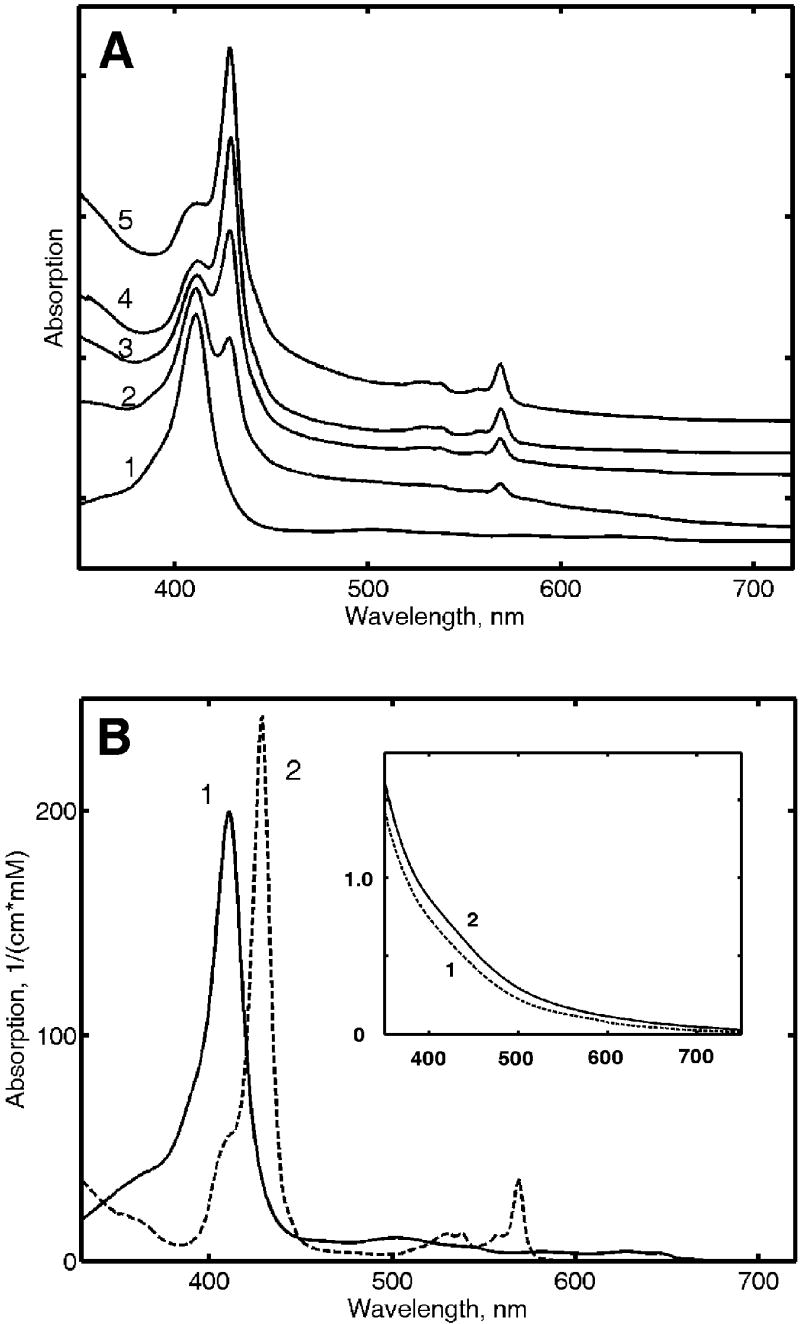
Figure 1.
A Absorption spectra of γ-irradiated met Mb measured at 77 K after photobleaching with white light. (1) Met Mb before irradiation; (2) 10 kGy dose; (3) 20 kGy; (4) 40 kGy; (5) 70 kGy. The samples were prepared from the same stock solution and irradiated at 77 K up to the shown dose. B Absorption spectra of met Mb (ferric) and cryoreduced Mb (ferrous) measured at 77 K and shown in millimolar absorption units. These spectra were used as the spectral basis for the cryoreduction yield measurements. Insert: Spectra of irradiated apo Mb at a dose 70 kGy after photobleaching. Protein concentration were: (1) 0.6 mM, (2) 0.15 mM.
The spectra of cryoreduced Mb and ferric Mb (Fig. 1) were used to construct a set of basis optical spectra. This set also included three smooth monotonous exponential curves and the linear slope used for the background subtraction. The resulting spectral matrix S (M by N elements) contained two spectra of Mb (Fig. 1) and 5 different background curves (total N=7 different spectra) digitized at 1 nm step in the range 320-720 nm (M=401 data points in each spectrum). For each sample the yield of cryoreduced ferrous Mb was determined as the best fit of the spectrum of this sample by the linear combination of the basis spectra defined above. This fit is equivalent to the optimal (in the sense of minimal least-square deviation) solution of the overdetermined system of linear equations:
where Sji is the absorption coefficient of the ith spectral component of the basis spectral matrix at the jth wavelength, Ci is the concentration corresponding to the ith spectral component, and Aj is the experimental absorption of the sample at the same the jth wavelength.
The solution of the system of linear equations and all concentrations of components with simultaneous background subtraction was found using a ‘left division’ subroutine implemented in MATLAB. For the samples with concentrations below 0.025 mM the spectra between 360 and 700 nm were used, for the samples with higher concentrations the absorption at Soret band was beyond instrumental linear response, and only the spectra from 460 to 700 nm were used for calculations.
Results
Radiolysis of aqueous-glycerol frozen glass produces trapped electrons with characteristic broad absorption band in the visible region (Willard 1973; Willard 1975; Rice, Kevan 1977). Hence, the samples appear deep purple immediately after irradiation, but this absorption can be easily photobleached using visible light, confirming that it is caused by trapped electrons (Fujii, Willard 1970; Rice, Kevan 1977). Because of the intense absorption of trapped electrons it was necessary to photobleach irradiated samples at 77 K before spectra of radiolytically reduced Mb could be obtained. For the thin layer samples, photobleaching was not necessary, and the absorption spectra could be measured before and after photobleaching.
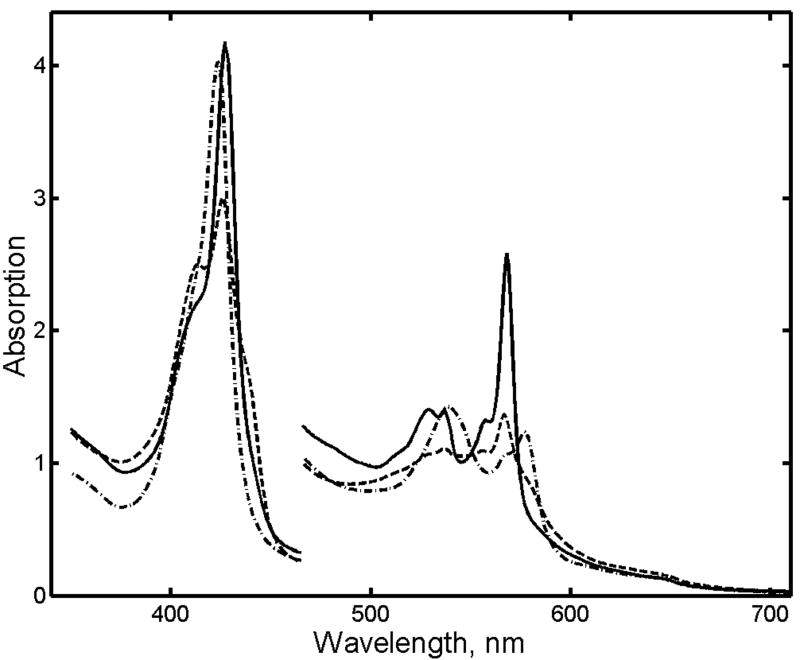
Representative spectra of an irradiated sample of Mb before and after photobleaching are shown at Fig. 2. Before photobleaching the sharp well-resolved spectrum of cryoreduced Mb appears on top of an intense background. After photobleaching the background disappears, and the characteristic absorption bands of cryoreduced Mb significantly increase. The Soret band is observed at 428 nm, and main components of split Q-bands are at 568 and 538 nm, all in a good agreement with earlier results (Gasyna 1979; Lamb et al. 1998; Engler et al. 2000).
Figure 2.
Absorption spectra of γ-irradiated metMb. (1) Cryoreduced met Mb after 90 kGy dose before photobleaching with white light; (2) Same sample after photobleaching; (3) Same sample before irradiation shown for comparison. Concentration of Mb was 4.7 mM, path length 0.05 mm.
The remaining smooth and featureless background is due to absorption spectra of multiple radicals formed in the glycerol solvent and protein matrix. To our knowledge, there is lack of information available on the absorption spectra of radicals which could be formed as a result of radiolysis at cryogenic temperatures although the absorption spectra of possible low-molecular weight analogs were studied in solution (Jonah 2001; Rappoport 2003). The detailed spectral assignment of this absorption is beyond the scope of our work. As a control, we have measured the spectra of two samples of apomyoglobin irradiated in the same conditions to the dose of 70 kGy. The spectra of these samples after photobleaching are shown at the Fig. 1(Insert). It is clear that both protein and solvent show absorption spectra due to radicals which are not photobleached, as well as to the products of secondary reactions between these radicals and electrons after photobleaching. After brief annealing of these samples at 140 K or higher, the absorption in visible region gradually decreases, as the radicals undergo further chemical reactions to form the end products of radiolysis.
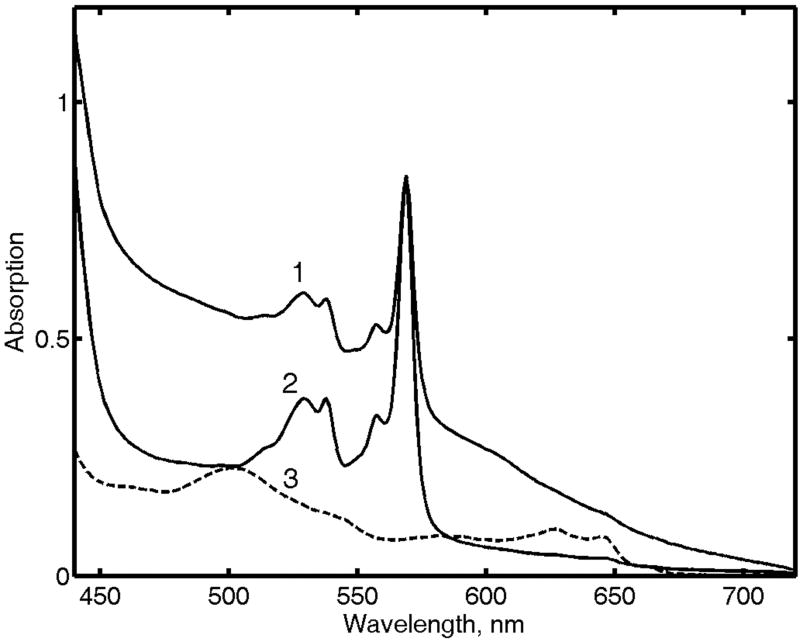
An essential feature of the method of cryogenic radiolytic reduction of proteins and other biological macromolecules is the stabilization of their structure by the glassy frozen solvent matrix (Symons, 1995). The primary product of radiolytic reduction, cryoreduced Mb, is immobilized at 77 K and maintains virtually all the structural features of the precursor met Mb (Lamb et al., 1998). After annealing at higher temperatures, the protein undergoes conformational relaxation. The spectra at Fig. 3 show at least two main sequential processes upon increase in temperature from 130 to 200 K. The conformational relaxation of the non-equilibrium low-spin cryoreduced ferrous Mb begins at ∼130 K, as revealed by the shift of Soret maximum from 428 to 438 nm. Above the glass transition temperature, where the translational diffusion of the small molecules is enabled, the complex of ferrous Mb with CO is gradually formed, with its characteristic Q-bands at 578 and 543 nm and Soret band at 423 nm.
Figure 3.
Annealing of cryoreduced met Mb (0.04 mM). Spectra were measured after photobleaching at different temperatures: (1) 90 K; (2) 153 K; (3) 188 K.
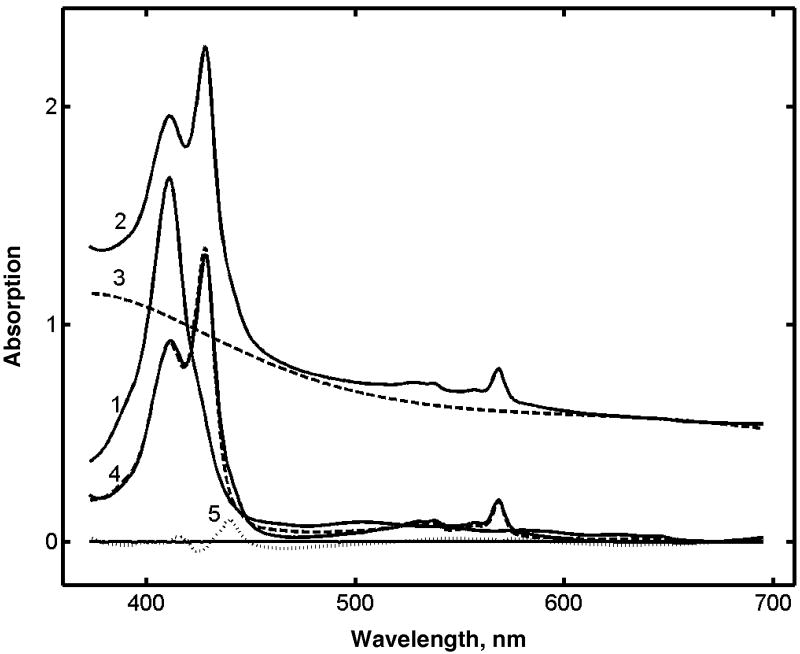
The yield of cryoreduction of met Mb was determined from the experimental spectra as shown at Fig. 4. Typically, after radiolysis and photobleaching, the spectrum of the partially reduced protein was observed in addition to a substantial background, due mainly to the absorption of trapped solvent radicals, and partly to the light scattering from the sample. Because of the diverse nature of the background, the latter was approximated as described in Materials and Methods. The representative result of such decomposition of experimental spectra is shown in Fig. 4 together with the initial experimental curve and the residuals of the data fitting. All spectra were processed in a similar manner, and the fractions of cryoreduced Mb were determined for different protein concentrations and irradiation doses. As described in Methods, the yield of cryoreduced Mb was calculated using spectra in the range 360 - 690 nm for the samples with concentrations lower than 0.025 mM, and the range 450 – 690 nm for higher concentrations, because of possible deviations from Lambert-Beer law.
Figure 4.
Calculation of the yield of radiolytic reduction from the experimental absorption spectra of γ-irradiated met Mb. (1) Spectrum of met Mb before irradiation; (2) Spectrum after γ-irradiation at 70 kGy and photobleaching with the white light; (3) Background subtracted as described in Materials and Methods; (4) Full line is the spectrum (2) with subtracted background (3); dotted line is the fit of this spectrum with two basis spectra shown in Fig. 1B. (5) Residuals.
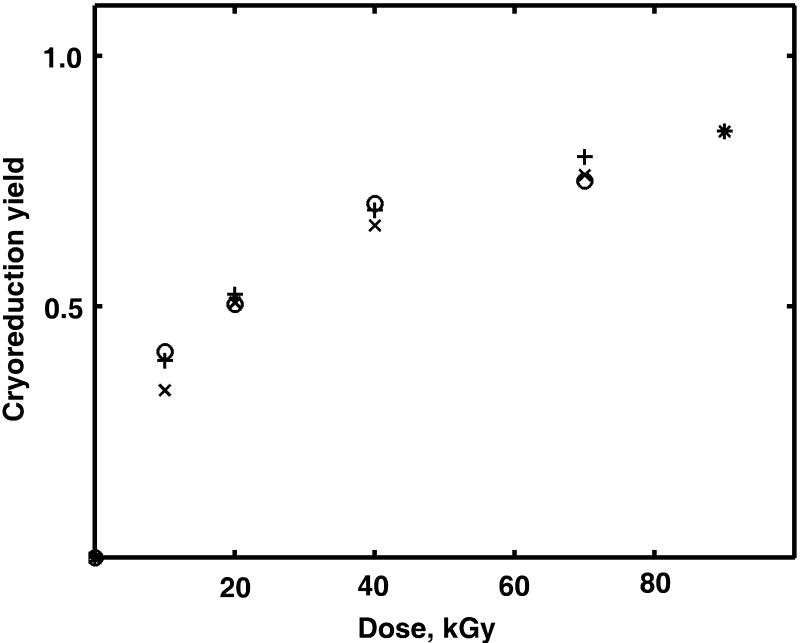
The fraction yield of cryoreduced Mb as a function of irradiation dose is shown in Fig. 5 for various protein concentrations. For all samples, we obtained high yields of cryoradiolytic reduction even at relatively low doses. Approximately 30% of the protein was reduced after 10 kGy of absorbed dose, and more than 70% - at 70 kGy. The reduction yield is not strongly dependent on Mb concentration in the range from 0.01 to 5 mM. At high doses the rate of cryoreduction decreases dramatically (Willard 1973; Willard 1975; Davydov et al. 1994). The fraction of reduced protein reached more than 95% at 160 kGy total dose, much higher than the 25% - 60% as previously reported (Davydov et al. 1994; Davydov et al. 1999; Krebs et al. 2000; Davydov et al. 2001).
Figure 5.
Yield of cryoreduction of met Mb as a function of γ-irradiation dose for different protein concentrations. (x) 0.02 mM; (+) 0.04 mM; (o) 0.15 mM; (*) 4.7 mM.
The lack of notable dependence of reduction yield on the protein concentration can be explained by the fact that the protein concentration in our samples is low compared to the concentration of trapped electrons generated by radiolysis. The yield of trapped electrons in aqueous glycerol glasses at 77 K, Ge, is approximately 1.5/100 eV (Makarov et al. 1969). Thus, under these conditions, approximately 1.5 mM/L of trapped electrons are generated at 10 kGy dose, and more than 10 mM/L at 70 kGy absorbed dose. The same concentrations of parent radicals are also trapped in the aqueous glycerol frozen matrix, which corresponds to an average distance of ∼50 Å between randomly distributed trapped electrons and radicals.
The highest protein concentrations used in this work are comparable to those encountered in protein crystals, where the solvent volume fractions may vary from 20% to 80%. At 5 mM concentration the volume fraction of myoglobin is 8%, and the direct radiolysis of the protein cannot be neglected. However, the yield of cryoreduced met Mb is the same as observed at low concentrations. Thus, a similar reduction by radiolytic electrons may induce redox state changes of the metal centers and protein cofactors during the data collection in cryogenic X-ray structural studies of protein crystals and this must be taken into consideration, especially in mechanistic interpretation of protein structures.
Discussion
The yield and reactions of trapped electrons during cryoradiolysis of aqueous – organic glasses in the presence of scavengers have been extensively studied (Fujii, Willard 1970; Sasaki, Ohno 1971; Zimbrick, Bowman 1972), but with little attention paid on the dynamics and target dependence of the average reduction yield. Importantly, for biological macromolecule designed to initiate chemical reaction by the addition of electron to intermediates stabilized at low temperature the target of radiolytic electrons also plays the role of scavenger. Hence, when cryoradiolysis is used as a method for the generation and accumulation of otherwise unstable intermediates in biological macromolecules such as metalloprotein, it is important to document the conditions under which the yield of cryoreduction of the scavenger can be maximized.
Under the conditions employed the yield of cryoreduced myoglobin is very high. As far as the maximal total yield depends on conditions, our results suggest that optimal conditions are realized in the presence of high concentration of glycerol and are independent of total protein concentration. The solvent composition, especially the presence of polyhydric alcohols (glycerol or ethylene glycol), is critically important for the yield of cryoreduction of metalloenzymes (Davydov et al. 1994; Davydov et al. 1999). At the same time these compounds are among the most popular cryoprotectants used in X-ray crystallography to prevent damage to protein crystal structure during flash-freezing at low temperatures. The results of this work demonstrate that the presence of glycerol at high concentrations may dramatically increase the yield of cryoradiolytic reduction of a metalloprotein such as met Mb. Thus, such processes must be controlled independently in protein X-ray crystallography, especially when mechanisms of redox-active metalloenzymes are addressed.
Several examples of studies where cryogenic X-ray crystallography of heme proteins was accompanied by the parallel measurements of optical absorption spectra of protein crystals have been published recently. For example, the reduction of oxy-ferrous complex of cytochrome P450 by cryogenic X-ray irradiation in crystal was used (Schlichting et al. 2000) to generate and trap at low temperature an otherwise unstable reactive enzyme - substrate complex. Subsequently, Hajdu and coworkers used this method to obtain X-ray structures of all five states of horseradish peroxidase (HRP) catalytic cycle (Berglund et al. 2002). The redox state of the enzyme was documented by optical spectroscopy of protein crystals before and after cryogenic data collection in the X-ray beam using a specially designed microspectrometer (Sjoegren et al. 2002). Notably, some of the reported heme enzyme intermediates, for example the “Compound I” oxo-ferryl complex porphyrin π-cation radical and “Compound III” (an analog of oxy-ferrous complex generated by reaction of ferric HRP with a large excess of hydrogen peroxide), proved to be very unstable with respect to radiolytic reduction. The others were able to withstand much higher radiolytic dose without any changes in visible spectra. Recently the crystal structure of oxyferrous complex of heme oxygenase from Corynebacterium diphtheriae was published by Ikeda-Saito and coworkers (Unno et al. 2004). In this system the oxy-complex was stable under X-ray irradiation during the data collection and not reduced to peroxo/hydroperoxo complex as confirmed by comparison of optical absorption spectra of irradiated crystals with the published spectra of hydroperoxo-ferric enzyme (Denisov et al. 2002c). These studies indicate that an appropriate control of protein redox centers is necessary in X-ray crystallographic analysis because their stability and the yield of reactions initiated by cryoradiolysis depends on many factors, including the chemical composition of the crystallization buffer and data collection conditions, which may be specific for each particular enzyme. It is worth noting, however, that the dose rate as well as the total dose ordinary used in protein X-ray crystallography are two orders of magnitude greater than that obtained from 60Co in the current study (Garman, Owen 2006), and hence our results may be considered as an upper margin for the dose-dependent radiolytic reduction.
In conclusion, the yield of cryoradiolytic reduction of met Mb in the frozen aqueous glycerol glassy matrix at 77 K was investigated as a function of 60Co γ-irradiation dose. Very high yield of cryoreduced products was observed virtually independent of protein concentration from 0.01 to 5 mM. The heme protein was reduced up to 50% at 20 kGy dose, and almost 100% at 160 kGy. These results may be useful for defining the experimental conditions to maximize the yield of cryoreduction of metalloproteins in frozen solutions for the isolation and spectroscopic characterization of mechanistically important but unstable redox intermediates.
Acknowledgments
We gratefully acknowledge help provided by Dr. S. Toshkov (Nuclear Radiation Lab, University of Illinois, Urbana-Champaign) when using the 60Co source and Dr. T. M. Makris for EPR spectroscopy in control experiments. This work was supported by NIH GM 33775.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. Amsterdam: North Holland Publishing Co.; 1971. [Google Scholar]
- Ausloos P, editor. Fundamental Processes in Radiation Chemistry. New York: Wiley Interscience Publishers; 1968. [Google Scholar]
- Berglund GI, Carlsson GH, Smith AT, Szoeke H, Henriksen A, Hajdu J. The catalytic pathway of horseradish peroxidase at high resolution. Nature. 2002;417(6887):463–468. doi: 10.1038/417463a. [DOI] [PubMed] [Google Scholar]
- Davydov R, Kofman V, Fujii H, Yoshida T, Ikeda-Saito M, Hoffman BM. Catalytic Mechanism of Heme Oxygenase through EPR and ENDOR of Cryoreduced Oxy-Heme Oxygenase and Its Asp 140 Mutants. J Amer Chem Soc. 2002;124(8):1798–1808. doi: 10.1021/ja0122391. [DOI] [PubMed] [Google Scholar]
- Davydov R, Kuprin S, Graeslund A, Ehrenberg A. Electron Paramagnetic Resonance Study of the Mixed-Valent Diiron Center in Escherichia coli Ribonucleotide Reductase Produced by Reduction of Radical-Free Protein R2 at 77 K. J Amer Chem Soc. 1994;116(24):11120–11128. [Google Scholar]
- Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. Hydroxylation of Camphor by Reduced Oxy-Cytochrome P450cam: Mechanistic Implications of EPR and ENDOR Studies of Catalytic Intermediates in Native and Mutant Enzymes. J Amer Chem Soc. 2001;123(7):1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- Davydov R, Valentine AM, Komar-Panicucci S, Hoffman BM, Lippard SJ. An EPR Study of the Dinuclear Iron Site in the Soluble Methane Monooxygenase from Methylococcus capsulatus (Bath) Reduced by One Electron at 77 K: The Effects of Component Interactions and the Binding of Small Molecules to the Diiron(III) Center. Biochemistry. 1999;38(13):4188–4197. doi: 10.1021/bi982391o. [DOI] [PubMed] [Google Scholar]
- Davydov RM, Khangulov SV. ESR spectroscopy of unstable intermediates of the reduction of oxygen complexes of cytochrome P 450 and other hemoproteins. Stud Biophys. 1983;95(2):97–106. [Google Scholar]
- Davydov RM, Magonov SN. Low-temperature reduction as a method for the generation and study of unstable complexes of transition metals. Cobalamines and tris(dipyridyl)cobalt(3+) Zhur Fizich Khim. 1981;55(1):264–265. [Google Scholar]
- Denisov IG, Makris TM, Sligar SG. Cryotrapped reaction intermediates of cytochrome P450 studied by radiolytic reduction with phosphorus-32. J Biol Chem. 2001;276(15):11648–11652. doi: 10.1074/jbc.M010219200. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Makris TM, Sligar SG. Cryoradiolysis for the study of P450 reaction intermediates. Methods in Enzymology. 2002a;357:103–115. doi: 10.1016/s0076-6879(02)57670-9. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Makris TM, Sligar SG. Formation and decay of hydroperoxo-ferric heme complex in horseradish peroxidase studied by cryoradiolysis. J Biol Chem. 2002b;277(45):42706–42710. doi: 10.1074/jbc.M207949200. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Ikeda-Saito M, Yoshida T, Sligar SG. Cryogenic absorption spectra of hydroperoxo-ferric heme oxygenase, the active intermediate of enzymatic heme oxygenation. FEBS Letters. 2002c;532(12):203–206. doi: 10.1016/s0014-5793(02)03674-8. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P 450. Chem Rev. 2005;105(6):2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- Engler N, Ostermann A, Gassmann A, Lamb DC, Prusakov VE, Schott J, et al. Protein dynamics in an intermediate state of myoglobin: optical absorption, resonance Raman spectroscopy, and x-ray structure analysis. Biophys J. 2000;78(4):2081–2092. doi: 10.1016/S0006-3495(00)76755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman VI. Radiation-induced transformations of isolated organic molecules in solid rare gas matrixes. Radiat Phys Chem. 1999;55(56):565–571. [Google Scholar]
- Fujii S, Willard JE. Reactions of electrons and free radicals in glassy ethanol. J Phys Chem. 1970;74(25):4313–4319. [Google Scholar]
- Garman EF, Owen RL. Cryocooling and radiation damage in macromolecular crystallography. Acta Crystallogr, Sect D: Biol Cryst. 2006;D62(1):32–47. doi: 10.1107/S0907444905034207. [DOI] [PubMed] [Google Scholar]
- Gasyna Z. Transient intermediates in the reduction of iron(III) myoglobin-ligand complexes by electrons at low temperature. Biochim Biophys Acta. 1979;577(1):207–216. doi: 10.1016/0005-2795(79)90022-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Denisov IG, Makris TM, Kincaid JR, Sligar SG. Resonance Raman Spectroscopic Studies of Hydroperoxo-Myoglobin at Cryogenic Temperatures. J Amer Chem Soc. 2003;125(45):13714–13718. doi: 10.1021/ja036949d. [DOI] [PubMed] [Google Scholar]
- Jonah CD, Rao BSM, editors. Studies in Physical and Theoretical Chemistry. Amsterdam: Elsevier; 2001. Radiation Chemistry Present Status and Future Trends. [Google Scholar]
- Kevan L. Current problems in the localization and solvation of excess electrons in glasses. J Phys Chem. 1980;84(10):1232–1240. [Google Scholar]
- Krebs C, Davydov R, Baldwin J, Hoffman BM, Bollinger JM, Jr, Huynh BH. Moessbauer and EPR Characterization of the S = 9/2 Mixed-Valence Fe(II)Fe(III) Cluster in the Cryoreduced R2 Subunit of Escherichia coli Ribonucleotide Reductase. J Amer Chem Soc. 2000;122(22):5327–5336. [Google Scholar]
- Lamb DC, Ostermann A, Prusakov VE, Parak FG. From metmyoglobin to deoxy myoglobin: relaxations of an intermediate state. Eur Biophys J. 1998;27(2):113–125. doi: 10.1007/s002490050117. [DOI] [PubMed] [Google Scholar]
- Levantino M, Huang Q, Cupane A, Laberge M, Hagarman A, Schweitzer-Stenner R. The importance of vibronic perturbations in ferrocytochrome c spectra: a reevaluation of spectral properties based on low-temperature optical absorption, resonance Raman, and molecular-dynamics simulations. J Chem Phys. 2005;123(5):054508. doi: 10.1063/1.1961556. [DOI] [PubMed] [Google Scholar]
- Lin DP, Kevan L, Steen HB. Electron scavenging in ethylene glycol-water glass at 4 and 77 K: scavenging of trapped vs mobile electrons. Int J Radiat Phys Chem. 1976;8(6):713–717. [Google Scholar]
- Magonov SN, Davydov RM, Blyumenfel'd LA, Vilu R, Arutyunyan AM, Sharonov YA. Absorption and magnetic circular dichroism spectra of nonequilibrium states of heme-containing proteins. II. Myoglobin and its complexes. Molek Biol (Moscow) 1978;12(5):1182–1190. [PubMed] [Google Scholar]
- Makarov IE, Ershov BG, Pikaev AK. EPR spectra of irradiated frozen aqueous solutions. 10. The captured electron in polyhydric alcohols and their aqueous solutions, gamma-irradiated at 77 K. ChemIzv Akad Nauk SSSR. 1969;(10):2170–2175. Engl. Transl. [Google Scholar]
- Murray J, Garman E. Investigation of possible free-radical scavengers and metrics for radiation damage in protein cryocrystallography. J Synchr Radiat. 2002;9(6):347–354. doi: 10.1107/s0909049502014632. [DOI] [PubMed] [Google Scholar]
- Murray JW, Garman EF, Ravelli RBG. X-ray absorption by macromolecular crystals: the effects of wavelength and crystal composition on absorbed dose. J Appl Crystallogr. 2004;37(4):513–522. [Google Scholar]
- Nave C, Garman EF. Towards an understanding of radiation damage in cryocooled macromolecular crystals. J Synchr Radiat. 2005;12(3):257–260. doi: 10.1107/S0909049505007132. [DOI] [PubMed] [Google Scholar]
- O'Neill P, Stevens DL, Garman EF. Physical and chemical considerations of damage induced in protein crystals by synchrotron radiation: a radiation chemical perspective. J Synchr Radiat. 2002;9(Pt 6):329–332. doi: 10.1107/s0909049502014553. [DOI] [PubMed] [Google Scholar]
- Rappoport Z, editor. Patai Series: The Chemistry of Functional Groups. Chichester: Wiley Interscience; 2003. The Chemistry of Phenols. [Google Scholar]
- Rice SA, Kevan L. Comparison of photoconductivity and optical spectra of the trapped electron in polar aqueous and alcoholic glasses. J Phys Chem. 1977;81(9):847–850. [Google Scholar]
- Sasaki T, Ohno S. Reactions of mobile electrons in the frozen solution of ethylene glycol-water at 77.deg.K. Bull Chem Soc Japan. 1971;44(10):2626–2630. [Google Scholar]
- Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, et al. The catalytic pathway of cytochrome P450cam at atomic resolution. Science. 2000;287(5458):1615–1622. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Takahashi H, Kaneko R, Kurita A, Kushida T. Conformational fluctuation of native-like and molten-globule-like cytochrome c observed by time-resolved hole burning. Biochemistry. 1999;38(6):1802–1810. doi: 10.1021/bi981568b. [DOI] [PubMed] [Google Scholar]
- Shintaku M, Matsuura K, Yoshioka S, Takahashi S, Ishimori K, Morishima I. Absence of a Detectable Intermediate in the Compound I Formation of Horseradish Peroxidase at Ambient Temperature. J Biol Chem. 2005;280(49):40934–40938. doi: 10.1074/jbc.M503472200. [DOI] [PubMed] [Google Scholar]
- Sjoegren T, Carlsson G, Larsson G, Hajdu A, Andersson C, Pettersson H, et al. Protein crystallography in a vapour stream: data collection, reaction initiation and intermediate trapping in naked hydrated protein crystals. J Appl Crystallogr. 2002;35(1):113–116. [Google Scholar]
- Sligar SG, Makris TM, Denisov IG. Thirty years of microbial P450 monooxygenase research: Peroxo-heme intermediates-The central bus station in heme oxygenase catalysis. Biochem Biophys Res Commun. 2005;338(1):346–354. doi: 10.1016/j.bbrc.2005.08.094. [DOI] [PubMed] [Google Scholar]
- Spinks JWT, Woods RJ. An Introduction to Radiation Chemistry. 1990. p. 574. [Google Scholar]
- Symons MCR. Electron spin resonance studies of radiation damage to DNA and to proteins. Radiat Phys Chem. 1995;45(6):837–845. [Google Scholar]
- Tanskanen H, Khriachtchev L, Rasanen M, Feldman VI, Sukhov FF, Orlov AY, et al. Infrared absorption and electron paramagnetic resonance studies of vinyl radical in noble-gas matrices. J Chem Phys. 2005;123(6):064318/1–064318/7. doi: 10.1063/1.2000907. [DOI] [PubMed] [Google Scholar]
- Unno M, Matsui T, Chu GC, Couture M, Yoshida T, Rousseau DL, et al. Crystal structure of the dioxygen-bound heme oxygenase from Corynebacterium diphtheriae Implications for heme oxygenase function. J Biol Chem. 2004;279(20):21055–21061. doi: 10.1074/jbc.M400491200. [DOI] [PubMed] [Google Scholar]
- Willard JE. Trapped free radicals and electrons in organic glasses. Science. 1973;180(4086):553–561. doi: 10.1126/science.180.4086.553. [DOI] [PubMed] [Google Scholar]
- Willard JE. Trapped electrons in organic glasses. J Phys Chem. 1975;79(26):2966–2973. [Google Scholar]
- Wojnarovits L. Radiation Chemistry. In: Vertes A, Nagy S, Klencsar Z, editors. Handbook of Nuclear Chemistry. Chemical Applications of Nuclear Reactions and Radiations. Vol. 3. Dordrecht: Kluwer Academic Publishers; 2003. pp. 1–55. [Google Scholar]
- Yano J, Kern J, Irrgang KD, Latimer MJ, Bergmann U, Glatzel P, et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc Natl Acad Sci USA. 2005;102(34):12047–12052. doi: 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SJ, Meyer J, Achim C, Peterson J, Hendrich MP, Munck E. Mossbauer, EPR, and MCD studies of the C9S and C42S variants of Clostridium pasteurianum rubredoxin and MDC studies of the wild-type protein. J Biol Inorg Chem. 2000;5(4):475–487. doi: 10.1007/s007750050008. [DOI] [PubMed] [Google Scholar]
- Zimbrick JD, Bowman MK. Concentrated electron scavenger effects on the yields of trapped species in g-irradiated alkaline glass. J Phys Chem. 1972;76(14):1962–1967. [Google Scholar]