Abstract
Myelodysplastic syndrome (MDS), previously known as preleukemia, comprises a spectrum of heterogeneous, clonal disorders of hematopoiesis. A patient’s life expectancy may range from a few months to more than a decade. Recent studies provide some insights into the pathophysiology of MDS. One mechanism contributing to the constellation of hypercellular marrow and peripheral blood cytopenia is a significant increase in programmed cell death (apoptosis) in hematopoietic cells. Tumor necrosis factor (TNF)-α, Fas-ligand, TNF-related apoptosis inducing ligand (TRAIL) and other pro-apoptotic cytokines are upregulated in early stage/low-risk MDS, and neutralization of these signals may improve hematopoiesis. TRAIL induces apoptosis preferentially in clonal cells, which may contribute to containment of the clone. In a proportion of patients, MDS will eventually evolve to acute leukemia. This progression has been correlated with upregulation of NFκB, altered expression of adaptor molecules such as Flice inhibitory protein (FLIP), and enhanced activity of anti-apoptotic members of the Bcl-2 and the inhibitors of apoptosis protein (IAP) families. Also, the ratio of TNF receptors 1 and 2 changes in favor of receptor 2. The role of the microenvironment in the pathophysiology and progression of MDS has remained controversial, although there is evidence that stroma and matrix components, and their interactions with clonal cells, play an important role. Microarray gene expression studies are consistent with dysregulation of apoptosis, but not all data are in agreement.
Introduction
The myelodysplastic syndrome (MDS) comprises a spectrum of clonal hematopoietic stem cell disorders. The French-American-British (FAB), the World Health Organization (WHO) classification, and the International Prognostic Scoring System (IPSS) have divided MDS into different stages based on marrow morphology, cytogenetic features and blood cytopenias, which confer prognostic significance [1–3]. While new insights have been gained in recent years, the precise mechanisms involved in the pathogenesis of MDS in general, and disease progression in particular, are poorly understood. There is evidence of autoimmune mediated marrow suppression in a proportion of patients, and factors in the marrow microenvironment including neoangiogenesis, cytokine signaling, and other components have been invoked [4–8]. The disease heterogeneity, the absence of pathognomonic disease markers, and the concurrent presence of normal and clonal (malignant) cells represent major hurdles to our understanding of the disease mechanisms. Further, no stable cell lines with specific MDS characteristics are available; the few cell lines with which investigators have worked have all been derived from patients in whom MDS had transformed into acute myeloid leukemia (tAML).
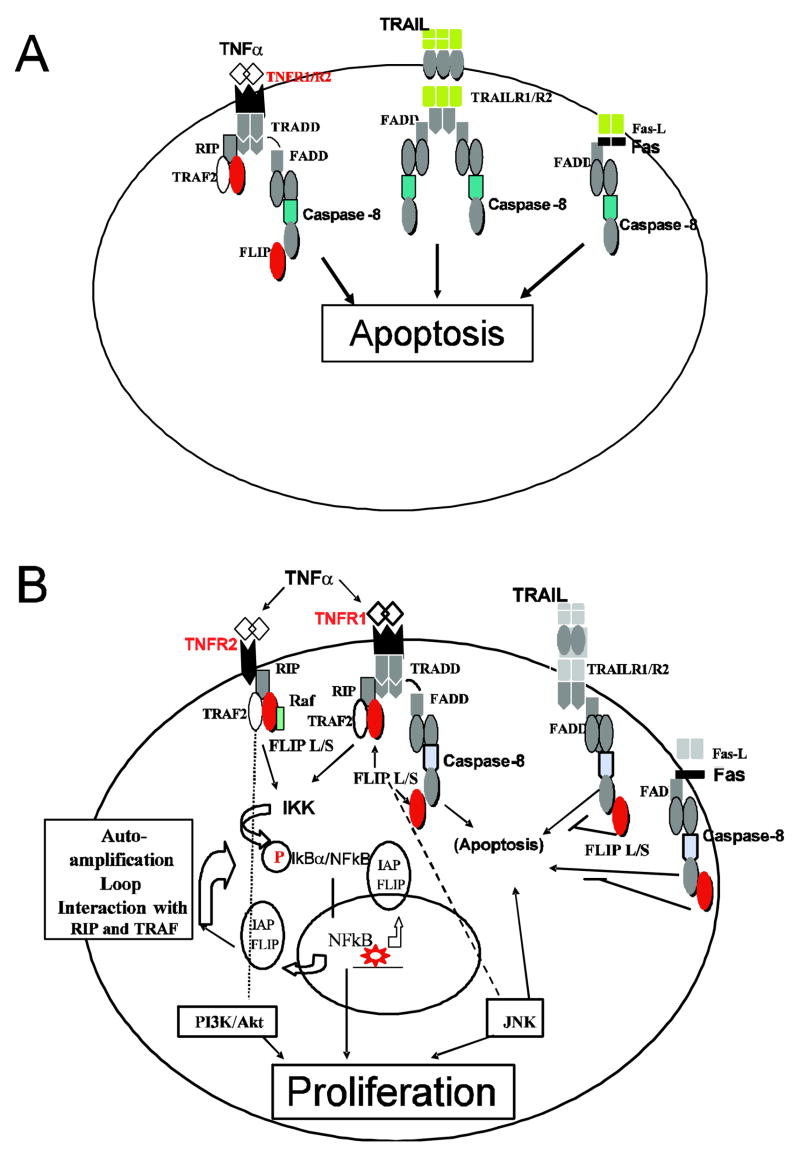
Several studies have shown increased rates of programmed cell death (apoptosis) in marrow cells of patients with low grade/early stage MDS [6,9–13] (Figure 1A). We and others described extensive dysregulation of pro-apoptotic pathways, including TNFα, Fas-ligand, and TRAIL (TNF-related apoptosis-inducing ligand)-mediated signaling in MDS [9–13]. (For abbreviations, see Table 1). FLIP (Flice [Caspase-8] inhibitory protein), an adaptor molecule that modulates TNFα-, Fas- and TRAIL-initiated signals, is abnormally expressed in MDS [14,15]. Recently we described a correlation of the expression of the transcription factor, nuclear factor kappa of B cells (NF-κB), which regulates the expression of several anti-apoptotic genes, with advanced stage MDS [16] when cells tend to be increasingly apoptosis resistant. These observations are consistent with a model in which a changing interplay of pro-apoptotic and anti-apoptotic signals is central to disease progression (Figure 1B). Such a model does not exclude, of course, a role for other factors. Here we focus our review on apoptotic pathways involved in MDS and specifically analyze evidence supporting a role of anti-apoptotic molecules, such as the Bcl-2 family proteins and the adaptor molecules that are recruited upon TNFα-, Fas- and TRAIL signaling. Other events involved in the pathophysiology of MDS and disease progression, such as epigenetic changes, oncogene expression, angiogenesis, FLT3 mutations, and telomere shortening have been reviewed elsewhere [17], and will not be addressed here.
Figure 1.
Signaling pathways involved in the regulation of apoptosis and proliferation of marrow cells in patients with MDS. In early stage disease pro-apoptotic signals predominate (1A). The interplay of pro-apoptotic, TNF/Fas/TRAIL, and anti-apoptotic signals, represented here by NF-κB and PI3K/AKT, is central to disease progression (1B).
TNF-α = tumor necrosis factor α; TRAIL = TNF-related apoptosis inducing ligand; R1/R2 = Receptor 1/Receptor 2. (For additional abbreviations, see text.)
Table 1.
Molecules involved in apoptosis and disease progression
| Molecule | Abbreviation |
|---|---|
| Tumor necrosis factor α | TNF-α |
| Tumor necrosis factor α Receptor 1/2 | TNF-α R1/R2 |
| TNF-related apoptosis inducing ligand | TRAIL |
| TNF-related apoptosis inducing ligand Receptor 1/2 | TRAILR1/R2 |
| Fas-Ligand | Fas-L |
| TNF receptor-associated death domain protein | TRADD |
| Fas-associated death domain protein | FADD |
| Receptor interacting protein | RIP |
| TNF receptor-associated factor 2 | TRAF2 |
| Flice [Caspase-8] inhibitory protein | FLIP |
| Inhibitors of apoptosis protein | IAP |
| nuclear factor kappa of B cells | NF-κB |
| IκB kinase | IKK |
| IκappaBα | IKBα |
| Phosphotidilinusitol (PI)3-kinase(PI3K)/Akt | PI3K/Akt |
MDS and Apoptosis
Ineffective hematopoiesis resulting in peripheral blood cytopenias is a hallmark of MDS, and excessive apoptosis of hematopoietic presursors in the marrow appears to be one of the underlying mechanisms. Raza et al., using in situ end labeling of fragmented DNA on marrow sections from patients with MDS, showed prominent apoptosis in 26 of 50 patients, in different MDS subtypes, including refractory anemia (RA) and RA with excess blasts (RAEB). However, in these cases apoptosis was present in differentiated cells, while myeloblasts did not appear to be involved [18]. We showed recently that apoptosis occurred primarily in non-clonal marrow cells (as determined by fluorescent in situ hybridization [FISH]), but also, albeit to a lesser extent, in clonal precursors, including myeloblasts [19].
These observations and the data on NF-κB activity are consistent with results by other investigators who showed that cells from patients with early stage MDS, including CD34+ precursors, are more prone to apoptosis, whereas marrow cells in advanced stages of MDS show less apoptosis and exhibit more proliferative features [20–24]. Such a pattern suggests that as the disease progresses, clonal cells [21,23–25] may acquire the ability to circumvent or escape the pro-apoptotic signals [20]. Rajapaska et al. showed higher apoptotic rates in CD34+ cells from patients with RA than from normal individuals and AML patients. They also observed an increase in the ratio of c-Myc (involved in apoptosis acceleration) to Bcl-2 proteins (with anti-apoptotic functions) [22]. Tsoplou et al. [21] noted that patients with higher apoptosis rates, as determined by DNA fragmentation, had a better overall survival than did patients with lower rates (median 66 months versus 30 months), and disease progression was associated with a reduction in apoptosis. The use of flow cytometry has been essential in characterizing apoptosis in distinct cell populations. Parker et al., determined apoptosis (by Annexin V staining), proliferation (by Ki-67) and Bcl-2 expression in MDS marrow and showed that the rate of apoptosis in marrow cells was higher among patients with early stage disease and always exceeded proliferation (apoptosis : proliferation ratio 2.08), while apoptosis declined and the ratio equalized with disease progression [20]. These findings support the concept that apoptosis is a central component in the pathophysiology of MDS. Regulatory molecules and cytokines that have been shown to play an essential role in this process are discussed below.
TNF-α, Fas-ligand and TRAIL mediated apoptosis
A major factor contributing to apoptosis and to hematopoietic failure in MDS appears to be the overexpression of negative regulators of hematopoiesis, such as TNFα, Fas-ligand, and TRAIL with their respective agonistic receptors. Bouscary et al. showed a significant upregulation of Fas antigen expression in CD34+, CD33+ and glycophorin + cells in patients with MDS in comparison to healthy controls [9]. Interestingly, the intensity of Fas expression on CD34+ cells correlated negatively with the number of myeloblasts, suggesting that as the disease advanced, Fas may have been down regulated. Conversely, the down regulation of pro-apoptotic signals may have led to clonal expansion and disease progression. However, in this study, no correlation was found between the rate of apoptosis and Fas expression. Evidence for upregulation of Fas and Fas-L at the message and protein levels has also been reported by others [10]. Fas and Fas-L were upregulated in CD34+, myeloid and erythroid cells, however, Fas-L was more prominent in CD68+ macrophage-derived cells than was Fas antigen. Importantly, Gersuk et al. showed that upregulation of these pro-apoptotic molecules in MDS marrow was of functional relevance, as long-term culture initiating cells (LTIC) propagated in the presence of the soluble Fas receptor, Fas-Ig, which is capable of neutralizing Fas-ligand, generated increased numbers of hematopoietic colonies, while the presence of an agonistic anti-Fas antibody resulted in reduced colony numbers [11]. In addition, and in agreement with previous data, the same investigators showed that TNF-α was upregulated in bone marrow plasma, and TNF-α levels correlated with Fas expression. Patients with RA had significantly higher TNF-α levels than did patients with RAEB or RAEB-t, and neutralization of TNF-α did enhance hematopoietic colony growth in vitro [11].
Evidence for a role of TNF-α in MDS derives from numerous additional studies. Koike et al. observed high levels of TNF-α in peripheral blood mononuclear cells from MDS patients. In agreement with our own data, levels were significantly higher in marrows from patients with RA/RARS than in RAEB/RAEB-t patients. Shetty et al. showed a positive correlation between TNF-α levels and apoptosis (p=0.0015) [26]. Kitagawa et al. demonstrated at the message level that TNF-α was upregulated in MDS in comparison to normal marrow controls: Using double immunostaining of MDS marrow biopsies, they observed that CD68+ macrophage-derived cells stained strongly positive for TNF-α, suggesting that this cell lineage was one of the sources of TNF production in MDS [27]. One additional source is activated T lymphocytes [8]. Stifter et al. also showed overexpression of TNF-α in MDS bone marrow, and levels of TNF-α positively correlated with the degree of anemia and microvessel density [27]. These data provide a rationale for the blockade of TNF-α as an approach to improve hematopoiesis in patients with MDS. In fact, in agreement with in vitro data, the soluble TNF receptor fusion protein, etanercept, showed biologic activity in MDS patients in one trial [28], albeit not in others [29,30].
The role of TRAIL, another TNF family member, in the pathophysiology of MDS has been studied less extensively. Zang et al. showed enhancement of apoptosis induced by TRAIL in MDS marrow cells, including myeloblasts [13]. The agonistic TRAIL receptors R1 and R2 were upregulated in MDS marrow cells, and the ratio between agonistic and decoy receptors was significantly higher in MDS than in marrow cells from healthy donors. Of note, TRAIL-induced apoptosis occurred primarily in clonal cells (as determined by FISH markers), particularly in patients with less advanced MDS. Resistance was noted in cells from patients with more advanced disease, again consistent with the concept that apoptosis resistance evolved with disease progression. Taken together, these findings suggested that TRAIL dysregulation was involved in the pathophysiology of MDS, and that TRAIL should be further investigated in clinical pilot trials in patients with MDS.
Several other cytokines have been shown to be overexpressed in MDS, including TGF-β, interferon-γ, IL-6 and IL-1β [26]. These observations point toward a pivotal role of these (pro)inflammatory cytokines as negative regulators of hematopoiesis in MDS.
Anti-apoptotic signals in disease progression
The mechanisms involved in leukemic transformation, which may be related to a shift of the balance of signals in favor of anti-apoptotic and proliferative signals, are essentially unknown. As discussed, Parker et al. documented increased rates of apoptosis in CD34+ cells from early stage MDS and showed at the same time that pro-apoptotic proteins such as Bax and Bad were present at higher levels than Bcl-2 and Bcl-xL, molecules with anti-apoptotic activity [20]. In more advanced MDS, however, Bcl-2 expression was significantly higher than in early stage MDS (levels were highest in patients with AML). These findings reflected prognostic features, where the Bax or Bad: Bcl-2 or Bcl-xL ratios inversely correlated with IPSS scores and cytogenetic risk groups. Boudard et al. also found that higher levels of expression of pro-apoptotic proteins, consistent with lower grade or less advanced MDS, were associated with longer life expectancy (see above) and lower risk of leukemic transformation [31]. However, the levels of expression of Bcl-2 family proteins were heterogeneous, suggesting that other factors in addition to those proteins played a role in the regulation of the apoptotic process.
We have shown, for example, that FLIP expression is dysregulated in MDS [14]. FLIP is an anti-apoptotic molecule, which exists in the form of several splice variants. It interferes with receptor-mediated activation of caspase 8, induced by TNF-α, Fas-L and TRAIL. In MDS, FLIPLong mRNA expression levels correlated negatively with apoptosis, while FLIPshort mRNA levels correlated positively with apoptosis. While FLIPLong protein levels were readily detectable in advanced disease, low levels were detected in early stage MDS, in excellent agreement with the potent anti-apoptotic activity of FLIPLong. Such a pattern would be consistent with a contribution of FLIP to disease progression [14]. However, these data have recently been challenged in a report by Campos et al. [15] who described reduced levels of FLIPshort in early stage MDS. FLIPLong and FLIPshort expression are differentially regulated and exhibit different kinetics [32]. FLIPshort is readily detectable 4 hours after TNF-α stimulation, while induction of FLIPLong is delayed. FLIPshort is also degraded more rapidly, apparently by ubiquitination mediated via its distinct 19 amino acid tail [33], which may explain differences observed at the mRNA and protein levels.
Recently, Yamamoto et al. evaluated the role of other inhibitors of apoptosis, in particular the inhibitor of apoptosis protein (IAP) family in MDS [34]. IAPs can either bind to and block caspase activation or potentiate NF-κB activation, and downstream anti-apoptotic signals [35]. The levels of expression of mRNA for survivin, cIAP1, NAIP and XIAP were significantly upregulated in MDS in comparison to control samples. In samples of patients with disease progression, survivin, and in the majority of cases, XIAP showed peak expression before leukemic transformation, suggesting a role for these molecules in the transforming process to overt leukemia [34]. Gianelli et al. also demonstrated higher levels of surviving mRNA in patients with MDS than in controls; however, mRNA levels were higher in low risk MDS than in high risk MDS. These contrasting findings are not easily reconciled and illustrate the complexity of the regulation of apoptosis [36].
FLIP, cIAPs, Bcl-2, Bcl-xL, are all under NF-κB transcriptional control [32,35,37,38]. NF-κB, which consists of homo- or heterodimers of the NF-κB/Rel family members, is activated in response to TNF-α and regulates transcription of a great diversity of genes involved in differentiation, inflammatory responses, and regulation of apoptosis and cell growth [37,39,40]. In many neoplastic cells (Hodgkin disease, lymphomas, myeloma, and acute leukemias) constitutive NF-κB activation is observed, which contributes to abnormal proliferation, resistance to apoptosis, and disease progression [38,41–44]. As TNF-α, which is up-regulated in MDS marrow, is a potent stimulus of NF-κB [45,46], and FLIP has been shown to be under NF-κB regulation, we hypothesized that this transcription factor was involved in the pathophysiology of MDS [37,40]. Our studies showed an increase in NF-κB nuclear translocation that correlated with MDS disease stage, i.e. cells from patients with more advanced MDS had higher NF-κB activity [16]. In early stage MDS, NF-κB activity was lower in CD34+ than in CD34 negative cells, consistent with a high rate of apoptosis in early (CD34+) precursors at that disease stage. In more advanced MDS, however, NF-κB activity was higher in CD34-positive than in CD34-negative cells. Such a pattern would be consistent with the upregulation of FLIP and other NF-κB dependent anti-apoptotic regulators (Bcl-xL, Bcl-2, XIAP), and resistance to apoptosis (in clonal MDS cells).
Interestingly, NF-κB activity also correlated with the extent of flow cytometric aberrancies of marrow cells, and the severity of “aberrancy” correlated inversely with survival in patients who underwent hematopoietic cell transplantation [47]. Braun et al. also found constitutive NF-κB activation in bone marrow cells from high-risk MDS patients, and its activation positively correlated with myeloblast counts [48]. These investigators showed, furthermore, that this finding was due to the constitutive activation of the IκB kinase (IKK) complex, which is formed by the IKKα, IKKβ and IKKγ/NF-κB essential modulator (NEMO) subunits. Inhibition of NEMO in CD34+ cells from high-risk MDS resulted in apoptosis [49]. These findings taken together point toward a pivotal role of NF-κB in disease progression. In fact, strategies to sensitize MDS cells to apoptosis through NF-κB inhibition are under investigation [50].
Levels of TNF-α are, as discussed, frequently upregulated in MDS but do not strictly correlate with NF-κB activity [26]. TNF-α levels are more commonly elevated in early stage disease, where NF-κB activity may be low, suggesting that an autocrine TNF-α stimulation is probably not the sole mechanism involved in the constitutive NF-κB activation. Shetty et al. hypothesized that TNF-α may have a dual role in the pathogenesis of MDS, as it could not only trigger apoptosis but also stimulate proliferation of early hematopoietic or clonal progenitors [26]. Moreover, other molecules may interfere with the role of TNF-α in the apoptosis process, including heat shock proteins that are constitutively expressed in MDS and inversely correlated with TNF-α and INF-γ induced apoptosis [51]. Another possible explanation is that NF-κB activation is not related to TNF-α levels and autocrine stimulation, but rather to “downstream” events that depend upon differential expression of adaptor molecules that shift towards anti-apoptotic mechanisms as the disease progresses. There is evidence of an autoamplification loop involving NF-κB and FLIP (see below), which may not require the continuous presence of TNF-α [52]. One additional possibility are changes in differential expression of TNF receptors as the disease evolves. While TNF R2 (p75) transmits cytoprotective signals via NF-κB, R1 (p55) transmits both cytoprotective (via NF-κB) and pro-apoptotic signals (via TRADD/FADD/Caspase-8). There appears to be a shift in the expression of TNF-α receptors R1 (p55) and R2 (p75) in favor of R2 as MDS progresses. Sawanabori et al. showed in marrow mononuclear cells that patients with RA expressed significantly higher levels of TNF R1 mRNA than controls, while patients with RAEB/RAEB-t showed significantly increased expression of R2 [53]. Our own data on TNF receptor R1 and R2 expression corroborate Sawanabori’s findings at the protein level, in both marrow mononuclear cells and CD34+ MDS cells [16]. TNF R1 overexpression in early stage MDS may favor apoptosis, consistent with higher apoptotic rates observed in early stage disease, and a shift in favor of R2 may lead to an anti-apoptotic response, mediated through NF-κB activation. In addition, Sawanabori et al. determined the mRNA expression levels of adaptor molecules involved in TNF-α signaling, including TRADD, FADD, RIP and TRAF2. In patients with RA they showed increased expression of mRNA for TRADD, FADD and RIP, while TRAF2 was decreased, which might be responsible for increasing TNF-induced apoptosis at this stage. In contrast, patients with AML showed low expression of TRADD/FADD and high expression of RIP (similar to RAEB/RAEB-t cases) and TRAF2. However, TRAF2 was not detected in a small group of four RAEB/RAEB-t patients studied. TRAF2 and RIP are required for NF-κB activation and downstream anti-apoptotic effects. Therefore, the pattern of TNF receptor expression in addition to differences in the level of adaptor molecules that mediate pro-apoptotic versus anti-apoptotic pathways may be essential for disease progression.
Other signaling pathways, such as PI3K/Akt can abrogate the apoptosis process, and have been shown to play a role in acute myeloid leukemias. In AML cells the constitutive NF-κB activation was mediated by PI3K/Akt, and blockade by the PI-3K inhibitor LY294002 abrogated NF-κB DNA binding activity. TF-1 cells, highly responsive to growth factors, when cultured with AML-conditioned medium did not activate NF-κB, and TNF-α autocrine production was thought to be unlikely the cause of NF-κB constitutive activation [54]. More recently, Nyakern et al. showed that levels of phosphorylated Akt (p-Akt) were higher in marrow mononuclear cells from patients with high risk than with low risk MDS [55]. Thus, the functional role of PI3K/Akt in MDS deserves further study. P-Akt may indeed participate in NF-κB activation, suggesting that different signaling pathways “cross-talk” and may lead to disease progression (Figure 1B). It has also been suggested that FLT3 ligand contributes to proliferation of blasts. However, determination of serum levels in 29 patients with MDS failed to show a clinical correlation with FLT3 ligand levels or with cytogenetic findings [56].
Does marrow stroma have a regulatory role?
The role of the microenvironment in the pathophysiology of MDS has remained controversial [5,7,57,58]. Certain components of the microenvironment, such as macrophages, are part of the MDS clone; fibroblast-like stromal cells or endothelial cells are not. Nevertheless, dysregulation of cytokines and functional alterations in stroma, including the in vitro “induction” of dysplastic features in normal hematopoietic precursors by MDS derived stroma, have been described [18,57]. It is generally thought that abnormalities in the stromal compartment are secondary to signals derived from marrow mononuclear cells, such as TNF-α [5].
Flores-Figueroa et al. showed functional abnormalities in macrophages and fibroblasts, including high production rates of TNF-α and IL-6 and a high apoptotic index. Stroma-induced levels of matrix metalloproteinase 9 (MMP9) in monocytes from MDS patients varied significantly and was inversely correlated with the number of clonally-derived monocytes [59]. Tennant et al. showed that adherent cell layers from MDS marrow were defective in supporting colony formation in comparison to normal layers [60], while others have shown enhanced colony formation from normal hematopoietic precursors when cultured on MDS marrow-derived supportive layers [5]. However, these layers were composed of a mix of cells rather than purified stroma cells. Tauro et al. also showed that MDS stroma was capable of supporting proliferation of hematopoietic progenitors. In vitro studies of co-cultured leukemic cell lines and a murine stroma cell line showed that apoptosis was prevented in stroma-supported leukemic cells, apparently via upregulation of Bcl-2 and Bcl-xL [61]. Studies in murine models suggest that the route of administration of MDS marrow cells, and contact with or access to human stroma cells (or their products) determine the survival and propagation of the MDS clone [62]. Gene expression profiling has provided further evidence for abnormalities in the stroma of MDS patients [63]. Thus, stroma cells, even though they are not part of the MDS clone, may support clonal proliferation. Identification of conditions that favor the clone will be important to develop effective regimens that allow for elimination of the clone in human patients.
Gene expression profiles
Several investigators have used cDNA microarray technology to determine gene expression profiles in marrow cells from patients with MDS. Qian et al. [64] showed that genes involved in cell cycle regulation, differentiation and cell growth, such as DNA-damage-inducible transcript 3 (DDIT3), and Ets variant gene 1 (ETV1) were abnormally expressed. They observed also that gene clustering could distinguish the expression profile of two clinically correlated risk groups. Moreover, some gene expression patterns appeared useful as differential markers for diagnosis [65]. The levels of RNAHP transcript, an RNA helicase, were differentially expressed in patients with RA, atypical aplastic anemia, and other hypocellular anemias. Distinctive gene expression profiles were observed among MDS cases with specific chromosome abnormalities, such as monosomy 7, trisomy 8 and del(5q) [66,67]. CD34+ cells from trisomy 8 patients showed upregulation of genes involved in immune-inflammatory responses and downregulation of genes involved in inhibition of apoptosis. These patterns correlated with the clinical behavior of these diseases and suggested that microarray analysis can provide new insights into the pathophysiology of MDS [66]. Among apoptosis-related genes TC21 (RRAS2), TNF-α induced gene (B94), and KET, genes that induce apoptosis, were up-regulated, and antiapoptotic genes such as Bcl-xL and Max were down-regulated in both trisomy 8 and monosomy 7 cases. In CD34+ cells of patients with monosomy 7, TRAIL, TRAILDR5, and FLICE, which induce apoptosis, were up-regulated. Inhibitors of apoptosis (IAP) genes, BIRC5 and NAIP, were down-regulated in trisomy 8. A possible correlation with blast count could not be evaluated.
Pellagatti et al. [67] studied CD34 cells from 55 patients with MDS and showed distinct expression patterns in specific FAB categories, such as RARS, and in cytogenetic risk groups. In RARS, the heme pathway and mitochondrial genes were differentially expressed, and in patients with del (5q), genes assigned to 5q were down-regulated as expected. There was up-regulation of many histone genes within the HITS1 gene cluster on chromosome 6p21. The other group of genes found to be up-regulated in patients with del(5q) are involved in the regulation/maintenance of the actin cytoskeleton, which are known to play a role in tumorigenesis. The most prominently up-regulated genes in this large group of patients with MDS were interferon stimulated genes, IFITM1 and IFITI. These data corroborate other findings related to overexpression of diverse cytokines in MDS that negatively regulate hematopoiesis (see above). Further, CASP3, the key effector caspase was expressed at higher levels in RA than in RAEB-2 patients. Thus, gene expression studies may provide valuable information for diagnosis and prognosis; however, further studies are needed, particularly in subgroups of patients with diagnostic uncertainties such as RA with normal karyotype. Gene expression profiling of highly purified 5q deleted CD34(+)CD38(−)Thy1(+) cells showed distinct differences in MDS and normal stem cells [68]. Molecular characterization can identify cell stage specific dysregulation. However, the diagnostic heterogeneity in MDS has limited the interpretation of the data generated by array approaches, and it should not be surprising that there are considerable discrepancies among the published data.
Summary
Studies by numerous investigators over the past decade have generated a model that suggests an important role for apoptosis in the pathophysiology of MDS. However, it is not well understood how a disease characterized by excessive apoptosis transforms into one with considerable apoptosis resistance. Here, we have summarized our current understanding of the interaction of pro- and anti-apoptotic signals in marrow cells of patients with MDS. Other studies have shown the development of new mutations in association with disease progression. Those mutations very likely contribut to disease progression and may do so by upregulating anti-apoptotic signals. Available data suggest that a complex network of cytokines, receptors, adaptor molecules and transcription factors determines the overall outcome. Gene expression profiling, at least in part, supports the interpretation of functional data; however, the broad spectrum of MDS and the limited supply of purified cells has rendered those studies difficult.
Acknowledgments
We thank Derek Stirewalt for discussions regarding gene expression profiling. We are indebted to Helen Crawford and Bonnie Larson for help with manuscript preparation.
Supported in part by HL082941, CA119599, and HL36444. DBK was also supported by a grant from the Fundação Coordenação de Aperfeiçamento de Pessoal de Nível Superior (Brazil) and by the Oncology Research Faculty Development Program of the Office of International Affairs of the NCI, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes (erratum appears in Blood 1998 Feb 1;91(3):1100) Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 3.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms (Review) Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa S, Nakano M, Iwase O, et al. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro. Leuk Res. 1999;23:239–246. doi: 10.1016/s0145-2126(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 5.Deeg HJ, Beckham C, Loken MR, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma. 2000;37:405–414. doi: 10.3109/10428190009089441. [DOI] [PubMed] [Google Scholar]
- 6.Sloand EM, Kim S, Fuhrer M, et al. Fas-mediated apoptosis is important in regulating cell replication and death in trisomy 8 hematopoietic cells but not in cells with other cytogenetic abnormalities. Blood. 2002;100:4427–4432. doi: 10.1182/blood-2002-01-0096. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy WT, Richter L, Sirjani D, et al. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427–1434. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- 8.Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99:699–705. doi: 10.1046/j.1365-2141.1997.4423249.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouscary D, De Vos J, Guesnu M, et al. Fas/Apo-1 (CD95) expression and apoptosis in patients with myelodysplastic syndromes. Leukemia. 1997;11:839–845. doi: 10.1038/sj.leu.2400654. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa M, Yamaguchi S, Takahashi M, Tanizawa T, Hirokawa K, Kamiyama R. Localization of Fas and Fas ligand in bone marrow cells demonstrating myelodysplasia. Leukemia. 1998;12:486–492. doi: 10.1038/sj.leu.2400980. [DOI] [PubMed] [Google Scholar]
- 11.Gersuk GM, Beckham C, Loken MR, et al. A role for tumor necrosis factor-α, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103:176–188. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa M, Saito I, Kuwata T, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–2054. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 13.Zang DY, Goodwin RG, Loken MR, Bryant E, Deeg HJ. Expression of tumor necrosis factor-related apoptosis-inducing ligand, Apo2L, and its receptors in myelodysplastic syndrome: effects on in vitro hemopoiesis. Blood. 2001;98:3058–3065. doi: 10.1182/blood.v98.10.3058. [DOI] [PubMed] [Google Scholar]
- 14.Benesch M, Platzbecker U, Ward J, Deeg HJ, Leisenring W. Expression of FLIPlong and FLIPshort in bone marrow mononuclear and CD34+ cells in patients with myelodysplastic syndrome: correlation with apoptosis. Leukemia. 2003;17:2460–2466. doi: 10.1038/sj.leu.2403180. [DOI] [PubMed] [Google Scholar]
- 15.de Melo Campos P, Traina F, da Silva A, et al. Reduced expression of FLIP(short) in bone marrow of low risk myelodysplastic syndrome. Leuk Res. doi: 10.1016/j.leukres.2006.11.017. In press. [DOI] [PubMed] [Google Scholar]
- 16.Kerbauy DMB, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ. NF-κB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs) Blood. 2005;106:3917–3925. doi: 10.1182/blood-2005-04-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catenacci DV, Schiller GJ. Myelodysplasic syndromes: a comprehensive review (Review) Blood Rev. 2005;19:301–319. doi: 10.1016/j.blre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Raza A, Gezer S, Mundle S, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood. 1995;86:268–276. [PubMed] [Google Scholar]
- 19.Li X, Bryant E, Deeg HJ. Simultaneous demonstration of clonal chromosome abnormalities and apoptosis in individual marrow cells in myelodysplastic syndrome. Int J Hematol. 2004;80:140–145. doi: 10.1532/ijh97.na0402. [DOI] [PubMed] [Google Scholar]
- 20.Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96:3932–3938. [PubMed] [Google Scholar]
- 21.Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC. Apoptosis in patients with myelodysplastic syndromes: differential involvement of marrow cells in ‘good’ versus ‘poor’ prognosis patients and correlation with apoptosis-related genes. Leukemia. 1999;13:1554–1563. doi: 10.1038/sj.leu.2401538. [DOI] [PubMed] [Google Scholar]
- 22.Rajapaksa R, Ginzton N, Rott LS, Greenberg PL. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996;88:4275–4287. [PubMed] [Google Scholar]
- 23.Bogdanovic AD, Trpinac DP, Jankovic GM, Bumbasirevic VZ, Obradovic M, Colovic MD. Incidence and role of apoptosis in myelodysplastic syndrome: morphological and ultrastructural assessment. Leukemia. 1997;11:656–659. doi: 10.1038/sj.leu.2400640. [DOI] [PubMed] [Google Scholar]
- 24.Parker JE, Mufti GJ. Excessive apoptosis in low risk myelodysplastic syndromes (MDS). [Review] Leuk Lymphoma. 2000;40:1–24. doi: 10.3109/10428190009054877. [DOI] [PubMed] [Google Scholar]
- 25.Tsoplou P, Kouralki-Symeonidis A, Thanopoulou E, Orphanos V, Zoumbos N. Apoptosis in patients iwth myelodysplastic syndromes (MDS): differential incolvment of marrow cells in early versus advanced cases and correlation with the expression of apoptosis-related genes [abstract] Blood. 1997;90(Suppl 1):203a–#895. [Google Scholar]
- 26.Shetty V, Mundle S, Alvi S, et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk Res. 1996;20:891–900. doi: 10.1016/s0145-2126(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 27.Stifter G, Heiss S, Gastl G, Tzankov A, Stauder R. Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol. 2005;75:485–491. doi: 10.1111/j.1600-0609.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 28.Deeg HJ, Gotlib J, Beckham C, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: A pilot study. Leukemia. 2002;16:162–164. doi: 10.1038/sj.leu.2402356. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld C, List A. A hypothesis for the pathogenesis of myelodysplastic syndromes: implications for new therapies (Review) Leukemia. 2000;14:2–8. doi: 10.1038/sj.leu.2401618. [DOI] [PubMed] [Google Scholar]
- 30.Maciejewski JP, Risitano AM, Sloand EM, et al. A pilot study of the recombinant soluble human tumour necrosis factor receptor (p75)-Fc fusion protein in patients with myelodysplastic syndrome (erratum appears in Br J Haematol 2002 Jun;117(4):1002 Note: Ristiano Antonio M [corrected to Risitano Antonio M]) Br J Haematol. 2002;117:119–126. doi: 10.1046/j.1365-2141.2002.03381.x. [DOI] [PubMed] [Google Scholar]
- 31.Boudard D, Sordet O, Vasselon C, et al. Expression and activity of caspases 1 and 3 in myelodysplastic syndromes. Leukemia. 2000;14:2045–2051. doi: 10.1038/sj.leu.2401959. [DOI] [PubMed] [Google Scholar]
- 32.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poukkula M, Kaunisto A, Hietakangas V, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005;280:27345–27355. doi: 10.1074/jbc.M504019200. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto K, Abe S, Nakagawa Y, et al. Expression of IAP family proteins in myelodysplastic syndromes transforming to overt leukemia. Leuk Res. 2004;28:1203–1211. doi: 10.1016/j.leukres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Chu Z-L, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κβ control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianello U, Francchiolla NS, Cortelezzi A, et al. Survivin expression in “low-risk” and “high-risk” myelodysplastic syndromes. Ann Hematol. 2007;86:185–189. doi: 10.1007/s00277-006-0215-0. [DOI] [PubMed] [Google Scholar]
- 37.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 38.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 41.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor κβ activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 43.Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-κB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bargou RC, Leng C, Krappmann D, et al. High-level nuclear NF-κB and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996;87:4340–4347. [PubMed] [Google Scholar]
- 45.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 46.Beg AA, Baltimore D. An essential role for NF-κβ in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 47.Wells DA, Benesch M, Loken MR, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hemopoietic stem cell transplantation. Blood. 2003;102:394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- 48.Braun T, Carvalho G, Coquelle A, et al. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood. 2006;107:1156–1165. doi: 10.1182/blood-2005-05-1989. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho G, Fabre C, Braun T, et al. Inhibition of NEMO, the regulatory subunit of the IKK complex, induces apoptosis in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26:2299–2307. doi: 10.1038/sj.onc.1210043. [DOI] [PubMed] [Google Scholar]
- 50.Fabre C, Carvalho G, Tasdemir E, et al. NF-kappaB inhibition sensitizes to starvation-induced cell death in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26:4071–4083. doi: 10.1038/sj.onc.1210187. [DOI] [PubMed] [Google Scholar]
- 51.Michalopoulou S, Micheva I, Karakantza M, Kouraklis-Symeonidis A, Mouzaki A, Zoumbos NC. Expression and inducibility of cytoprotective heat shock proteins in the bone marrow of patients with myelodysplastic syndrome: correlation with disease progression. Haematologica. 2006;91:1714–1716. [PubMed] [Google Scholar]
- 52.Micheau O, Thome M, Schneider P, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 53.Sawanobori M, Yamaguchi S, Hasegawa M, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res. 2003;27:583–591. doi: 10.1016/s0145-2126(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 54.Birkenkamp KU, Geugien M, Schepers H, Westra J, Lemmink HH, Vellenga E. Constitutive NF-κB DNA-binding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia. 2004;18:103–112. doi: 10.1038/sj.leu.2403145. [DOI] [PubMed] [Google Scholar]
- 55.Nyakern M, Tazzari PL, Finelli C, et al. Frequent elevation of Akt kinase phosphorylation in blood marrow and peripheral blood mononuclear cells from high-risk myelodysplastic syndrome patients. Leukemia. 2006;20:230–238. doi: 10.1038/sj.leu.2404057. [DOI] [PubMed] [Google Scholar]
- 56.Zwierzina H, Anderson JE, Rollinger-Holzinger I, Torok-Storb B, Nuessler V, Lyman SD. Endogenous FLT-3 ligand serum levels are associated with disease stage in patients with myelodysplastic syndromes. Leukemia. 1999;13:553–557. doi: 10.1038/sj.leu.2401378. [DOI] [PubMed] [Google Scholar]
- 57.Flores-Figueroa E, Gutiérrez-Espíndola G, Montesinos JJ, Arana-Trejo RM, Mayani H. In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndromes. Leuk Res. 2002;26:677–686. doi: 10.1016/s0145-2126(01)00193-x. [DOI] [PubMed] [Google Scholar]
- 58.Deeg HJ. Marrow stroma in MDS: culprit or bystander? Leuk Res. 2002;26:687–688. doi: 10.1016/s0145-2126(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 59.Iwata M, Pillai M, Ramakrishnan A, et al. Reduced expression of inducible gelatinase B/matrix metalloproteinase-9 in monocytes from patients with myelodysplastic syndrome: correlation of inducible levels with the percentage of cytogenetically marked cells and with marrow cellularity. Blood. 2007;109:85–92. doi: 10.1182/blood-2006-05-020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tennant GB, Walsh V, Truran LN, Edwards P, Mills KI, Burnett AK. Abnormalities of adherent layers grown from bone marrow of patients with myelodysplasia. Br J Haematol. 2000;111:853–862. [PubMed] [Google Scholar]
- 61.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 62.Kerbauy DMB, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-β2microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells (Letter to the Editor) Blood. 2004;104:2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 63.Mayani H. Abnormal stromal cells in myelodysplastic syndromes: genomics presents further evidence. Leuk Res. 2007;31:577–578. doi: 10.1016/j.leukres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Qian Z, Fernald AA, Godley LA, Larson RA, Le Beau MM. Expression profiling of CD34+ hematopoietic stem/progenitor cells reveals distinct subtypes of therapy-related acute myeloid leukemia. Proc Natl Acad Sci USA. 2002;99:14925–14930. doi: 10.1073/pnas.222491799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roela RA, Carraro DM, Brentani HP, et al. Gene stage-specific expression in the microenvironment of pediatric myelodysplastic syndromes. Leuk Res. 2007;31:579–589. doi: 10.1016/j.leukres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Chen G, Zeng W, Miyazato A, et al. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood. 2004;104:4210–4218. doi: 10.1182/blood-2004-01-0103. [DOI] [PubMed] [Google Scholar]
- 67.Pellagatti A, Esoof N, Watkins F, et al. Gene expression profiling in the myelodysplastic syndromes using cDNA microarray technology. Br J Haematol. 2004;125:576–583. doi: 10.1111/j.1365-2141.2004.04958.x. [DOI] [PubMed] [Google Scholar]
- 68.Nilsson L, Eden P, Olsson E, et al. The molecular signature of MDS stem cells supports a stem cell origin of 5q- myelodysplastic syndromes. Blood. doi: 10.1182/blood-2007-03-079368. In press. [DOI] [PubMed] [Google Scholar]