Abstract
Objective
To determine if indomethacin used as a tocolytic agent is associated with adverse neonatal outcomes.
Study Design
We used published guidelines of the Meta-analysis of Observational Studies in Epidemiology Group (MOOSE) to perform the meta-analysis. The search strategy employed included computerized bibliographic searches of MEDLINE (1966–2005), PubMed (1966–2005), abstracts published in Obstetrics and Gynecology (1991–2005), abstracts published in Pediatric Research (1991–2005) and references of published manuscripts. Study inclusion criteria were publication in English, >30 deliveries <37 weeks’ gestation, and meeting diagnostic criteria for individual neonatal outcomes. Exclusion criteria included case reports, case series and multiple publications from the same author. Meta-analysis was performed using random effects model if there were > 2 observational studies for a specific outcome. Eggers test was performed to exclude publication bias. Sensitivity analysis was performed to evaluate the effect of antenatal steroid exposure, gestation and recent antenatal indomethacin exposure (≤ 48 hours duration between the last dose and delivery).
Results
Fifteen retrospective cohort studies and 6 case controlled studies met inclusion criteria. Antenatal indomethacin was associated with an increased risk of periventricular leukomalacia (OR 2.0, 95% CI 1.3 – 3.1). Recent exposure to antenatal indomethacin was associated with necrotizing enterocolitis (OR 2.2, 95% CI 1.1 – 4.2). Antenatal indomethacin was not associated with intraventricular hemorrhage, patent ductus arteriosus, respiratory distress syndrome, bronchopulmonary dysplasia and mortality.
Conclusion
Antenatal indomethacin may be associated with an increased risk of periventricular leukomalacia and necrotizing enterocolitis in premature infants and, therefore, should be used judiciously for tocolysis.
Keywords: premature infants, antenatal indomethacin, meta-analysis of observational studies, periventricular leukomalacia, necrotizing enterocolitis
INTRODUCTION
Prematurity is associated with adverse neonatal outcomes, including intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and patent ductus arteriosus (PDA). Tocolytic agents to delay delivery are routinely used. Prostaglandins modulate the onset and maintenance of labor; indomethacin, a potent inhibitor of prostaglandin synthesis, has been used as tocolytic agent.1–4 Because indomethacin crosses the placenta freely, however, indomethacin also can affect the fetus causing adverse neonatal effects.5, 6
Most of the theorized mechanisms for indomethacin-induced adverse neonatal effects are based on animal studies and are thought to be secondary to inhibition of prostaglandin synthesis. Antenatal indomethacin (AI) may predispose neonates to NEC and intestinal perforation by decreasing mesenteric blood flow, blocking autoregulation of terminal ileum oxygen consumption and altering defense mechanisms in the neonatal gastrointestinal tract.7–10 Renal dysfunction is explained by indomethacin’s effect on decreasing renal blood flow.11 Similarly, by decreasing fetal cerebral blood flow, AI may cause fetal central nervous system hypoperfusion injury resulting in PVL.12 The profound effects of indomethacin on platelet and neutrophil function may lead to increased incidence of IVH and sepsis.13 AI is associated with in utero constriction of the fetal ductus arteriosus.14 This initial constriction is associated with ischemic damage to the intimal layer of the ductal wall and may explain its failure to respond to oxygen resulting in a PDA.15 The incidence of RDS and BPD may be also increased by the inhibitory effects of indomethacin on surfactant production and its stimulatory effects on proinflammatory mediators in the lung.16
Despite biological plausibility, clinical studies have not consistently shown that individual adverse neonatal effects are associated with the use of AI except for renal dysfunction.17–42 Two recent randomized double blind controlled studies evaluating the effect of AI on neonatal outcomes were aborted due to recruitment problems.17, 21 A recent meta-analysis of 10 randomized trials involving AI for preterm labor cited several limitations including:1) absence of neonatal outcome data for PVL, 2) no clear definitions for most outcome measures, 3) inadequate number of neonates for precise estimations of effect size, and 4) inclusion of neonates > 34 weeks’ gestation.43 The incidence of adverse neonatal outcomes is inversely proportional to gestation and is extremely low after 34 weeks’ gestation. For 80% power to detect a 2-fold increased risk from 1% to 2% in an individual neonatal outcome, 2515 infants are required in each arm. Similarly, 1239 infants are required in each arm to detect a 2-fold increased incidence from 2% to 4%. Therefore, most of the published studies on the effect of AI on neonatal outcomes are observational and include premature infants < 34 weeks’ gestation.
We performed a meta-analysis of observational studies because there remains controversy regarding the adverse neonatal effects from the use of AI based on inadequately powered studies. Secondly, we hypothesized that AI’s effect on neonatal outcome may be dependent on timing of AI before delivery. A meta-analysis of observational and randomized studies on the effect of AI on neonatal outcomes was published recently.44 This publication concluded that AI is not associated with adverse neonatal outcomes. However, this report failed to include two published observational studies, inadvertently included one study with data on postnatal indomethacin, did not evaluate PVL and RDS and did not use established diagnostic criteria for neonatal outcomes. We therefore completed a meta-analysis of observational studies to determine if AI is associated with specific adverse neonatal outcomes including PVL and RDS.
MATERIAL AND METHODS
We used published guidelines of the Meta-analysis of Observational Studies in Epidemiology Group (MOOSE) to perform this meta-analysis.45
Eligibility Criteria
Inclusion criteria: 1) > two observational studies for each neonatal outcome, 2) gestational age <37 weeks at delivery, 3) publication in English, 4) sample size ≥30, and 5) studies met established diagnostic criteria routinely used for individual neonatal outcomes. Specific diagnostic criteria included: a) RDS, based on clinical findings and chest x-ray, b) BPD, based on oxygen requirement at 28 days of life or at 36 weeks post-menstrual age, c) IVH, based on head ultrasonography performed during the hospital stay and graded based on Papile’s classification46, d) NEC, based on x-ray findings of pneumatosis intestinalis or intestinal perforation, e) PVL, based on findings on radiologic imaging, f) PDA, based on echocardiography. Exclusion criteria: case reports, case series studies, multiple publications of the same material from the same author, and sample size < 30 infants. The latter criterion was used because the asymptotic method for meta-analysis tends to overestimate the precision of small studies which would carry too much weight in a meta-analysis. 47
Identification of studies
Our search strategy sought to identify all published and unpublished completed studies until December 2005. The search strategy included computerized bibliographic searches of Cochrane Controlled Trials Register, MEDLINE (1966–2005), PubMed (1966–2005), abstracts published in Obstetrics and Gynecology (1991–2005), abstracts published in Pediatric Research (1991–2005) and references of published manuscripts. EMBASE was not included because it was not accessible through University of Rochester Miner Library. Index terms included indomethacin, antenatal, pregnancy, complications and infant. The search was performed by two physicians and a medical school librarian.
Data extraction
Data were collected from each study independently by two physicians using pre-designed forms including first author, publication year, study design, study matching criteria, gestational age (GA) of subjects, number of individuals in the AI and control groups, neonatal outcomes that met diagnostic criteria, raw data using 2×2 tables for each neonatal outcome in the exposed (AI) and unexposed groups, data on antenatal steroid exposure for both exposed and unexposed groups, data on the duration of AI and time period from the last dose to delivery, data on whether indomethacin was used as a single agent or part of combination tocolysis and if used as a combination tocolysis whether indomethacin was used as a first choice tocolytic agent or for preterm labor recalcitrant to other tocolytic agents. Each study was assessed with respect to the definition of the individual neonatal outcome used in reporting that specific outcome.
Statistical analysis
Meta-analysis was performed for each individual neonatal outcome using Stata 8.0 (Stata Corporation, TX). Briefly, the analysis software produces Forrest plots as a schematic description of the meta-analysis results. Summary random effect estimates are reported using pooled odds ratios (OR), and 95% confidence intervals (CI) were calculated around each summary effect estimate. The random effect model assumes that analyzed studies are a random sample of a hypothetical population of studies. Heterogeneity testing using Q statistics was performed to evaluate variance between the studies. If the between-study variance was large (i.e. when there is heterogeneity) enough to make the test of heterogeneity significant (p < 0.05), random effects models are considered most appropriate. As these tests of heterogeneity are relatively insensitive, a more conservative p-value of < 0.10 was used. Each of these studies was examined for sources of clinical heterogeneity because these studies may differ in design and in subject characteristics such as GA at birth, antenatal steroid exposure, and duration and timing of AI exposure that can modify the incidence of each neonatal outcome. Reasons for clinical heterogeneity were explored for each neonatal outcome using sensitivity analysis. Sensitivity analysis included evaluation of effects of AI on neonatal outcomes by performing meta-analysis on the following: 1) retrospective cohort studies, 2) observational studies that reported matching of cases and controls for antenatal steroid exposure and gestation, and 3) observational studies that reported that the AI exposed group was recently (≤ 72 hours before delivery) exposed. Finally, Begg’s funnel plot was produced and Eggers test was performed to exclude publication bias. A p-value < 0.05 was considered significant for publication bias.
RESULTS
Meta-analysis of Observational Studies
Twenty one observational studies met inclusion criteria (Table I). Of these 21 studies, 15 were retrospective cohorts and six were case-control studies. Two case series, 10 case reports, and 4 studies involving only renal insufficiency were excluded (the latter because of nonuniform definition).6, 48–52 Data from one research project were published twice and therefore one of the manuscripts was excluded.53 As shown in table I, a majority of the studies (n =13) used indomethacin for preterm labor recalcitrant to other tocolytic agents. Table II lists the studies (reference) that met diagnostic criteria for each neonatal outcome. Five studies (589 infants) met diagnostic criteria for BPD as defined by oxygen requirement at 28 days of life, 11 studies (2700 infants) met criteria for NEC, 14 studies (2936 infants) met criteria for PDA, 12 studies (1677 infants) met criteria for IVH, 12 studies (4226 infants) met criteria for severe IVH (Grade III and IV), five studies (950 infants) met criteria for PVL, 10 studies (1927 infants) met criteria for RDS and 11 studies (1942 infants) reported information on mortality. Seven studies reported neonatal outcomes following recent exposure to antenatal indomethacin. Of these seven studies, six(27,31,32,35,37,41) reported exposure to AI within 48 hours of delivery while one study (Norton et al23) reported exposure to AI within 72 hours of delivery with more than 80% exposed within 48 hours of delivery.
Table I.
Observational studies included in meta-analysis
| Author/Reference # | Year | Study Group | Control Group | N | GA | Antenatal steroid | Recent AI Exposure |
|---|---|---|---|---|---|---|---|
| Neibyl22 | 1986 | AI* | None | 135 | <35 wks, Matched | No data | No data |
| Baerts37 | 1990 | AI+ Fenoterol | None or Fenoterol | 159 | < 30 wks, Matched | No data | Yes |
| Gerson26 | 1990 | AI + Mg or AI + β-mimetic | Mg or β-mimetic | 57 | < 34 wks, Matched | Matched | No |
| Norton23 | 1993 | AI + Mg or AI + β-mimetic | None, Mg, or β-mimetic | 114 | < 30 wks, Matched | Matched | Yes |
| Major27 | 1994 | AI + Mg | Mg ± β-mimetic | 759 | < 30 wks | Unmatched | Yes |
| Gardner29 | 1995 | AI + Mg ± β-mimetic | None, Mg, or β-mimetic | 124 | < 32 wks, Matched | Matched | No |
| Iannucci30 | 1996 | AI + Mg | Mg | 56 | < 30 wks | Unmatched | No data |
| al-Alaiyan28 | 1996 | AI* | None | 30 | < 34 wks | Unmatched | No data |
| Souter31 | 1998 | AI + Salbutamol or AI + Nifedipine | None, Salbutamol, or Nifedipine | 79 | < 30 wks, Matched | Matched | Yes |
| Pietrantoni33 | 1995 | AI** | None | 280 | < 32 wks, Matched | Matched | No data |
| Van Overmeire41 | 1998 | AI + β-mimetic | β-mimetic | 76 | < 33 wks, Matched | Unmatched | Yes |
| Hammerman24 | 1998 | AI* | None | 105 | < 34 wks, Matched | No data | No data |
| Vermillion32 | 1999 | AI + Mg | Mg | 225 | < 32 wks, Matched | Matched | Yes |
| Ojala25 | 2000 | AI* | None | 176 | < 32 wks | Unmatched | No data |
| Parilla40 | 2000 | AI ± Mg* | Mg or None | 120 | < 37 wks | Unmatched | No data |
| Weintraub34 | 2001 | AI* | None | 219
0 |
< 32 wks | Unmatched | No data |
| Suarez39 | 2001 | AI ± Mg* | Mg | 112 | < 32 wks | Unmatched | No data |
| Abbasi35 | 2003 | AI + Mg ± Terbutaline | None, Mg, or Terbutaline | 248 | < 34 wks, Matched | Matched | Yes |
| Friedman42 | 2005 | AI | Mg or Ritodrine or Nifedipine | 284 | < 32 wks | Unmatched | No data |
| Murata38 | 2005 | AI + Ritodrine | Ritodrine + Mg | 201 | < 33 wks | Unmatched | No |
| Doyle 36 | 2005 | AI + Mg | Mg or none | 549 | < 32 wks | Unmatched | No data |
GA denotes gestational age; AI denotes antenatal indomethacin; N denotes number of subjects; Mg denotes magnesium sulfate;
First choice tocolytic or sole agent.
Unclear about first choice tocolytic.
Table II.
Meta-analysis of observational studies: effect of antenatal indomethacin (AI) on neonatal outcomes
| Neonatal Outcome | Reference | # Infants exposed to AI | # Infants unexposed to AI | Random-effects Odds ratio (95% C.I) | Heterogeneity Test p-value | Publication bias p- value |
|---|---|---|---|---|---|---|
| IVH | 23,25,26, 28,29,31, 32,33,35, 37,39,41 | 812 | 865 | 1.31 (0.86 – 2.02) | 0.003 | 0.83 |
| Grade III–IV IVH | 23,25,28, 29,30,31, 32,33,34, 35,36,37 | 952 | 3274 | 1.37 (0.83 – 2.3) | 0.001 | 0.74 |
| PVL | 31,35,37, 38, 42 | 413 | 558 | 2.08 (1.38 – 3.14) | 0.98 | 0.95 |
| NEC | 23,25,27, 28,29,31, 32,33,35, 36, 40 | 755 | 1945 | 1.4 (0.91– 2.3) | 0.08 | 0.99 |
| BPD | 23,26,28, 31,41 | 164 | 177 | 1.17 (0.65 – 2.1) | 0.25 | 0.80 |
| PDA | 22,23,24, 26,27, 28,29,31, 32,33,35, 36,37,41 | 864 | 2072 | 1.03 (0.78 – 1.37) | 0.02 | 0.95 |
| RDS | 22,23,25, 26,27,29, 31,35,37, 41 | 604 | 1323 | 1.08 (0.67 – 1.74) | 0.001 | 0.09 |
| Mortality | 22,23,25, 26,29,31, 32,35,36, 37, 41 | 708 | 1234 | 1.08 (0.72 – 1.63) | 0.06 | 0.91 |
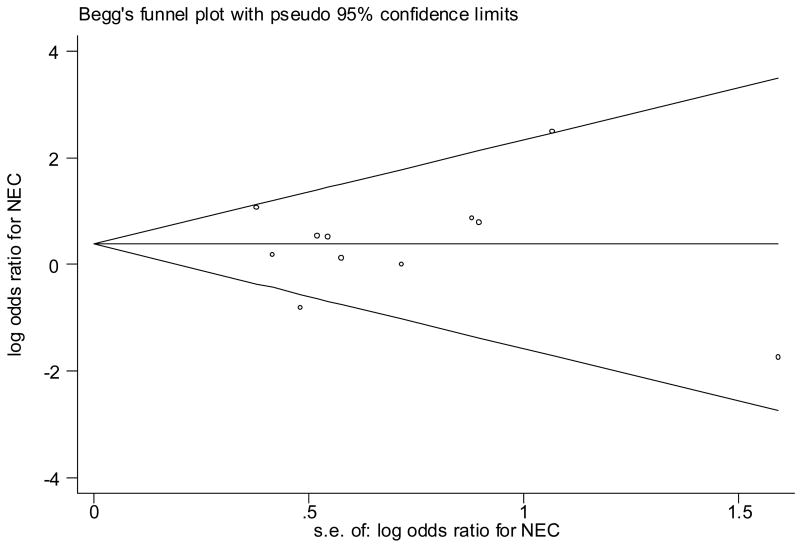
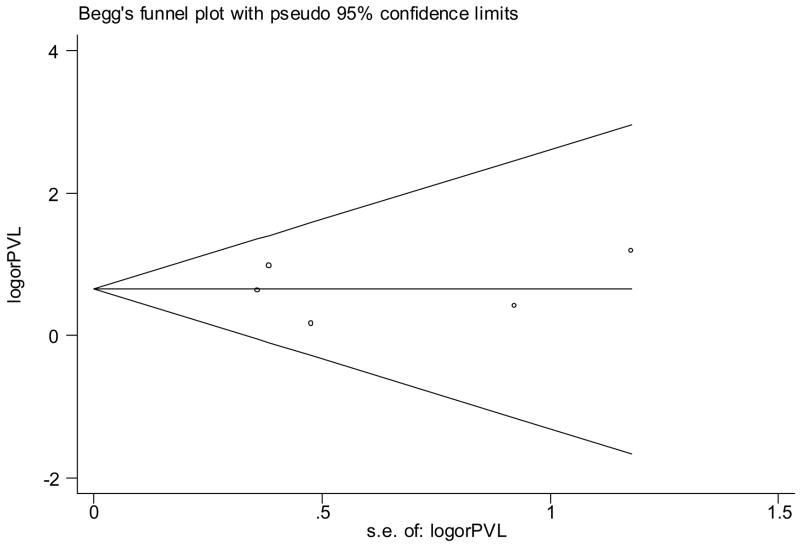
Significant heterogeneity between studies was noted for all neonatal outcomes except PVL and BPD (Table II). There was no evidence of publication bias for any of the neonatal outcomes (Table II, Figure 1, Figure 2).
Figure 1.
Begg’s funnel plot (with pseudo 95% CI) of the log odds ratio versus the standard errors of log odds ratio in studies that evaluated the effect of antenatal indomethacin on NEC. There was no publication bias (no asymmetry).
Figure 2.
Begg’s funnel plot (with pseudo 95% CI) of the log odds ratio versus the standard errors of log odds ratio in studies that evaluated the effect of antenatal indomethacin on periventricular leukomalacia. There was no publication bias (no asymmetry).
Effect of AI on NEC
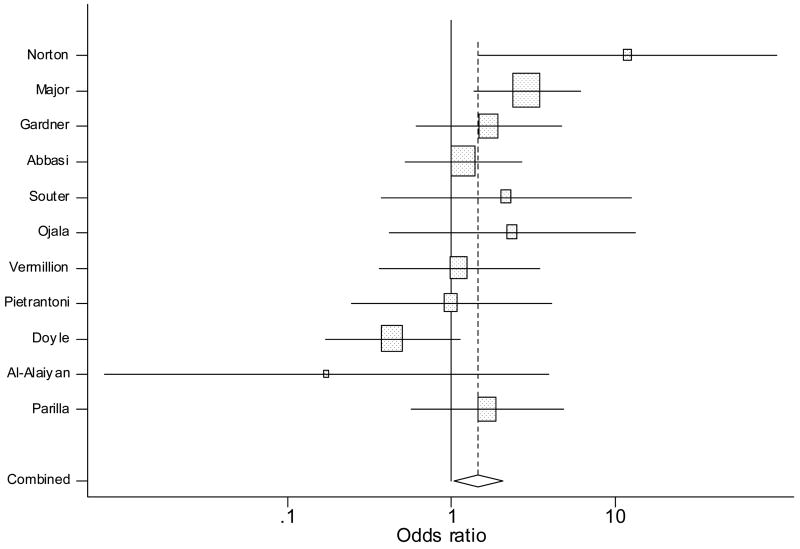
Meta-analysis demonstrated a non-significant positive trend associating AI and NEC (OR 1.4, 95% CI 0.91 – 2.3, Figure 3). There was statistical heterogeneity (Q = 16.57, P = 0.08). Regarding sources of clinical heterogeneity, the two studies demonstrating the largest positive association involved premature infants <30 weeks’ gestation. 23, 27 while the two studies that demonstrated the largest negative association involved less-premature infants (32–34 weeks).28, 36 Because NEC is uncommon in less-premature infants, the GA difference between the subjects between the positive and negative studies could explain the heterogeneity. On sensitivity analysis including only the retrospective cohort studies (n = 10, Table III), the non-significant positive trend associating AI and NEC persisted. The association between AI and NEC was significant (OR 2.23, 95% CI 1.16 – 4.25) when including only retrospective cohort studies with recent exposure to AI (n = 5, Table IV).. On sensitivity analysis including only observational studies with the AI and control groups matched for antenatal steroid exposure and gestation at birth (n = 6, Table V), the trend for positive association remained but was not statistically significant (OR 1.47, 95% CI 0.9 – 2.3). On sensitivity analysis including only observational studies with AI used as the first tocolytic agent (n =3), AI was not associated with NEC (OR 1.52, 95% CI 0.6–3.8).
Figure 3.
Forrest plot showing individual and combined effect size estimates and 95% CIs in the studies that evaluated the effect of antenatal indomethacin on NEC. The weighting ( ) given to the study in the overall pooled estimate, taking into account the number of subjects and the amount of between-study variation (heterogeneity). Error bars (−) indicate 95% CIs. The rhombus indicates combined effect size of 1.4.
Table III.
Meta-analysis of retrospective cohort studies: effect of antenatal indomethacin (AI) on neonatal outcomes
| Neonatal Outcome | Reference | # Infants exposed to AI | # Infants unexposed to AI | Random-effects Odds ratio (95% C.I) | Heterogeneity Test p-value |
|---|---|---|---|---|---|
| IVH | 23,25,26, 28,29,31, 32,33,35, 37,41 | 730 | 834 | 1.25 (0.79 – 1.97) | 0.003 |
| Grade III–IV IVH | 23,25,28, 29,30,31, 32,33, 35,36,37 | 775 | 1261 | 1.48 (0.83 – 2.6) | 0.003 |
| PVL | 31,35,37 | 239 | 247 | 1.9 (1.01 – 3.58) | 0.9 |
| NEC | 23,25,27, 28,29,31, 32,33,35, 36 | 733 | 1847 | 1.43 (0.84 – 2.43) | 0.06 |
| BPD | 23,26,28, 31,41 | 164 | 177 | 1.17 (0.65 – 2.1) | 0.25 |
| PDA | 22,23,26, 27,28,29, 31,32,33, 35,36,37, 41 | 835 | 1996 | 0.96 ( 0.75 – 1.23) | 0.11 |
| RDS | 22,23,25, 26,27,29, 31,35,37,41 | 604 | 1323 | 1.08 (0.67 – 1.74) | 0.001 |
| Mortality | 22,23,25, 26,29,31, 32,35,36, 37,41 | 708 | 1234 | 1.08 (0.72 – 1.63) | 0.06 |
Table IV.
Meta-analysis of observational studies with recent exposure to antenatal indomethacin (AI)
| Neonatal Outcome | Reference | # Infants exposed to AI | # Infants unexposed to AI | Random-effects Odds ratio (95% C.I) | Heterogeneity Test p-value |
|---|---|---|---|---|---|
| IVH | 23,31,32, 35,37,41 | 337 | 424 | 1.18 (0.57 – 2.4) | 0.003 |
| Grade III–IV IVH | 23,31,32, 35,37 | 299 | 396 | 1.3 ( 0.5 – 3.2) | 0.04 |
| PVL | 35,37 | 144 | 151 | N/A | N/A |
| NEC | 23,27,31, 32,35 | 268 | 1016 | 2.23 (1.16 – 4.25) | 0.23 |
| BPD | 23,41 | 86 | 89 | N/A | N/A |
| PDA | 23,31,32, 35,37,41 | 337 | 434 | 1.05 (0.61 – 1.8) | 0.01 |
| RDS | 23,35,37,41 | 239 | 246 | 2.2 (0.94 – 5.12) | 0.02 |
| Mortality | 23,31,32, 35,37,41 | 339 | 436 | 1.15 (0.7 – 1.88) | 0.2 |
N/A denotes not applicable
Table V.
Meta-analysis of observational studies with antenatal indomethacin (AI) group and control group matched for antenatal steroid exposure and gestation at birth
| Neonatal Outcome | Reference | # Infants exposed to AI | # Infants unexposed to AI | Random-effects Odds ratio (95% C.I) | Heterogeneity Test p-value |
|---|---|---|---|---|---|
| IVH | 23,26,29, 31,32,33,35 | 519 | 604 | 1.52 (0.85 – 2.7) | 0.007 |
| Grade III–IV IVH | 23,29,31, 32,33,35 | 495 | 571 | 1.57 (0.63 – 3.9) | 0.01 |
| PVL | 31,35 | 163 | 164 | N/A | N/A |
| NEC | 23,29,31, 32,33,35 | 495 | 571 | 1.47 (0.9 – 2.3) | 0.43 |
| BPD | 23,26,31 | 111 | 124 | 0.96 (0.53 – 1.7) | 0.61 |
| PDA | 23,26,29, 31,32,33,35 | 519 | 604 | 1.04 (0.77 – 1.4) | 0.27 |
| RDS | 23,26,29, 31,35 | 306 | 316 | 0.69 (0.39 – 1.2) | 0.03 |
| Mortality | 23,26,29, 31,32,35 | 381 | 466 | 0.99 (0.5 – 1.7) | 0.15 |
N/A denotes not applicable
Effect of AI on PVL
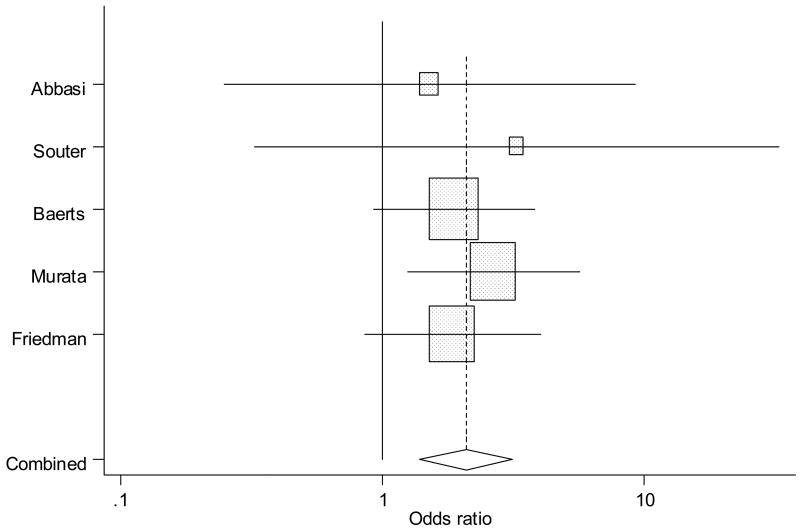
There was a statistically significant association between AI and greater incidence of PVL (OR 2.08, 95% CI 1.38 – 3.14; Q = 2.0, p = 0.98, Table II, Figure 4), without evidence of heterogeneity. On sensitivity analysis including only the retrospective cohort studies (n = 3, Table III), the positive association between AI and PVL persisted (OR 1.9, 95% CI 1.01 – 3.58). There were only 2 studies (Table IV) with recent exposure to AI and PVL, 2 studies matched for gestation and antenatal steroid exposure (Table V) and only one study with exposure to AI as a first choice tocolytic agent and PVL, therefore, sensitivity analyses were not performed.
Figure 4.
Forrest plot showing individual and combined effect size estimates and 95% CIs in the studies that evaluated the effect of antenatal indomethacin on periventricular leukomalacia. The weighting ( ) given to the study in the overall pooled estimate, taking into account the number of subjects and the amount of between-study variation (heterogeneity). Error bars (−) indicate 95% CIs. The rhombus indicates combined effect size of 2.08.
Effect of AI on IVH
There was no significant effect of AI on severe IVH. (Table II) On exploring for the sources of heterogeneity (Table II), the study with the largest negative association involved a small sample size and more mature infants (< 34 weeks’ gestation) 28 compared to the study with a strong positive association that involved infants < 28 weeks’ gestation.30 The difference in GA of study subjects may explain heterogeneity. On sensitivity analysis, there was no statistically significant effect of AI on severe IVH when including studies (n = 5, Table IV) with recent exposure to AI, including studies (n = 6, Table V) matched for GA and antenatal steroid exposure and including studies (n =3) with AI used as a first choice tocolytic agent (OR 0.8, 95% CI 0.46 – 1.41).
There was also no significant association between AI and any IVH (n = 12, Table II). On sensitivity analysis, there was no statistically significant effect of AI on any IVH when including studies (n = 6, Table IV) with recent exposure to AI, including studies (n = 7, Table V) matched for GA and antenatal steroid exposure and including studies (n = 3) with AI used as a first choice tocolytic agent (OR 1.83, 95%.CI 0.86 – 3.9).
Effect of AI on PDA
There was no significant effect of AI on PDA (Table II). On sensitivity analysis, there was no effect of AI on PDA when including studies (n = 6, Table IV) with recent exposure to AI, including studies (n = 7, Table V) matched for GA and antenatal steroid exposure and including studies (n = 3) with AI used as a first tocolytic agent (OR 1.5, 95% CI 0.45 – 5.02). Examining for sources of heterogeneity, however, the studies by Norton et al (AI-to-delivery interval, 1–79 days) and Souter et al (AI-to-delivery interval, 1–10 days) with positive association had longer exposure to AI compared to the studies by Vermillion et al with negative associations that had - <3 days AI-to-delivery.23, 31, 32 This may explain the heterogeneity between the studies.
Effect of AI on RDS
There was no significant effect of AI on RDS. The study by Neibyl et al demonstrating a positive association between AI and RDS had no subjects exposed to antenatal steroids (which are known to prevent RDS). 22 Niebyl differed from studies by Sorya et al., Souter et al. and Gardner et al. with negative associations in which subjects received antenatal steroids.29, 31, 35 The differences in antenatal steroid exposure between positive and negative studies may explain the heterogeneity.
On sensitivity analysis, there was a non-significant trend for positive association between AI and RDS when including studies (n = 4, Table IV) with recent exposure to AI (OR 2.2, 95% CI 0.94 – 5.12). However, when including studies (n = 5, Table V) matched for GA and antenatal steroid exposure, AI was associated with a non-significant trend for protective effect on RDS (OR 0.69, 95% CI 0.39 – 1.2). There were only two studies with AI as first choice tocolytic agent and RDS, therefore, sensitivity analysis was not performed.
Effect of AI on BPD
There was no significant effect of AI on BPD. There was no statistically significant effect of AI on BPD when including studies (n = 3, Table V) matched for GA and antenatal steroid exposure. There were only 2 studies with recent exposure to AI and one study with AI used as first choice tocolytic agent, therefore, sensitivity analyses were not performed.
Effect of AI on Mortality
There was no significant effect of AI on mortality. On sensitivity analysis, there was no statistically significant effect of AI on mortality when including studies (n = 6, Table IV) with recent exposure to AI and including studies (n = 6, Table V) matched for GA and antenatal steroid exposure.
COMMENTS
Although AI may provide enough time for antenatal steroids to improve fetal maturation, the results of our meta-analysis of observational studies suggest that these benefits may come with a price. Our study suggests that AI used as a tocolytic agent is associated with PVL in premature infants. The results also suggest that exposure of AI within 72 hours prior to delivery is associated with NEC in premature infants. AI does not appear to be associated with PDA, RDS, BPD, IVH and mortality in premature infants.
Although meta-analysis on randomized studies is a gold standard, the recent meta-analysis of randomized studies evaluating the effect of AI on neonatal outcomes cited several limitations and was inconclusive because of small pooled numbers and lack of clear definitions used for each neonatal outcome.43, 44 Moreover, the neonates studied with randomized studies usually were >35 weeks’ gestation when the neonatal adverse outcomes of interest are rare. The observational studies performed to date involved premature infants <35 weeks’ gestation to study neonatal effects secondary to AI usage, but have shown conflicting results.22–41 Some of these studies were insufficiently powered to evaluate the association of AI usage with individual adverse neonatal outcomes. For these reasons, we chose to analyze observational studies.
Compared to Loe’s published meta-analysis on observational studies44, we used strict diagnostic criteria for each neonatal outcome in order to determine true association between exposure to AI and neonatal outcome. In addition, we conducted sensitivity analysis to evaluate the effect of possible confounding variables, such as antenatal steroids, gestational age of the infant, use of indomethacin as first choice tocolytic agent and timing of antenatal indomethacin prior to delivery. Another notable difference is that we evaluated the effect of antenatal indomethacin on PVL and RDS in addition to the neonatal outcomes reported by Loe. We did not include the effects of AI on pulmonary hypertension and renal insufficiency because there are no uniform criteria available for these diagnoses in premature infants. There is increased risk of ductal constriction and secondary pulmonary hypertension after 32 weeks’ gestation and, therefore, use of AI is limited < 32 weeks’ gestation.54 In addition, fetal echocardiography is recommended to detect any evidence of ductal constriction if indomethacin is continued for more than 72 hours. 54 All the studies reporting on renal insufficiency employed differing criteria.49–51 The current literature suggests that AI may be associated with renal insufficiency in premature infants.
Recent exposure to AI was associated with NEC which can be explained based on its effect on mesenteric blood flow10. A possible increased risk of NEC (OR 2.43, 95% CI 0.73–8.03) was also suggested by the meta-analysis of randomized clinical trials but failed to reach statistical significance because of small sample size.44 Antenatal indomethacin was associated with an increased risk of PVL that can be explained by its known effect on cerebral blood flow.12 The trend for an increased risk of RDS with recent exposure to antenatal indomethacin can be explained by its known inhibitory effect on surfactant production.16 The trend for a protective effect on RDS when including studies that controlled for antenatal steroid exposure and gestation at birth suggest that the inhibitory effect on surfactant production may be an acute effect and is probably mitigated by the use of antenatal steroids.
Similar to Loe44, we found no effect of antenatal indomethacin on PDA, BPD, IVH and mortality. The inclusion of studies that met established diagnostic criteria for neonatal outcomes in our meta-analysis may explain differences in effect size for individual neonatal outcomes between the two meta-analysis of observational studies. For example, compared to Loe44, we did not include Ojala et al., Abbasi et al., and Vermillion et al. in our meta-analysis for BPD because these studies did not define BPD.25, 32, 35 Moreover, we included five additional studies, two of which were not included in the published meta-analysis and three of which were reported after the published meta-analysis.33, 36–38, 42 Also, we did not include a study that reported neonatal outcomes secondary to the use of postnatal indomethacin and was inadvertently included in Loe’s meta-analysis of AI. 44, 55
A limitation of meta-analysis involving observational studies is that it is difficult to control for the confounding factors and selection bias often associated with observational studies. However, the majority of studies matched on two of the most important confounding factors, GA and antenatal steroid exposure. We conducted sensitivity analysis to control for confounding variables that could affect the neonatal outcomes. Although there are statistical reasons to control for confounding variables in individual observational studies, adjusting for gestation at birth in studies using AI tocolysis could lead to an underestimate of benefit associated with postponing preterm delivery. It is more appropriate to adjust for gestation at onset of preterm labor. However, the information on the age at onset of preterm labor is not reported for most observational studies involving AI. It is unlikely that the results would have differed if the findings were adjusted for gestation at the onset of labor since majority of observational studies included in this meta-analysis used AI for recalcitrant labor. In the face of recalcitrant labor, prolongation of pregnancy for a few hours to days allows time to administer antenatal steroids, a confounding variable, which was adjusted in most of the observational studies included in this analysis.
The other limitation of our meta-analysis is that the results mainly reflect the effect of AI when used for recalcitrant labor. A potential cause for recalcitrant labor could be overt or silent chorioamnionitis56 which has recently been associated with PVL, NEC and BPD.57, 58 Most observational studies did not provide information on silent chorioamnionitis. Whether the adverse neonatal effects are secondary to recent exposure to AI or to underlying silent chorioamnionitis, a marker of recalcitrant labor, remains to be answered. In addition, the few studies using indomethacin as the first agent for tocolysis prevented the performance of a sensitivity analysis evaluating the risk of PVL and BPD after its exposure.
Another limitation is that we were not able to evaluate the independent risk of a spontaneous intestinal perforation after exposure to AI. Most observational studies included in this meta-analysis did not elaborate on their definition of NEC so that a spontaneous intestinal perforation was not differentiated from an intestinal perforation that accompanied NEC.
Since not every computer assisted search will be exhaustive, there is a possibility of missing important publications. However we did not find publication bias for any of the neonatal outcomes studied in this meta-analysis. Despite all these limitations, meta-analysis can improve the conclusions of multiple small studies, especially if individual studies are not powered sufficiently to independently study neonatal outcomes and provide meaningful conclusions. In this context, the findings of this meta-analysis allow investigators to derive more accurate conclusions than those from individual studies or non-quantitative systematic reviews. We conclude that AI used for recalcitrant labor may be associated with PVL and NEC in premature infants and recommend that AI be used judiciously as a tocolytic agent. There is a need to conduct well designed randomized trials to confirm the findings of our meta-analysis.
Acknowledgments
The study was performed as part of a clinical investigational training towards Masters in Clinical Research and was funded by K-23 NIH grant DC06229. We are thankful to our reference librarian, Marilyn Rosen, who helped in the search process.
ABBREVIATIONS
- PVL
periventricular leukomalacia
- IVH
intraventricular hemorrhage
- RDS
respiratory distress syndrome
- BPD
bronchopulmonary dysplasia
- NEC
necrotizing enterocolitis, and PDA, patent ductus arteriosus
- GA
gestational age
- OR
odds ratio
- CI
confidence interval
- AI
antenatal indomethacin
Footnotes
Presented at the Pediatric Academic Society Meeting held in San Francisco on May 1, 2006
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuckerman H, Reiss U, Rubinstein I. Inhibition of human premature labor by indomethacin. Obstet Gynecol. 1974;44(6):787–792. [PubMed] [Google Scholar]
- 2.Niebyl JR, Blake DA, White RD, et al. The inhibition of premature labor with indomethacin. Am J Obstet Gynecol. 1980;136(8):1014–1019. doi: 10.1016/0002-9378(80)90629-8. [DOI] [PubMed] [Google Scholar]
- 3.Van den Veyver IB, Moise KJ., Jr Prostaglandin synthetase inhibitors in pregnancy. Obstet Gynecol Surv. 1993;48(7):493–502. doi: 10.1097/00006254-199307000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Moise KJ, Jr, Huhta JC, Sharif DS, et al. Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl J Med. 1988;319(6):327–331. doi: 10.1056/NEJM198808113190602. [DOI] [PubMed] [Google Scholar]
- 5.Moise KJ, Jr, Ou CN, Kirshon B, Cano LE, Rognerud C, Carpenter RJ., Jr Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol. 1990;162(2):549–554. doi: 10.1016/0002-9378(90)90427-9. [DOI] [PubMed] [Google Scholar]
- 6.Dudley DK, Hardie MJ. Fetal and neonatal effects of indomethacin used as a tocolytic agent. Am J Obstet Gynecol. 1985;151(2):181–184. doi: 10.1016/0002-9378(85)90008-0. [DOI] [PubMed] [Google Scholar]
- 7.Neu J, Wu-Wang CY. Eicosanoids in the developing gastrointestinal tract. Semin Perinatol. 1987;11(1):22–30. [PubMed] [Google Scholar]
- 8.Wallace JL, Cohen MM. Gastric mucosal protection with chronic mild restraint: role of endogenous prostaglandins. Am J Physiol. 1984;247(2 Pt 1):G127–132. doi: 10.1152/ajpgi.1984.247.2.G127. [DOI] [PubMed] [Google Scholar]
- 9.Peskar BM. On the synthesis of prostaglandins by human gastric mucosa and its modification by drugs. Biochim Biophys Acta. 1977;487(2):307–314. doi: 10.1016/0005-2760(77)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Meyers RL, Alpan G, Lin E, Clyman RI. Patent ductus arteriosus, indomethacin, and intestinal distension: effects on intestinal blood flow and oxygen consumption. Pediatr Res. 1991;29(6):569–574. doi: 10.1203/00006450-199106010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Gleason CA, Clyman RI, Heymann MA, Mauray F, Leake R, Roman C. Indomethacin and patent ductus arteriosus: effects on renal function in preterm lambs. Am J Physiol. 1988;254(1 Pt 2):F38–44. doi: 10.1152/ajprenal.1988.254.1.F38. [DOI] [PubMed] [Google Scholar]
- 12.Leffler CW, Busija DW, Fletcher AM, Beasley DG, Hessler JR, Green RS. Effects of indomethacin upon cerebral hemodynamics of newborn pigs. Pediatr Res. 1985;19(11):1160–1164. doi: 10.1203/00006450-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Friedman Z, Whitman V, Maisels MJ, Berman W, Jr, Marks KH, Vesell ES. Indomethacin disposition and indomethacin-induced platelet dysfunction in premature infants. J Clin Pharmacol. 1978;18(5–6):272–279. doi: 10.1002/j.1552-4604.1978.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph AM, Heymann MA. Hemodynamic changes induced by blockers of prostaglandin synthesis in the fetal lamb in utero. Adv Prostaglandin Thromboxane Res. 1978;4:231–237. [PubMed] [Google Scholar]
- 15.Moise KJ., Jr Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168(5):1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- 16.Bustos R, Ballejo G, Giussi G, Rosas R, Isa JC. Inhibition of fetal lung maturation by indomethacin in pregnant rabbits. J Perinat Med. 1978;6(5):240–245. doi: 10.1515/jpme.1978.6.5.240. [DOI] [PubMed] [Google Scholar]
- 17.Eronen M, Pesonen E, Kurki T, Teramo K, Ylikorkala O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. J Pediatr. 1994;124(5 Pt 1):782–788. doi: 10.1016/s0022-3476(05)81374-5. [DOI] [PubMed] [Google Scholar]
- 18.Morales WJ, Smith SG, Angel JL, O’Brien WF, Knuppel RA. Efficacy and safety of indomethacin versus ritodrine in the management of preterm labor: a randomized study. Obstet Gynecol. 1989;74(4):567–572. [PubMed] [Google Scholar]
- 19.Kurki T, Eronen M, Lumme R, Ylikorkala O. A randomized double-dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstet Gynecol. 1991;78(6):1093–1097. [PubMed] [Google Scholar]
- 20.Besinger RE, Niebyl JR, Keyes WG, Johnson TR. Randomized comparative trial of indomethacin and ritodrine for the long-term treatment of preterm labor. Am J Obstet Gynecol. 1991;164(4):981–986. doi: 10.1016/0002-9378(91)90569-d. discussion 986–988. [DOI] [PubMed] [Google Scholar]
- 21.Panter KR, Hannah ME, Amankwah KS, Ohlsson A, Jefferies AL, Farine D. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo-controlled trial. Br J Obstet Gynaecol. 1999;106(5):467–473. doi: 10.1111/j.1471-0528.1999.tb08300.x. [DOI] [PubMed] [Google Scholar]
- 22.Niebyl JR, Witter FR. Neonatal outcome after indomethacin treatment for preterm labor. Am J Obstet Gynecol. 1986;155(4):747–749. doi: 10.1016/s0002-9378(86)80012-6. [DOI] [PubMed] [Google Scholar]
- 23.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329(22):1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 24.Hammerman C, Glaser J, Kaplan M, Schimmel MS, Ferber B, Eidelman AI. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics. 1998;102(5):E56. doi: 10.1542/peds.102.5.e56. [DOI] [PubMed] [Google Scholar]
- 25.Ojala R, Ikonen S, Tammela O. Perinatal indomethacin treatment and neonatal complications in preterm infants. Eur J Pediatr. 2000;159(3):153–155. doi: 10.1007/s004310050040. [DOI] [PubMed] [Google Scholar]
- 26.Gerson A, Abbasi S, Johnson A, Kalchbrenner M, Ashmead G, Bolognese R. Safety and efficacy of long-term tocolysis with indomethacin. Am J Perinatol. 1990;7(1):71–74. doi: 10.1055/s-2007-999450. [DOI] [PubMed] [Google Scholar]
- 27.Major CA, Lewis DF, Harding JA, Porto MA, Garite TJ. Tocolysis with indomethacin increases the incidence of necrotizing enterocolitis in the low-birth-weight neonate. Am J Obstet Gynecol. 1994;170(1 Pt 1):102–106. doi: 10.1016/s0002-9378(94)70392-2. [DOI] [PubMed] [Google Scholar]
- 28.al-Alaiyan S, Seshia MM, Casiro OG. Neurodevelopmental outcome of infants exposed to indomethacin antenatally. J Perinat Med. 1996;24(4):405–411. doi: 10.1515/jpme.1996.24.4.405. [DOI] [PubMed] [Google Scholar]
- 29.Gardner MO, Owen J, Skelly S, Hauth JC. Preterm delivery after indomethacin. A risk factor for neonatal complications? J Reprod Med. 1996;41(12):903–906. [PubMed] [Google Scholar]
- 30.Iannucci TA, Besinger RE, Fisher SG, Gianopoulos JG, Tomich PG. Effect of dual tocolysis on the incidence of severe intraventricular hemorrhage among extremely low-birth-weight infants. Am J Obstet Gynecol. 1996;175(4 Pt 1):1043–1046. doi: 10.1016/s0002-9378(96)80050-0. [DOI] [PubMed] [Google Scholar]
- 31.Souter D, Harding J, McCowan L, O’Donnell C, McLeay E, Baxendale H. Antenatal indomethacin--adverse fetal effects confirmed. Aust N Z J Obstet Gynaecol. 1998;38(1):11–16. doi: 10.1111/j.1479-828x.1998.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 32.Vermillion ST, Newman RB. Recent indomethacin tocolysis is not associated with neonatal complications in preterm infants. Am J Obstet Gynecol. 1999;181(5 Pt 1):1083–1086. doi: 10.1016/s0002-9378(99)70085-2. [DOI] [PubMed] [Google Scholar]
- 33.Pietrantoni MWJ, Bridges S, Johnson I, Spinnato JA, Gall SA. Adverse neonatal outcomes following antenatal indomethacin use. Am J Obstet Gynecol. 1995:172. [Google Scholar]
- 34.Weintraub Z, Solovechick M, Reichman B, et al. Effect of maternal tocolysis on the incidence of severe periventricular/intraventricular haemorrhage in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F13–17. doi: 10.1136/fn.85.1.F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbasi S, Gerdes JS, Sehdev HM, Samimi SS, Ludmir J. Neonatal outcome after exposure to indomethacin in utero: a retrospective case cohort study. Am J Obstet Gynecol. 2003;189(3):782–785. doi: 10.1067/s0002-9378(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 36.Doyle NM, Gardner MO, Wells L, Qualls C, Papile LA. Outcome of very low birth weight infants exposed to antenatal indomethacin for tocolysis. J Perinatol. 2005;25(5):336–340. doi: 10.1038/sj.jp.7211256. [DOI] [PubMed] [Google Scholar]
- 37.Baerts W, Fetter WP, Hop WC, Wallenburg HC, Spritzer R, Sauer PJ. Cerebral lesions in preterm infants after tocolytic indomethacin. Dev Med Child Neurol. 1990;32(10):910–918. doi: 10.1111/j.1469-8749.1990.tb08104.x. [DOI] [PubMed] [Google Scholar]
- 38.Murata Y, Itakura A, Matsuzawa K, Okumura A, Wakai K, Mizutani S. Possible antenatal and perinatal related factors in development of cystic periventricular leukomalacia. Brain Dev. 2005;27(1):17–21. doi: 10.1016/j.braindev.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Suarez RD, Grobman WA, Parilla BV. Indomethacin tocolysis and intraventricular hemorrhage. Obstet Gynecol. 2001;97(6):921–925. doi: 10.1016/s0029-7844(01)01356-4. [DOI] [PubMed] [Google Scholar]
- 40.Parilla BV, Grobman WA, Holtzman RB, Thomas HA, Dooley SL. Indomethacin tocolysis and risk of necrotizing enterocolitis. Obstet Gynecol. 2000;96(1):120–123. doi: 10.1016/s0029-7844(00)00846-2. [DOI] [PubMed] [Google Scholar]
- 41.Van Overmeire B, Slootmaekers V, De Loor J, et al. The addition of indomethacin to betamimetics for tocolysis: any benefit for the neonate? Eur J Obstet Gynecol Reprod Biol. 1998;77(1):41–45. doi: 10.1016/s0301-2115(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 42.Friedman S, Flidel-Rimon O, Steinberg M, Shinwell ES. Indomethacin tocolysis and white matter injury in preterm infants. J Matern Fetal Neonatal Med. 2005;18(2):87–91. doi: 10.1080/14767050500199160. [DOI] [PubMed] [Google Scholar]
- 43.King J, Flenady V, Cole S, Thornton S. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005;(2):CD001992. doi: 10.1002/14651858.CD001992.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Loe SM, Sanchez-Ramos L, Kaunitz AM. Assessing the neonatal safety of indomethacin tocolysis: a systematic review with meta-analysis. Obstet Gynecol. 2005;106(1):173–179. doi: 10.1097/01.AOG.0000168622.56478.df. [DOI] [PubMed] [Google Scholar]
- 45.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 46.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 47.Petitti DB. Statistical methods in meta-analysis. In: Petitti DB, editor. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. 2. New York: Oxford University Press; 2000. p. 83. [Google Scholar]
- 48.Jacqz-Aigrain E, Guillonneau M, Boissinot C, Bavoux F, Hartmann JF, Blot P. Maternal and neonatal effects of indomethacin administrated during pregnancy Apropos of 18 cases. Arch Fr Pediatr. 1993;50(4):307–312. [PubMed] [Google Scholar]
- 49.Nishikubo T, Takahashi Y, Nakagawa Y, et al. Renal impairment in very low birthweight infants following antenatal indomethacin administration. Acta Paediatr Jpn. 1994;36(2):202–206. doi: 10.1111/j.1442-200x.1994.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 50.Butler-O’Hara M, D’Angio CT. Risk of persistent renal insufficiency in premature infants following the prenatal use of indomethacin for suppression of preterm labor. J Perinatol. 2002;22(7):541–546. doi: 10.1038/sj.jp.7210790. [DOI] [PubMed] [Google Scholar]
- 51.Wurtzel D. Prenatal administration of indomethacin as a tocolytic agent: effect on neonatal renal function. Obstet Gynecol. 1990;76(4):689–692. [PubMed] [Google Scholar]
- 52.Vanhaesebrouck P, Thiery M, Leroy JG, et al. Oligohydramnios, renal insufficiency, and ileal perforation in preterm infants after intrauterine exposure to indomethacin. J Pediatr. 1988;113(4):738–743. doi: 10.1016/s0022-3476(88)80392-5. [DOI] [PubMed] [Google Scholar]
- 53.Salokorpi T, Eronen M, von Wendt L. Growth and development until 18 months of children exposed to tocolytics indomethacin or nylidrin. Neuropediatrics. 1996;27(4):174–177. doi: 10.1055/s-2007-973782. [DOI] [PubMed] [Google Scholar]
- 54.Vermillion ST, Landen CN. Prostaglandin inhibitors as tocolytic agents. Semin Perinatol. 2001;25(4):256–262. doi: 10.1053/sper.2001.27549. [DOI] [PubMed] [Google Scholar]
- 55.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23(4):278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 56.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22(2):281–342. [PubMed] [Google Scholar]
- 57.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34(1):5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 58.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23-to-32 week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]