Abstract
We tested the hypothesis that amphetamine- (AMPH) induced conditioned motor sensitization is accompanied by cellular activation (measured by Fos immunoreactivity) and synaptophysin immunoreactivity in reward-related brain areas. Forty-eight rats were tested for conditioned motor sensitization using a conditioning paradigm that was performed in a three-chambered apparatus. Rats underwent two drug pairings with 1.0 mg/kg AMPH in one outer chamber and, on alternate days, were paired with saline in the other. On the fifth day, relative to the first AMPH treatment, AMPH administration increased motor activity in the AMPH-paired context but not in the saline-paired context. Relative to the first saline treatment, saline on the fifth day produced a conditioned increase in motor activity when given in the chamber previously paired with AMPH, and saline given in the saline-paired context produced a conditioned decrease in motor activity. AMPH administered in the AMPH-paired context increased the density of both Fos and synaptophysin immunoreactivity in the dentate gyrus, cornu ammonis (CA)1, CA3, basolateral amygdala, and dorsolateral striatum. This pairing between context and drug increased Fos but not synaptophysin immunoreactivity in the nucleus accumbens core and shell. Saline administered in the AMPH-paired context increased the density of Fos immunoreactivity in the basolateral amygdala and nucleus accumbens core. These data indicate that the basolateral amygdala-nucleus accumbens core pathway is necessary for the context-elicited conditioned motor responses, while the hippocampus encodes the spatial context.
Keywords: amphetamine, classical conditioning, conditioned motor sensitization, Fos, synaptophysin
Introduction
Sensitization is an enhancement of neuronal and behavioral function as a result of repeated drug exposure. In experimental animals, psychostimulant-induced sensitized behaviors, including the motoric and incentive motivational effects, last for months to years after drug treatment is discontinued (Castner & Goldman-Rakic, 1999; Paulson et al., 1991; Robinson & Berridge, 1993). Sensitization to these effects has been proposed as a mechanism to explain the transition from a regular pattern of voluntary drug intake to compulsive drug seeking and drug taking behavior (Berridge & Robinson, 1998; Robinson & Berridge, 2003).
When sensitization occurs in a distinct context, the conditioned stimulus (CS) can contribute to the development and expression of that sensitization (Robinson & Berridge, 2003). In fact, a higher degree of motor sensitization often is observed when drug (unconditioned stimulus (UCS)) is administered in the context that has been previously paired with the drug and subjects may fail to express motor sensitization if they are tested in the context that has never been paired with the drug; in this case, motor sensitization is said to be context-specific or is referred to as conditioned motor sensitization (CMS) (Pert et al., 1990; Robinson et al., 1998). Models that help explain how context modulates motor sensitization include: (a) An excitatory conditioning model in which environmental stimuli acquire excitatory CS properties (Hinson & Paulos, 1981; Pert et al., 1990); (b) An inhibitory conditioning model in which environmental stimuli acquire inhibitory CS properties (Stewart & Vezina, 1988; 1991); and (c) An occasion setting model in which associative learning determines whether sensitization is expressed. Thus, the expression of the behavior is enhanced or prevented in environmental contexts where the drug is or is not expected, respectively (Anagnostaras & Robinson, 1996).
Persistent changes in behavior as a result of experience are thought to reflect the reorganization and/or strengthening of synaptic connections in specific neuronal circuits. Psychostimulant drugs produce such morphological changes in reward-related brain regions (Robinson & Kolb 1997; 1999). Synaptophysin is a vesicular membrane protein found in presynaptic terminals (Wiedenmann & Franke, 1985), and immunoreactive (IR) puncta are markers for synapses (Hiscock et al., 2000). Increases in reference memory errors correlate with decreases in synaptophysin in the frontal cortex (Denisova et al., 2002). The degree of amphetamine- (AMPH) induced conditioned place preference (CPP) is positively correlated with increased synaptophysin immunoreactivity in the basolateral amygdala (BLA) and hippocampus (Rademacher et al., 2006). Thus, synaptic density could be altered by the learned association between contextual CS and drug UCS.
Fos, the protein product of the early immediate gene, c-fos, is expressed at low levels in the unstimulated brain (Harlan & Garcia, 1998). An evaluation of Fos expression provides a “map” of activated neurons (Dragunow & Robertson, 1987), and changes in Fos expression have been used to map regions engaged by the CS (Miller & Marshall, 2005a; Rademacher et al., 2006). In the present study, Fos and synaptophysin were used to map neuronal activation and synaptogenesis or increased synapse size.
Materials and methods
Animals
Forty-eight male Sprague Dawley rats (Harlan, Indianapolis, IN, USA), weighing 180-200 g at the start of the experiment, were housed in groups of three and allowed 2 weeks of habituation to the housing room prior to the beginning of the experiment. Animals were handled for 5 days before behavioral conditioning. Rats were maintained on a 12 h light:dark cycle with lights on at 0700 h, and food and water were available ad libitum in their home cages. All studies were carried out in accordance with the Declaration of Helsinki and with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No.80-23, revised 1996)and were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Drugs
D-amphetamine (AMPH) sulfate (Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% saline. Injections were administered at a concentration of 1 mg/ml/kg i.p. and doses refer to the drug base.
Conditioning apparatus
Conditioned motor sensitization studies were performed in a three-compartment apparatus (AccuScan Instruments, Inc., Columbus, OH, USA). The two larger, outer compartments (25 × 30 × 32 cm) were separated by a central compartment (10 × 25 × 32 cm) and differed in both visual and tactile cues. One outer compartment had white vertical stripes on the walls and the other, white horizontal stripes. The compartments had floors with different textures. The central compartment had white walls and a Plexiglas floor, and allowed free movement between the two outer compartments unless barred by two white partitions. Infrared sensors along all four sides of the conditioning apparatus recorded the movement and location of the animals. Conditioning sessions were conducted with dim illumination and in the presence of white noise.
Behavioral conditioning
Conditioning occurred according to previous published protocols (Rademacher et al., 2006; Shen et al., 2006) with some modifications. Conditioning sessions lasted for 45 min. On days 1 and 3, the rats (n=48) were injected with AMPH (1.0 mg/kg, i.p.) immediately before placement into one of the two outer compartments (AMPH-paired compartment). On days 2 and 4, the rats (n=48) were injected with saline (1.0 ml/kg, i.p.) and immediately confined to the opposite outer compartment (saline-paired compartment). On day 5, rats were randomly assigned to one of four groups with 12 rats in each. Group one received a challenge injection of AMPH immediately before being placed into the AMPH-paired compartment. Group two received a challenge injection of AMPH before being placed into the saline-paired compartment. Group three received a challenge injection of saline before being placed into the AMPH-paired compartment. four received a challenge injection of saline before being placed into the saline-paired compartment. Between conditioning sessions, the walls of the conditioning apparatus were thoroughly washed and floors were replaced. Horizontal locomotor activity was recorded throughout each conditioning session. Motor sensitization was calculated as locomotor activity on day 5 minus that on day 1 for rats receiving AMPH on day 5, and day 5 minus that on day 2 for rats that received saline on day 5. To determine if motor sensitization was context-specific and if the context elicited conditioned motor responses, one-sample t-tests, with a theoretical mean set at zero, were used. An α level of 0.05 was required for statistical significance. Data are presented as the mean ± S.E.M.
Immunohistochemistry (IHC)
The objectives of this study were: (1) to ascertain the brain regions that are engaged by the environmental context on the CMS challenge day; (2) to be able to compare the effects of the conditioning history on this map; and (3) to indicate if synaptogenesis or increased synapse size were correlated with and, thus, contribute to this process. To address the first two objectives, Fos IHC was performed. Fos expression appears rapidly after neuronal activation and, thus for this marker, animals were sacrificed 90 min after behavioral conditioning, i.e. on day 5 (n = 6/group). In contrast to Fos, synaptophysin levels canaccumulate with repeated treatment/learning sessions (Frick & Fernandez, 2003). Thus, to substantiate the second objective, and address the third, rats used for synaptophysin IHC were sacrificed 20 h after behavioral conditioning, i.e. on day 6 (n = 6/group).
All rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 20 min. The brains were removed and postfixed for 2.5 h at 4°C, rinsed and transferred to 30% sucrose in 0.1 M phosphate buffer (pH 7.4) at 4°C until saturated. Coronal sections (35 μm) were cut on a cryostat at -22°C and either immunoreacted immediately or stored in cryoprotectant (ethylene glycol and glycerol in 0.1 M phosphate buffer) at -20°C until IHC processing.
For Fos IHC, free-floating sectionswere rinsed in 0.1 M phosphate-buffered saline (pH 7.4), blocked in 0.2% Triton X-100 (TX) and 5% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h at room temperature, and incubated in rabbit antibodies against Fos (PC-38; Calbiochem, La Jolla, CA, USA) diluted 1:20,000 in 0.3% TX plus 3% normal goat serum for 60 h at 4°C. Following extensive rinsing, sections were incubated in biotinylated goat anti-rabbit IgG (1:200, Vector Labs, Burlingame, CA, USA), 0.2% TX, and 5% normal goat serum for 2 h at room temperature and, after rinsing, were incubated with avidin-biotin peroxidase complex (Vectastain kit; Vector Labs, Burlingame, CA, USA) for 1 h at room temperature. The sections were reacted with 0.05% 3,3’diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) containing 0.01% H2O2 for 25 min. Rinsing in 0.05 M Tris-HCl terminated the reaction. Fos staining was absent when control sections were incubated without the primary antibody (data not shown), suggesting that the antibody was Fos-specific.
For synaptophysin IHC, rinsed sections were first blocked in 0.5% TX with 5% normal horse serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at room temperature, and then incubated in monoclonal mouse anti-synaptophysin (MAB5258; Chemicon, Temecula, CA, USA), diluted 1:5000 in 0.5% TX and 5% normal horse serum for 60 h at 4°C. This was followed by an incubation in biotinylated horse anti-mouse IgG (1:250), 0.5% TX, and 3% normal horse serum for 2 h at room temperature and avidin-biotin peroxidase complex for 1 h at room temperature. The sections were rinsed, reacted with 0.05% 3,3’diaminobenzidine tetrahydrochloride containing 0.01% H2O2, mounted onto slides, dried, dehydrated, and topped with coverslips. Synaptophysin staining was absent in control sections incubated without the primary antibody (data not shown), suggesting that the antibody was synaptophysin-specific.
Quantification of IHC
An observer blind to the treatment groups captured images using a Nikon Eclipse E600 microscope and digital camera (Optronics, Goleta, CA, USA). A rectangular counting frame of the same size was placed over each region under investigation. Fos-IR cells and synaptophysin punctae were photographed at 10X (Fos) or 20X (synaptophysin) in different target regions of the mesolimbic dopamine pathway and in the dorsolateral striatum (DLS) (Fig. 1). One level of the DLS and nucleus accumbens (NAc), bregma +1.6 (intermediate) (Paxinos & Watson, 1998) was captured, and both core (NAcC) and shell (NAcS) were analyzed. For the ventral pallidum (VP), two coronal planes, bregma +0.20 (rostral) and −0.26 (caudal), were studied. The BLA, hippocampus (i.e., cornu ammonis (CA)1 and CA3 fields, and dentate gyrus (DG)), were sampled at three different levels in the coronal plane, bregma −2.12 (rostral), −2.8 (intermediate), and −3.8 (caudal). The sampled area in the BLA primarily consisted of the basal nucleus (see Fig. 1). Digital photos were analyzed with ImageJ software for Macintosh (Scion Corp, Frederick, MD, USA). A threshold intensity for Fos immunoreactivity was set to allow all positive cells to be counted while minimizing counts due to background labeling. The density of Fos-IR cells was quantified and then divided by the area of the corresponding rectangular counting frame to express the density as cells per square millimeter. Synaptophysin immunoreactivity was analyzed as follows: After setting a threshold to minimize background, the mean optical density of pixels was computed based on a scale of 0 to 256 relative units. Background values were taken from a white matter structure (corpus callosum) and subtracted from the mean optical density of grey level. The density of Fos-IR cells and synaptophysin-IR puntae were analyzed using two-way ANOVA with challenge injection (saline, AMPH) and context (saline-paired, AMPH-paired) as factors. Between-group comparisons were assessed using Newman-Keuls Multiple Comparison Tests, with an α level of 0.05 required for statistical significance. Separate analyses were conducted for each brain region. Data are presented as mean ± S.E.M.
Figure 1.
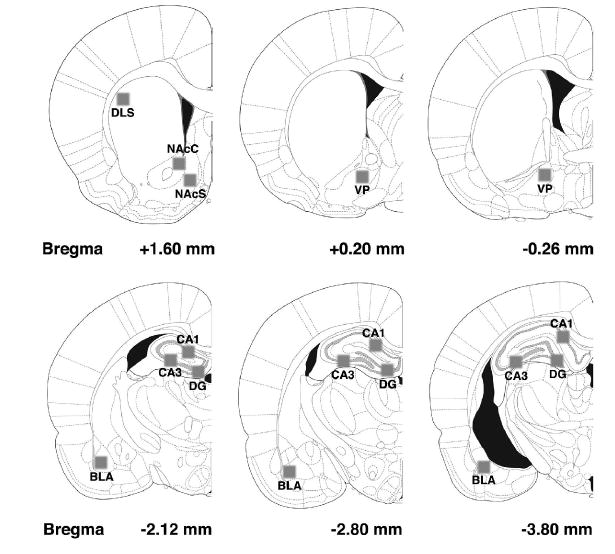
The areas analyzed for Fos and synaptophysin immunoreactivity (indicated by the shaded boxes). Stereotaxic maps were reproduced, with permission, from the brain atlas of Paxinos & Watson (1998).
Results
AMPH-induced conditioned motor sensitization (CMS) and context-elicited conditioned motor responses
Our prior work has shown that the motor response in this behavioral paradigm is context-dependent, for the enhancement of the motor response after 1.0 mg/kg AMPH did not occur if the rat is placed into the saline-paired compartment for the final AMPH injection (Shen et al., 2006). Likewise, in the current study, the change in locomotor activity (day 5 − day 1) was significantly greater than zero when AMPH was administered in the AMPH-paired context (t11 = 4.74, P < 0.001) but not when AMPH was administered in the saline-paired context (t11 = 1.33, P > 0.05) (Fig. 2). When saline was administered in the AMPH-paired context, the change in locomotor activity (day 5 − day 2) also was significantly greater than zero (t11 = 2.33, P < 0.05) (Fig. 2). The change in locomotor activity (day 5 − day 2) was significantly less than zero when saline was administered in the saline-paired context (t11 = 3.05, P < 0.05; Fig. 2).
Figure 2.
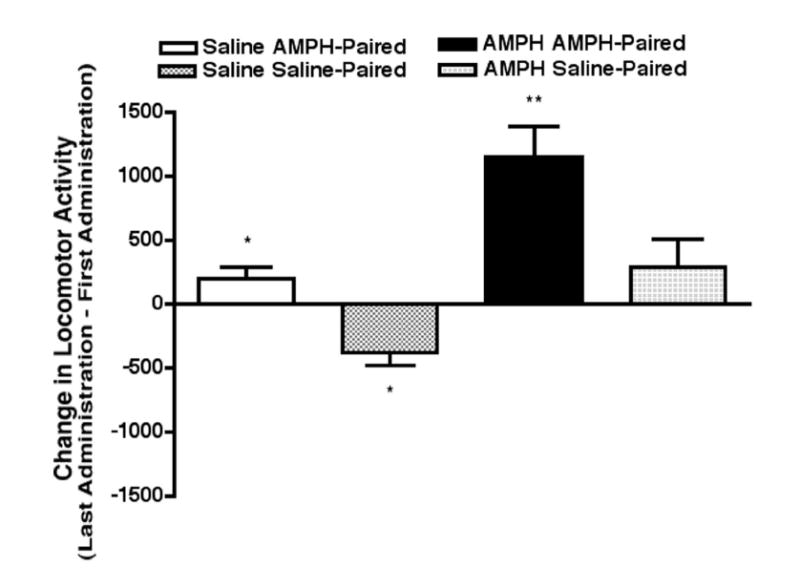
AMPH-induced CMS and context elicited conditioned motor responses. Motor sensitization was observed when AMPH was administered in the AMPH-paired context (** P < 0.01 compared to a theoretical mean equal to zero) but not when AMPH was administered in the saline-paired context. A conditioned increase in motor activity was observed when saline was administered in the AMPH-paired context; * P < 0.05 compared to a theoretical mean equal to zero. A conditioned decrease in motor activity was observed when saline was administered in the saline-paired context; * P < 0.05 compared to a theoretical mean equal to zero.
Density of Fos-IR cells
Qualitative evaluations suggested that there are differences in Fos-IR density between animals receiving different challenge injections in different contexts (see Fig. 3). Two-way ANOVA revealed a significant effect of the challenge injection on the density of Fos-IR cells in the DG (F1,20 = 8.88, P < 0.01), CA1 field (F1,20 = 28.90, P < 0.0001), CA3 field (F1,20 = 19.83, P < 0.001), BLA (F1,20 = 22.46, P < 0.001), NAcC (F1,20 = 11.44, P < 0.01), NAcS (F1,20 = 9.29, P < 0.01), and DLS (F1,20 = 22.04, P < 0.0001), but not the VP. There was a significant effect of context on the density of Fos-IR cells in the DG (F1,20 = 22.46, P < 0.001), CA1 field (F1,20 = 4.01, P < 0.05), CA3 field (F1,20 = 9.82, P < 0.01), BLA(F1,20 = 14.37, P < 0.01), NAcC (F1,20 = 12.60, P < 0.01), NAcS (F1,20 = 6.44, P < 0.05), and DLS (F1,20 = 6.10, P < 0.05), but not the VP. There was a statistically significant interaction between challenge injection and context in the CA1 field (F1,20 = 4.35, P < 0.05), CA3 field (F1,20 = 5.47, P < 0.05), and DLS (F1,20 = 6.78, P < 0.05).
Figure 3.
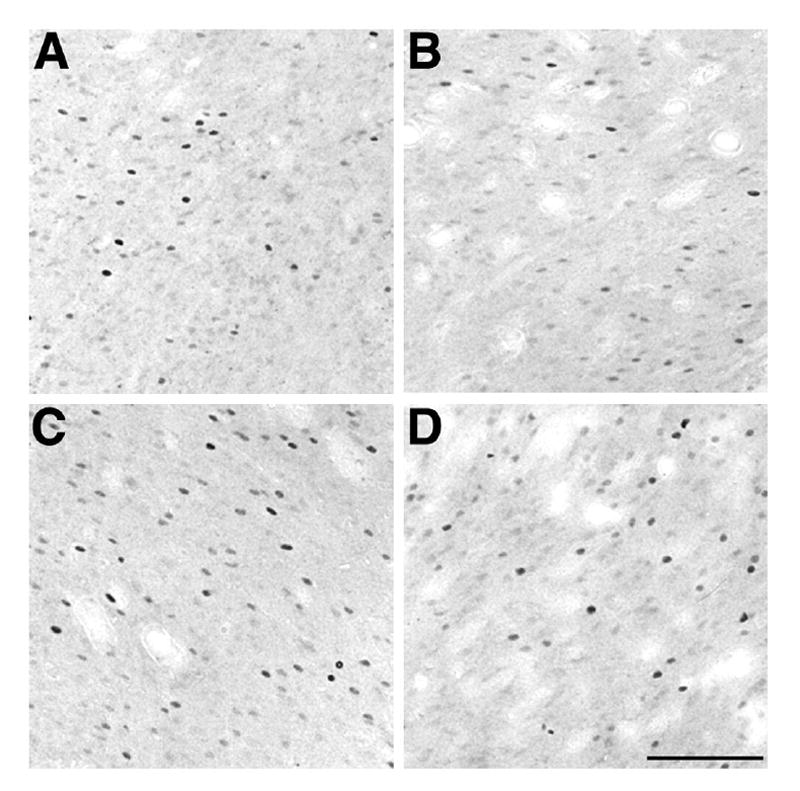
Brightfield photomicrographs depicting the effect of AMPH and saline conditioning on Fos immunoreactivity in the NAcC. Density of Fos-IR cells when A. saline was administered in the AMPH-paired context; B. saline was administered in the saline-paired context; C. AMPH was administered in the AMPH-paired context; and D. AMPH was administered in the saline-paired context. Compare the Fos-IR cell density in A with B and C with D. Scale bar in D is valid for A-C = 100 μm.
Post hoc analyses (Fig. 4) revealed which regions were affected by the environmental context. The density of Fos-IR cells was significantly greater in the BLA and NAcC for rats that received a saline injection in the AMPH-paired compartment compared to rats given a saline injection in the saline-paired compartment. The density of Fos-IR cells was significantly greater in the DG, CA1 field, CA3 field, BLA, NAcC, NAcS, and DLS for rats that received an AMPH injection in the AMPH-paired compartment compared to rats that received AMPH in the saline-paired compartment.
Figure 4.
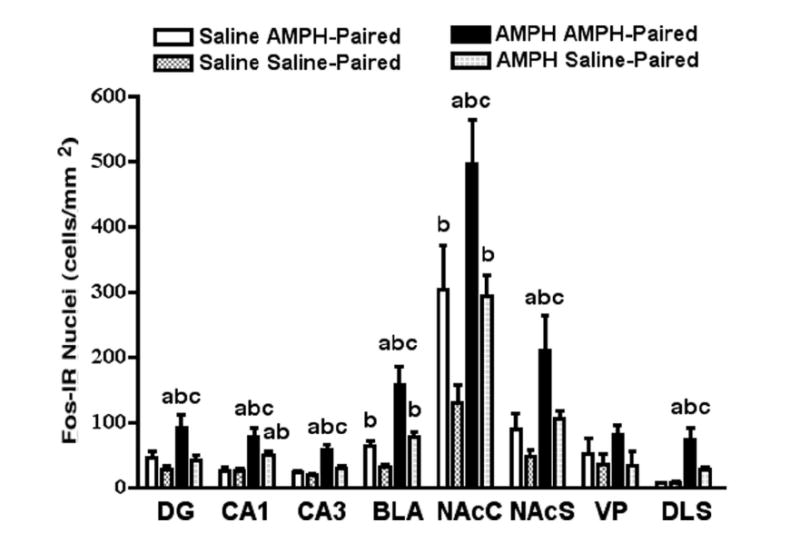
Brain region comparisons for the effects of AMPH- and saline-conditioning on Fos immunoreactivity. Statistically significant results are marked with the letters a, b, and c. Each letter represents a significant increase in Fos-IR cell density for rats that received a challenge injection (AMPH or saline) in a specific compartment (see legend) compared to those that received a challenge injection of (a) saline in the AMPH-paired compartment, (b) saline in the saline-paired compartment, and (c) AMPH in the saline-paired compartment.
Post hoc analyses (Fig. 4) revealed which regions were affected by the challenge injection. The density of Fos-IR cells was significantly greater in the CA1 field, BLA, and NAcC for rats that received an AMPH injection in the saline-paired compartment compared to rats that received a saline injection in the saline-paired compartment. The density of Fos-IR cells was significantly greater in the DG, CA1 field, CA3 field, BLA, NAcC, NAcS, and DLS for rats that received an AMPH injection in the AMPH-paired compartment compared to rats that received a saline injection in the AMPH-paired compartment.
Post hoc analyses (Fig. 4) revealed which regions where affected by both environmental context and challenge injection. The density of Fos-IR cells was significantly greater in the DG, CA1 field, CA3 field, BLA, NAcC, NAcS, and DLS for rats that received an AMPH injection in the AMPH-paired compartment compared to rats that received a saline injection in the saline-paired compartment. Finally, the density of Fos-IR cells was significantly greater in the CA1 field for rats that received an AMPH injection in the saline-paired compartment compared to rats that received a saline injection in the AMPH-paired compartment.
Density of synaptophysin-IR varicosities
The pattern of synaptophysin immunoreactivity in saline-treated control animals was similar to that reported previously (Grillo et al., 2005; Navone et al., 1986; Rademacher et al., 2006). Two-way ANOVA revealed no effect of challenge injection on the density of synaptophysin-IR varicosities in any of the brain regions examined. There was a significant effect of context on the density of synaptophysin-IR varicosities in the DG (F1,20 = 11.56, P < 0.01), CA1 field (F1,20 = 5.31, P < 0.05), CA3 field (F1,20 = 9.09, P < 0.01), BLA(F1,20 = 5.15, P < 0.05), and DLS (F1,20 = 5.18, P < 0.05), but not in the NAcC, NAcS, or VP. However, there was a significant interaction between the challenge injection and context in the DG (F1,20 = 4.50, P < 0.05), CA1 field (F1,20 = 6.42, P < 0.05), CA3 field (F1,20 = 4.84, P < 0.05), and DLS (F1,20 = 4.56, P < 0.05).
Post hoc analyses (Fig. 5) revealed which brain regions were affected by the environmental context. The density of synaptophysin-IR varicosities was significantly greater in the DG, CA1 field, CA3 field, BLA, and DLS for animals that received an AMPH injection in the AMPH-paired compartment compared to animals that received an AMPH injection in the saline-paired compartment.
Figure 5.
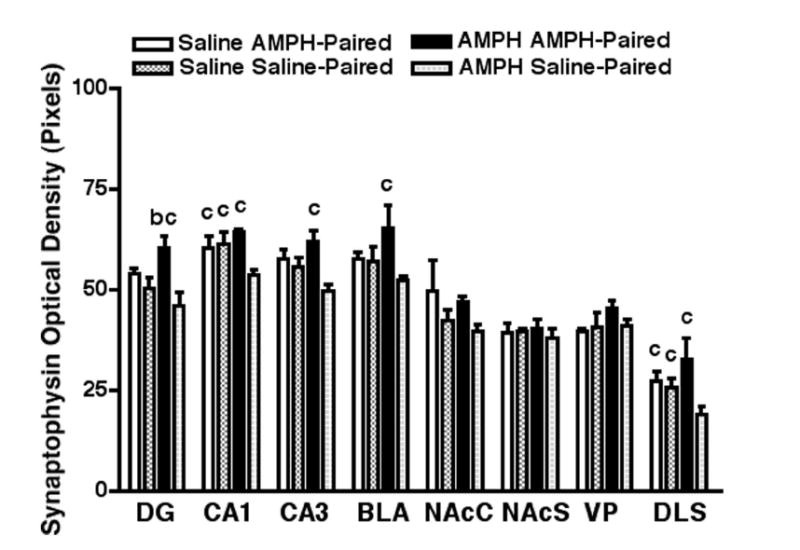
Brain region comparisons for the effects of AMPH- and saline-conditioning on synaptophysin immunoreactivity. Statistically significant results are marked with the letters a, b, and c. Each letter represents a significant increase in synaptophysin-IR varicosities for rats that received a challenge injection (AMPH or saline) in a specific compartment (see legend) compared to those that received a challenge injection of (a) saline in the AMPH-paired compartment (not represented), (b) saline in the saline-paired compartment, and (c) AMPH in the saline-paired compartment.
Post hoc analyses (Fig. 5) revealed which brain regions were affected by the challenge injection. The density of synaptophysin-IR varicosities was significantly greater in the CA1 field and DLS for animals that received a saline injection in the saline-paired compartment compared to animals that received a AMPH injection in the saline-paired compartment.
Post hoc analyses (Fig. 5) revealed which brain regions were affected by both the environmental context and the challenge injection. The density of synaptophysin-IR varicosities was significantly greater in the DG for animals that received an AMPH injection in the AMPH-paired compartment compared to animals that received a saline injection in the saline-paired compartment. The density of synaptophysin-IR varicosities was significantly greater in the CA1 field and DLS for animals that received a saline injection in the AMPH-paired compartment compared to animals that received an AMPH injection in the saline-paired compartment.
Discussion
There are three principle findings arising from these investigations. First, we demonstrate that a conditioned motor response with this behavioral paradigm was context-specific, results that are consistent with other studies (Ahmed et al., 1995; Badiani et al., 1995; Jodogne et al., 1994; Shen et al., 2006; Tirelli & Terry, 1998). Secondly, non-contingent AMPH administration activated Fos in all brain regions examined except the VP, results that are consistent with other studies (Cadoni et al., 1995; Dalia & Wallace, 1995; Engber et al., 1998; Niles et al., 1997). Thirdly, AMPH administered in the context that reliably predicted AMPH (i.e., the AMPH-paired context) activated Fos in the hippocampus, BLA, NAcC, NAcS, and DLS. However, the administration of saline in the AMPH-paired context also activated Fos, but only in the BLA and NAcC. Finally, AMPH, but not saline, given in the AMPH-paired context was associated with increased immunoreactivity for synaptophysin in the hippocampus, BLA, and DLS, but not in the NAcC. Considered together, these data suggest thatcellular activation of the BLA and NAcC are involved in the context-elicited conditioned motor activity, but the change in the synaptic marker associated with CMS is restricted to the BLA, hippocampus, and the DLS.
We found, in an earlier investigation, in which we assayed the same brain regions, that the 1.0 mg/kg dose of AMPH and a similar conditioning procedure produced a significant increase in both Fos and synaptophysin immunoreactivity in the NAcC for rats that showed a significant CPP (Rademacher et al., 2006). In the present study, we found an increase in Fos, but not synaptophysin, immunoreactivity in the NAcC and NAcS for rats that received an AMPH challenge injection in the AMPH-paired compartment. This important difference could be related to the two experimental paradigms. In the earlier study, all rats were tested for CPP, drug- and saline-free, 72 hours after the final conditioning session. During the test for CPP, animals had access to all three compartments of the place conditioning apparatus, and contextual cues presumably elicited approach behavior to the preferred compartment (see e.g., Bardo & Bevins, 2000; Everitt et al., 1991; Rademacher et al., 2000). In contrast, for the current report, there was no test for CPP, and animals were confined to one compartment of the apparatus after the challenge injection. Thus, it is possible, that at least for the NAcC, synaptic change, as measured by an increase in synaptophysin immunoreactivity, only occurs when the memory trace is being reactivated and reconsolidated at the time of the test for CPP (Miller & Marshall, 2005b). The strength of the initial context-drug memories and the nature of the reactivation events, important contributors to the degree of memory destabilization and reconsolidation (Bozon et al., 2003; Milekic et al., 2006; Suzuki et al., 2004), could also have differed between the two studies. However, this possibility seems unlikely considering that memory reactivation and reconsolidation appear to be independent of the initial memory consolidation (Lee et al., 2004). Finally, the expression of CPP could engage brain systems that include the NAcC, but these systems are not activated in the absence of a need to retrieve contextual cues, as at the time of the evaluation of CMS, a possibility that requires further investigation.
The finding that AMPH-administered in the AMPH-paired context increased cellular activation and synaptophysin expression in the DLS is consistent with the view that the DLS mediates the acquisition of stimulus-response (S-R) associations or habits (Everitt et al., 2001; Packard & Knowlton, 2002). Glutamatergic corticostriatal projections provide the DLS with sensory information underlying the formation of S-R associations (Alloway et al., 2006). Dopaminergic input to the DLS provides a signal that effectively imprints S-R associations (White, 1989). Glutamate and dopamine are involved in synaptogenesis (Petrak et al., 2005; Spencer et al., 1998) and activation of these two systems increase Fos expression in the DLS (Graybiel et al., 1990; Liste et al., 1995). The current findings indicate that the associative learning processes subsumed during AMPH-induced CMS may engage these, or similar systems, to alter synaptically mediated function in the DLS.
Augmented synaptophysin immunoreactivity could mean an increase in the number of vesicles, or, as been shown with electron microscopy, represent a true increase in the number of synapses (see e.g., Calhoun et al., 1996; Liu & Ju, 2001). Clearly one of the limitations of the present study was that it was conducted at the light microscopy level and so we are unable to distinguish synaptogenesis from enlarged boutons. Nevertheless, either outcome could result in synaptic enhancement. We have studies in progress at the electron microscopic level to establish the nature of this change.
A puzzling set of findings in the present study are those that demonstrate that the density of synaptophysin immunoreactivity is significantly greater in the CA1 field and DLS for animals that received a saline challenge injection in the AMPH- or saline-paired compartment compared to animals that received an AMPH challenge injection in the saline-paired compartment. One explanation is the density of synaptophysin immunoreactivity is dependent upon the pairing between the contextual CS and drug UCS. Certainly, the density of synaptophysin immunoreactivity generally increased whenever the AMPH challenge injections were administered in the “expected” compartment and decreased when given in the “unexpected” compartment. In addition, there was no difference in the density of synaptophysin immunoreactivity in any brain region for rats given saline in the AMPH-paired compartment compared to rats given saline in the saline-paired compartment. Further evaluation is required to determine whether or not the decrease in synaptophysin immunoreactivity for animals that received AMPH in the saline-paired compartment reflects a true decrease in the number of vesicles or synapses.
The AMPH-associated context significantly increased the expression of Fos in the BLA and NAcC, which is consistent with other reports of AMPH- or cocaine-paired environments activating this immediate early gene in these regions (Brown et al., 1992; Ciccocioppo et al., 2001; Franklin & Druhan, 2000; Mead et al., 1999; Miller & Marshall, 2004, 2005a; Neisewander et al., 2000). However, as a context-elicited increase in synaptophysin density also occurs in the BLA but not in the NAcC, the formation of contextual CS-drug UCS associations may require adaptations in the BLA neurons for the appropriate motor output from the NAcC to be elicited (Rademacher et al., 2006, Sellings & Clarke, 2006; Sutton et al., 2000). Supporting this concept, Di Ciano and Everitt (2004) demonstrated that if the BLA-NAcC pathway is interrupted, cue-elicited cocaine seeking on a second order schedule of reinforcement is greatly attenuated.
Both Fos and synaptophysin are significantly elevated the BLA and hippocampus after AMPH-induced CMS. These two regions serve important roles in appetitive cue and contextual conditioning. Whereas the formation of associations between discrete, elemental CS occurs in the BLA (Everitt et al., 1991; Ito et al., 2006; McDonald & White, 1995), the representation of spatial context probably resides in the hippocampus (Holland & Bouton, 1999; Ito et al., 2006). Thus, lesions of either the dorsal hippocampus (Ferbinteanu & McDonald, 2001; Meyers et al., 2003) or the BLA (Brown & Fibiger, 1993; Everitt et al., 1991; Hiroi & White, 1991) block drug-induced CPP. We hypothesize that contextual CS-UCS associations, formed in the BLA and hippocampus, may gain control over the motor response through convergent input onto NAc neurons (see Pennartz et al., 1994 for review).
Finally, it is useful to describe our results in terms of an occasion-setting model. Critical to this consideration is our finding that saline given in the AMPH-paired and saline–paired context enhances and inhibits the expression of behavioral sensitization, respectively, by setting the occasion for the response. Whereas the AMPH-paired context reliably predicts drug administration and has taken-on occasion setting properties that act to enhance the motor response, the saline-paired context reliably predicts the absence of drug administration and has taken on occasion setting properties that act to diminish the motor response. Thus, the context-specific expression of AMPH-induced motor sensitization may, in part, be due to differential activation of the BLA-NAcC pathway by these occasion setters.
Acknowledgments
These studies were funded by a USPHS grant DA016662. The authors thank Mrs. Jennifer Jackolin for technical assistance.
Abbreviations
- AMPH
amphetamine
- BLA
basolateral amygdala
- CMS
conditioned motor sensitization
- CPP
conditioned place preference
- CS
conditioned stimulus
- CA
cornu ammonis
- DG
dentate gyrus
- DLS
dorsolateral striatum
- IHC
immunohistochemistry
- IR
immunoreactive
- MEK
mitogen-activated protein kinase kinase
- NAc
nucleus accumbens
- NAcC
nucleus accumbens core
- NAcS
nucleus accumbens shell
- S-R
stimulus-response
- TX
Triton X-100
- UCS
unconditioned stimulus
- VP
ventral pallidum
References
- Ahmed SH, Cador M, Le Moal M, Stinus L. Amphetamine-induced conditioned activity in rats: comparison with novelty-induced activity and role of the basolateral amygdala. Behav Neurosci. 1995;109:723–733. doi: 10.1037//0735-7044.109.4.723. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. doi: 10.1002/cne.21039. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychostimulant effects of cocaine and amphetamine. Brain Res. 1995;674:291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology. 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Pinna A, Russi G, Consolo S, Di Chiara G. Role of vesicular dopamine in the in vivo stimulation of striatal dopamine transmission by amphetamine: evidence from microdialysis and Fos immunohistochemistry. Neuroscience. 1995;65:1027–1039. doi: 10.1016/0306-4522(94)00507-2. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A, Wallace LJ. Amphetamine induction of c-fos in the nucleus accumbens is not inhibited by glutamate antagonists. Brain Res. 1995;694:299–307. doi: 10.1016/0006-8993(95)00794-q. [DOI] [PubMed] [Google Scholar]
- Denisova NA, Shukitt-Hale B, Rabin BM, Joseph JA. Brain signaling and behavioral responses induced by exposure to (56)Fe-particle radiation. Radiat Res. 2002;158:725–734. doi: 10.1667/0033-7587(2002)158[0725:bsabri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987;329:441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett. 1998;241:95–98. doi: 10.1016/s0304-3940(97)00962-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock JJ, Murphy S, Willoughby JO. Confocal microscopic estimation of GABAergic nerve terminals in the central nervous system. J Neurosci Methods. 2000;95:1–11. doi: 10.1016/s0165-0270(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, McNaughton BL, Everitt BJ. Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci. 2006;23:3071–3080. doi: 10.1111/j.1460-9568.2006.04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodogne C, Marinelli M, Le Moal M, Piazza PV. Animals predisposed to develop amphetamine self-administration show higher susceptibility to develop contextual conditioning of both amphetamine-induced hyperlocomotion and sensitization. Brain Res. 1994;657:236–244. doi: 10.1016/0006-8993(94)90973-3. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Liste I, Rozas G, Guerra MJ, Labandeira-Garcia JL. Cortical stimulation induces Fos expression in striatal neurons via NMDA glutamate and dopamine receptors. Brain Res. 1995;700:1–12. doi: 10.1016/0006-8993(95)00958-s. [DOI] [PubMed] [Google Scholar]
- Liu YY, Ju G. Quantitative evaluation of synaptophysin-like immunoreactive nerve terminals or varicosities in anterior pituitary of normal and adrenalectomized rats. J Neuroendocrinol. 2001;13:967–974. doi: 10.1046/j.1365-2826.2001.00720.x. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- Mead AN, Vasilaki A, Spyraki C, Duka T, Stephens DN. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur J Neurosci. 1999;11:4089–4098. doi: 10.1046/j.1460-9568.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005a;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005b;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JA, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles LP, Smith LJ, Tenn CC. Modulation of c-fos expression in the rat striatum by diazepam. Neurosci Lett. 1997;236:5–8. doi: 10.1016/s0304-3940(97)00755-6. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Ann Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes de Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophyiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss SR. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. NIDA Res Monogr. 1990;97:208–241. [PubMed] [Google Scholar]
- Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J Comp Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Anders KA, Riley MG, Nesbitt JT, Steinpreis RE. Determination of the place conditioning and locomotor effects of amperozide in rats: a comparison with cocaine. Exp Clin Psychopharmacol. 2000;8:434–443. doi: 10.1037//1064-1297.8.3.434. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse. 2006;59:374–377. doi: 10.1002/syn.20247. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–11051. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer GE, Klumperman J, Syed NI. Neurotransmitters and neurodevelopment. Role of dopamine in neurite outgrowth, target selection and specific synapse formation. Perspect Dev Neurobiol. 1998;5:451–467. [PubMed] [Google Scholar]
- Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the nervous system. Telford Press; Caldwell, NJ: 1988. pp. 207–224. [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- Sutton MA, McGibney K, Beninger RJ. Conditioned locomotion in rats following amphetamine infusion into the nucleus accumbens: blockade by coincident inhibition of protein kinase A. Behav Pharmacol. 2000;11:365–376. doi: 10.1097/00008877-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Terry P. Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav Pharmacol. 1998;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- White NM. A functional hypothesis concerning the striatal matrix and patches: mediation of S-R memory and reward. Life Sci. 1989;45:1943–1957. doi: 10.1016/0024-3205(89)90569-9. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38, 000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]


