Abstract
Background
Syphilis is caused by the spirochetal pathogen Treponema pallidum. The local and systemic cellular immune responses elicited by the bacterium have not been well studied in humans.
Methods
We used multiparameter flow cytometry to characterize leukocyte immunophenotypes in skin and peripheral blood from 23 patients with secondary syphilis and 5 healthy control subjects recruited in Cali, Colombia. Dermal leukocytes were obtained from fluid aspirated from epidermal suction blisters raised over secondary syphilis skin lesions.
Results
Compared with peripheral blood (PB), blister fluids (BFs) were enriched for CD4+ and CD8+ T cells, activated monocytes/macrophages, and CD11c+ monocytoid and CD11c− plasmacytoid dendritic cells (mDCs and pDCs, respectively). Nearly all mDCs in BFs expressed the human immunodeficiency virus (HIV) coreceptors CCR5 and DC-specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN) and high levels of human leukocyte antigen (HLA)–DR. Dermal pDCs expressed both HIV coreceptors without increases in HLA-DR intensity. Compared with normal blood, circulating mDCs in patients with syphilis expressed higher levels of both CCR5 and DC-SIGN, whereas circulating pDCs in patients expressed only higher levels of DC-SIGN. Most dermal T cells were CCR5+ and displayed a memory (CD27+/CD45RO+) or memory/effector (CD27−/CD45RO+) immunophenotype. A corresponding shift toward memory and memory/effector immunophenotype was clearly discernible among circulating CD4+ T cells. Compared with PB from control subjects, a larger percentage of CD4+ T cells in PB from patients with syphilis expressed the activation markers CD69 and CD38.
Conclusions
During secondary syphilis, T. pallidum simultaneously elicits local and systemic innate and adaptive immune responses that may set the stage for the bidirectional transmission of HIV.
Syphilis is a chronic inflammatory disorder caused by the sexually transmitted spirochetal pathogen Treponema pallidum [1]. The inability to cultivate T. pallidum in vitro, coupled with the lack of a suitable inbred animal model for immunological studies, has greatly hindered efforts to elucidate the basic immunobiological aspects of syphilis. As a consequence, many questions remain unanswered about the biological characteristics of the spirochete—most importantly, why T. pallidum can persist for extended periods in tissues, despite evoking vigorous cellular and humoral immune responses [1]. Venereal syphilis also is well recognized as a risk factor for the transmission of HIV [2]. Evidence has suggested that the epidemiological synergy between syphilis and HIV infection is due not only to the association of shared high-risk behaviors or the breakdown of the epithelial barrier associated with genital ulcers [3] but also to the ability of T. pallidum and its constituent lipoproteins to activate immune cells that are likely to be present in syphilitic genital lesions [4–7].
Early studies of systemic cell-mediated responses to T. pallidum in humans often led investigators to conclude that cellular immunity is suppressed during early syphilis [8, 9]—a viewpoint that is clearly at odds with the histological abnormalities that characterize syphilitic lesions [10]. Much of our understanding about the importance of cellular immunity during early syphilis evolved from studies in the rabbit model. First, T. pallidum has been shown to induce the antigen-specific proliferation of splenic and lymph-node T cells harvested within days of infection and to maintain this high proliferative responsiveness for many weeks to months [11, 12]. Second, the appearance of T. pallidum reactive lymphocytes correlates with the progression of mononuclear cell infiltration and activated macrophages at the site of inoculation [13–15]. Last, during acute infection, high levels of interleukin (IL)–2 and interferon (IFN)–γ and low levels of IL-10 transcripts have been obtained from rabbit splenocytes incubated with treponemal sonicates or recombinant proteins [12]. Although the cellular immune response is less well understood in humans with early syphilis, available data generally support findings from the rabbit model. Immunocytochemical and reverse-transcription polymerase chain reaction analysis of biopsy specimens from primary and secondary syphilis lesions has revealed that, as in the rabbit, early syphilis infiltrates contain large numbers of macrophages and T cells and that they express mRNA for the Th1 cytokines IL-2, IFN-γ, and IL-12 [16, 17]. Interestingly, the relative proportion of CD4+ and CD8+ T cells in tissue has been reported to shift from a CD4+ predominance in chancres to a CD8+ preponderance in secondary lesions [18, 19]. The latter finding is open to question, given that cytotoxic T cells are usually not associated with the immune response to extracellular pathogens, such as T. pallidum. Two groups have analyzed circulating T cell immunophenotypes in early syphilis using flow cytometry [20, 21]. In both studies, a mild decrease in the overall percentage of CD4+ T cells, with a proportional increase in CD8+ T cells, was seen. The significance of these alterations remains unclear.
It is evident that the studies described above have provided only a bare outline of the local and systemic cellular immune response in humans with early syphilis. In the present study, we used our previously described blister fluid (BF) methodology [4, 7, 22], in conjunction with multiparameter flow cytometry, to better characterize the responses elicited in blood and skin by T. pallidum in HIV-seronegative individuals with secondary syphilis. Here, we confirm previous reports that skin lesions are composed mostly of CD4+ and CD8+ T cells, activated macrophages, and dendritic cells (DCs) [18, 19, 23] but not a predominance of CD8+ T cells. We also show that lesional T cells and DCs express CCR5, the coreceptor for sexually transmitted, M-tropic strains of HIV-1 [24] and a key chemokine receptor for leukocyte trafficking into inflamed skin, and DC-specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN), a member of the C-type lectin family involved in DC–T cell interactions and the transmission of HIV [25]. Additionally, we provide evidence of systemic T cell activation and differentiation and the prominent involvement of memory and memory effector CD4+ T cells during secondary syphilis. Taken as a whole, our results demonstrate that, during the course of early syphilis, T. pallidum has the ability to elicit coordinated innate and adaptive immune processes in blood and tissue. In addition, the resulting high level of HIV coreceptor expression in tissue-based T cells and DCs and of circulating CD4+ T cells creates an environment optimal for the bidirectional transmission of HIV.
SUBJECTS, MATERIALS, AND METHODS
Study subjects
The institutional review boards of both the University of Connecticut Health Center and Centro Internacional de Entrenamiento e Investigaciones Médicas approved the study. Informed, written consent was obtained from all 23 patients with syphilis and from 5 healthy control subjects enrolled in Cali, Colombia. Patients were identified and referred for enrollment by health care professionals from a citywide sexually transmitted disease clinic network. The diagnosis of secondary syphilis was based on a compatible history, the appearance of the skin lesions, and the results of nontreponemal and treponemal tests. Subjects (including control subjects) were excluded if they were known to be HIV positive, were receiving anti-inflammatory medications, had recently used antibiotics or immunosuppressive medications, had a history of a chronic dermatitis (i.e., eczema or psoriasis), or had other underlying acute or chronic disease. At study entry, all subjects provided baseline demographic characteristics and relevant clinical and epidemiological information by means of a standardized questionnaire. All subjects had a complete physical examination performed by a study dermatologist (A.R.C. or R.T.); both are well versed in the recognition of the manifestations of secondary syphilis. Peripheral blood (PB) was drawn for a complete blood count with differential, erythrocyte sedimentation rate, and serological tests for HIV and syphilis. This was followed, when feasible, by the elicitation of blisters over secondary syphilis lesions. All patients with syphilis received 2.4 × 106 U of intramuscular benzathine penicillin therapy, in accordance with Centers for Disease Control and Prevention treatment guidelines [26]. Patients who underwent the BF procedure were asked to return the following day for aspiration of blisters.
Serological assays
The serological tests for syphilis used were the rapid plasma reagin card test and the fluorescent treponemal antibody test absorbed. HIV ELISA serum tests were done using a standard methodology. All serological tests were performed at a reference laboratory in Colombia (Clinica Colsanitas).
Elicitation of epidermal blisters
Epidermal blisters were elicited over syphilitic lesions as described elsewhere [4, 7, 22, 27] by the application of mild suction (200 mm Hg) through acrylic cups (5 blisters/cup) applied to the skin surface and gentle warming with a 125 W infrared lamp for 2 h. Two or 3 suction cups were applied over skin lesions, depending on the location and size of the lesions. Fluid was aspirated from each blister (5 blisters/cup) within 24 h, and samples were pooled together for flow-cytometric analysis. Six patients had secondary lesions that were not amenable to blistering. We did not raise blisters over genital lesions because of both ethical and technical considerations. Blisters were not raised over unaffected healthy skin from healthy volunteers, because, in our experience, the cellular infiltrate obtained is so sparse that flow-cytometric analysis is not feasible [22].
Cell staining and flow cytometry
Erythrocyte-depleted leukocytes from PB and BF were prepared for flow-cytometric analysis using antibody panels, as described elsewhere [4, 7, 22]. A minimum of 50,000 events was collected for each staining panel performed in BF and/or PB. The high yield of cells obtained from PB, as opposed to that from BF, enabled us to examine circulating cells with more antibody panels than could be applied to skin-derived leukocytes. We therefore had to devise a strategy to sequentially perform specific panels in order of relevance to our primary objectives. We moved on to the next panel once a minimum of 5 patients with syphilis had completed each panel.
Statistical analysis
Statistical analysis was performed using GraphPad Instat software (version 3.06, 32 Bit for Windows; GraphPad Software). The Wilcoxon matched-pairs test was used to compare the median percentage of cells expressing each cell surface receptor studied between PB and BF from patients with secondary syphilis. Likewise, the Mann-Whitney U test was used to compare PB from patients with syphilis with PB from 5 healthy Colombian volunteers. All tests were 2-tailed, and P ≤ .05 was considered to indicate significance.
RESULTS
Patient enrollment and clinical data
We enrolled 31 patients, and 23 met final inclusion criteria. Two subjects (2/31) were excluded because of a previously undiagnosed HIV infection. As indicated in table 1, the mean age of subjects analyzed was 42 years (range, 19–51 years), and the male:female ratio was 1:1.6. Most subjects had minimal constitutional symptoms, and none reported fever. Although dermal lesions were universally distributed, hyperkeratotic plaques and macules on palms and soles were the most common dermatological finding. Two patients had alopecia with the classic “moth-eaten appearance,” and 2 women had multiple genital ulcers in addition to skin lesions. Most of our patients (20/23) had lymphopenia, and 3 had anemia (hemoglobin level, <11.0 g/dL). The lymphopenia was corroborated by flow-cytometric analysis and affected both CD4+ and CD8+ T cell counts without altering their ratio. Lymphopenia has been reported elsewhere in secondary syphilis [21, 28]. For those patients who were seen at follow-up 4–6 weeks later, the lymphocyte values in PB returned to normal (19.9% vs. 27.3%; P = .001), which indicates that this anomaly was directly related to the disease process.
Table 1.
Clinical and epidemiological features of study subjects.
| Characteristic | Value
(n = 23) |
|---|---|
| Male sex | 9 (39) |
| Female sex | 14 (61) |
| Age, mean (range), years | 42 (19–51) |
| Race | |
| White | 3 (13) |
| Mestizo | 7 (30) |
| Black | 13 (57) |
| Duration of signs and symptoms at enrollment, mean (range), days | 43 (30–240) |
| Dermal lesions | |
| Plaques on palms and/or soles | 11 (48) |
| Plaques other areas | 7 (30) |
| Moth-eaten alopecia | 2 (9) |
| Condyloma latum | 1 (4) |
| Genital ulcers | 2 (9) |
| RPR titer, no./total (%) | |
| >1:64 | 9/23 (39) |
| >1:16 but <1:64 | 13/23 (57) |
| >1:8 but <1:16 | 1/23 (1) |
NOTE. Data are no. (%) of subjects, unless otherwise indicated. RPR, rapid plasma reagin card test.
Cellular elements of innate and adaptive immunity in secondary syphilis skin lesions
Yields from secondary syphilis lesion BFs averaged 1.9 ×105 cells (range, 5 × 104–5 × 105 cells). As shown in figure 1, dermal mononuclear cell infiltrates contained T cells, monocytes/macrophages, and DCs but were virtually devoid of B cells. Dermal infiltrates contained very few plasma cells (0.03%) and many granulocytes (61.1%). Monocytic cells from infected skin were larger and more granular according to forward and side scatter than their circulating counterparts and expressed higher levels of CD14 (306.1 vs. 240.5 mean fluorescence intensity, P <.05), indicating that they had differentiated into macrophages. The proportions of CD4+, CD8+, and γδ T cells obtained from skin were very similar in both BF and PB (data not shown), indicating no selective recruitment of any one subset into the skin. These findings contrast with previous observations made using immunocytochemical techniques, which indicated that CD8+ T cells are the predominant lymphocyte in secondary syphilis infiltrates [18, 19].
Figure 1.
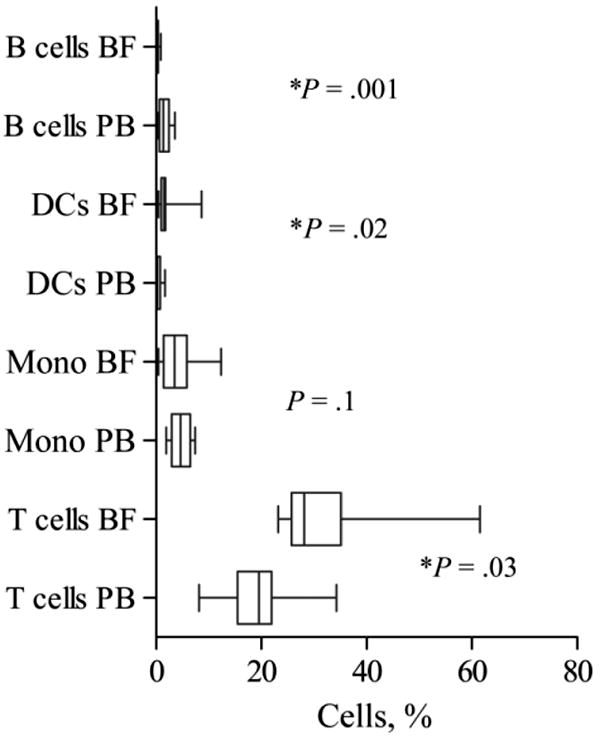
Mononuclear cell populations in blister fluid (BF) and peripheral blood (PB) obtained from patients with secondary syphilis. Circulating and dermal T cells, B cells, and monocytes (Mono) were identified on the basis of CD14, CD20, CD45, and CD38 surface expression; dendritic cells (DCs) were identified as being lineage mixture negative and HLA-DR+. List mode multiparameter files (consisting of forward and orthogonal scatter and 3 or 4 fluorescence parameters) were analyzed using PAINT-A-GATEPRO (version 3.0) software (BD Immunocytometry Systems). Median percentage, interquartile range, and both minimum and maximum values are shown for each cell population studied. The Wilcoxon signed-ranks paired test was used to determine statistical differences between median percentage values in BF and PB. P values are shown for each paired comparison.
Recruitment and activation of 2 DC subsets in both PB and skin during secondary syphilis
DCs are bone marrow–derived professional antigen-presenting cells (APCs) that initiate specific cellular immune responses [29]. Two subsets—CD11c+ monocytoid and CD11c− plasmacytoid DCs (mDCs and pDCs, respectively)—have been characterized in blood and tissues on the basis of CD11c expression [30, 31]. Both subsets were proportionately recruited from PB into skin (median percentage of CD11c+ DCs, 67.2% in PB vs. 53.64% in BF; P = .6). The median HLA-DR level quantified on dermal mDCs was 10 times higher (P = .004) than that on circulating mDCs; by contrast, pDCs did not show any increases in HLA-DR levels (P = .9). We then examined DCs for surface expression of CCR5 and DC-SIGN (figure 2). The large majority of skin-derived mDCs and approximately half of all circulating mDCs expressed CCR5; both median percentages from patients with syphilis were statistically higher than those in PB from control subjects. As with mDCs, pDCs in BF were predominantly CCR5+; however, the median percentage of CCR5+ pDCs in PB from patients with syphilis did not differ from that in PB from control subjects. The preponderance of skin-derived mDCs expressed DC-SIGN, as did a significant number of circulating mDCs. DC-SIGN surface expression was also higher in both PB- and BF-derived pDCs, and the median percentage of pDCs expressing this molecule in PB from patients with syphilis was also statistically higher than that in PB from healthy control subjects.
Figure 2.
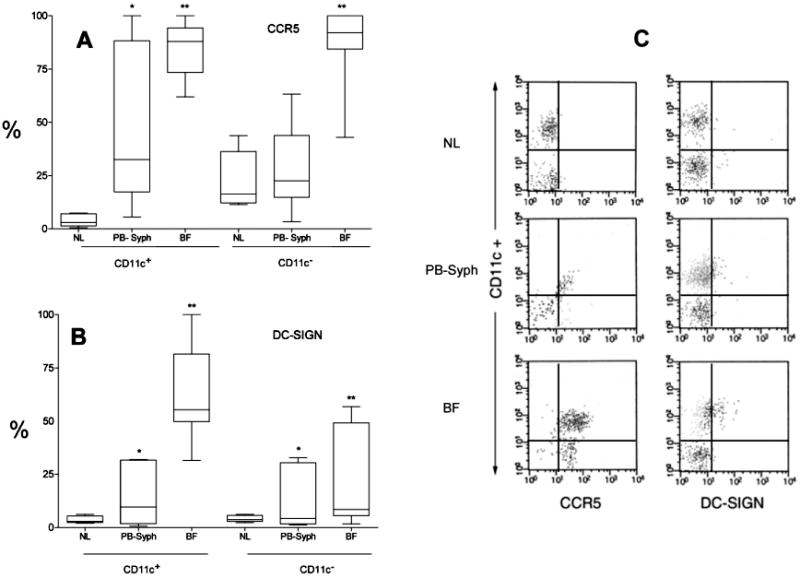
Expression of dendritic cell (DC)–specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN) (A) and CCR5 (B) on the surface of CD11c+ (monocytoid) and CD11c− (plasmacytoid) DCs obtained from secondary syphilis dermal lesions (BF) and peripheral blood (PB-syph), compared with those obtained from blood from healthy control subjects (NL). DCs were first identified as lineage mixture negative and HLA-DR+. Median percentage, interquartile range, and both minimum and maximum values are shown for each cell population studied. The Wilcoxon signed-ranks paired test was used to compare the median percentage of cells expressing each cell surface receptor expression between patients with secondary syphilis' PB and blister fluid. Likewise, the Mann-Whitney U test was used to compare PB from patients with syphilis with that obtained from 5 healthy Colombian volunteers. *P < .05, patient's skin vs. blood. **P < .05, patients' blood vs. blood from healthy control subjects. C, Representative flow-cytometric dot plot for DC CCR5 and DC-SIGN vs. CD11c surface expression on cells obtained from a normal volunteer's PB, and skin and PB from 1 patient with secondary syphilis.
Biased memory/effector T cell subsets in the skin and PB from patients with secondary syphilis
We next sought to better define the immunophenotypes of the infiltrating T cell subsets and their counterparts in PB (table 2). The majority of dermal T cells, CD4+ and CD8+, expressed CCR5. Although the median percentage of CD4+ T cells expressing CCR5 was considerably lower in PB from patients with syphilis than that in skin, it was significantly greater than that in PB from control subjects. By contrast, CCR5 expression by circulating CD8+ T cells from patients and control subjects did not differ. Also noteworthy was that, for skin-derived CD4+ and CD8+ T cells, CCR5 mean fluorescence intensity levels were significantly greater than those in PB-derived T cells (data not shown), indicating that the cutaneous milieu was not just attracting CCR5+ T cells but also inducing increased expression of this chemokine receptor. CCR5 is known to be a marker for memory T cells [32]; in this regard, it is worth noting that, in all instances, CCR5 expression was confined almost exclusively to cells expressing CD45RO (indicative of a memory immunophenotype). As another means to distinguish T cells, we doubly stained them for the expression of CD27 and CD45RO—surface markers commonly used to distinguish the naive from the antigen-experienced T subset [33]. There was an unambiguous bias in skin-derived CD4+ and CD8+ T cells toward the memory (CD27+/CD45RO+) and memory/effector (CD27−/CD45RO+) immunophenotypes. Interestingly, a shift toward the memory and memory/effector subsets also was observed for circulating CD4+, but not CD8+, T cells in PB from patients with syphilis. These results provide indirect evidence that CD4+ T cells are newly sensitized in response to the infection. To confirm this notion, we examined circulating T cells for the expression of CD27 and CD28. During T cell differentiation, the down-regulation of CD28 precedes that of CD27 [33]; thus, a decrease in the percentage of CD27+/CD28+ cells would be expected when large numbers of newly sensitized T cells are being released into the circulation. Consistent with this prediction, compared with control subjects, the median percentage of CD28+ cells was significantly lower (P = .04) within the CD4+/CD27+ subset, whereas a comparable down-regulation was not observed among the corresponding CD8+ subset.
Table 2.
T cell immunophenotype distribution in blood from healthy control subjects and in blood and dermal lesions (blister fluid [BF]) from patients with syphilis.
| Peripheral blood | |||
|---|---|---|---|
| Surface antigens | Control subjects
(n = 5) |
Patients with syphilis | BF |
| CD45RO/CCR5 | (n = 8) | (n = 8) | |
|
|
|||
| CD4+CCR5+CD45RO− | 0.4 (0.2–0.8) | 0.01 (0.0–0.4) | 0.4 (0.2–1.8)a |
| CD4+CCR5+CD45RO+ | 6.0 (4.7–8.6) | 16.1 (13.1–17.9)b | 65.9 (54.4–84.0)a |
| CD4+CCR5−CD45RO+ | 26.7 (21.8–34.5) | 47.6 (40.5–56.0)b | 20.6 (12.4–30.8)a |
| CD4+CCR5−CD45RO− | 67.2 (46.3–80.4) | 31.1 (21.0–35.4)b | 3.9 (1.1–10.5)a |
| CD8+CCR5+CD45RO− | 6.7 (4.5–8.3) | 5.4 (1.7–6.8) | 9.8 (2.5–16.7) |
| CD8+CCR5+CD45RO+ | 21.7 (20.2–26.7) | 22.9 (17.9–33.8) | 56.9 (42.7–72.6)a |
| CD8+CCR5−CD45RO+ | 15.0 (10.6–28.1) | 33.9 (30.1–38.8) | 21.2 (5.9–25.0)a |
| CD8+CCR5−CD45RO− | 52.9 (17.9–60.9) | 36.8 (24.2–45.5) | 12.5 (7.1–15.3)a |
|
|
|||
| CD27/CD45RO | (n = 6) | (n = 6) | |
|
|
|||
| CD4+CD27+CD45RO− | 65.9 (52.9–69.7) | 32.1 (21.6–41.2)b | 1.8 (0.6–8.3)a |
| CD4+CD27+CD45RO+ | 28.2 (24.9–40.0) | 52.2 (43.5–63.3)b | 62.8 (53.3–72.7)a |
| CD4+CD27−CD45RO+ | 4.9 (4.0–5.8) | 13.7 (12.2–15.8)b | 24.9 (18.6–43.4) |
| CD4+CD27−CD45RO− | 0.5 (0.3–2.5) | 0.4 (0.01–1.0) | 0.7 (0.2–2.9) |
| CD8+CD27+CD45RO− | 49.8 (42.6–62.7) | 29.6 (20.5–41.0)b | 15.5 (4.4–25.4)a |
| CD8+CD27+CD45RO+ | 35.2 (28.3–44.5) | 41.2 (32.7–50.1) | 61.3 (43.7–79.5)a |
| CD8+CD27−CD45RO+ | 3.6 (2.7–5.9) | 12.9 (7.3–19.1)b | 12.6 (8.9–19.8) |
| CD8+CD27−CD45RO− | 5.4 (3.7–12.3) | 14.0 (8.6–18.7)
|
4.8 (2.8–16.2) |
| CD27/CD28 | (n = 13) | ||
| CD4+CD27+CD28− | 0.5 (0.2–0.6) | 0.2 (0.1–2.3) | |
| CD4+CD27+CD28+ | 93.0 (91.5–95.0) | 85.8 (75.6–91.0)b | |
| CD4+CD27−CD28+ | 4.2 (3.8–6.0) | 9.0 (3.0–15.2) | |
| CD4+CD27−CD28− | 0.8 (0.1–3.8) | 4.0 (1.4–7.7) | |
| CD8+CD27+CD28− | 14.5 (9.0–16.4) | 11.3 (3.5–14.8) | |
| CD8+CD27+CD28+ | 76.0 (68.1–82.2) | 66.0 (51.5–75.5) | |
| CD8+CD27−CD28+ | 2.1 (1.1–2.6) | 2.6 (1.0–14.8) | |
| CD8+CD27−CD28− | 7.7 (5.0–14.9) | 15.4 (6.9–23.8)b | |
NOTE. Data are median percentage (25th–75th percentile).
P < .05, BF vs. peripheral blood in patients with secondary syphilis (Wilcoxon paired signed-ranks test).
P < .05, patients with secondary syphilis vs. healthy control subjects (Mann-Whitney U test).
Evidence of T cell activation in PB
To extend the T cell differentiation data presented above, we also stained PB for markers indicative of systemic T cell activation (table 3). Compared with control subjects, the mean percentage of circulating memory (CD45RO+) CD4+ T cells expressing CD69 was statistically higher (P < .05) in patients with syphilis, whereas a comparable change was not seen for CD8+ T cells. CD38, another early activation marker, was likewise increased only on CD4+ HLA-DR− T cells (P < .05). HLA-DR, on the other hand, was not elevated in either subset.
Table 3.
T cell immunophenotypes in peripheral blood from patients with secondary syphilis and healthy control subjects.
| Surface antigens | Healthy control subjects (n = 5) | Patients with secondary syphilis |
|---|---|---|
| CD69/CD45RO | (n = 9)
|
|
| CD4+CD69+CD45RO− | 0.1 (0.08–0.3) | 0.2 (0.1–1.3) |
| CD4+CD69+CD45RO+ | 0.2 (0.2–0.7) | 0.9 (0.6–3.3)a |
| CD4+CD69−CD45RO+ | 37.4 (31.3–50.6) | 53.7 (49.5–71.1)a |
| CD4+CD69−CD45RO− | 62.3 (48.7–68.2) | 39.5 (22.3–49.8)a |
| CD8+CD69+CD45RO− | 0.7 (0.4–1.4) | 1.1 (0.8–2.2) |
| CD8+CD69+CD45RO+ | 2.4 (1.5–2.5) | 2.3 (1.4–5.8) |
| CD8+CD69−CD45RO+ | 36.7 (34.5–49.4) | 35.8 (28.7–55.5) |
| CD8+CD69−CD45RO− | 59.2 (47.2–63.4) | 60.5 (35.2–66.8)
|
| HLA-DR/CD38 | (n = 12)
|
|
| CD4+CD38−HLA-DR+ | 1.3 (0.9–2.0) | 1.3 (0.8–2.4) |
| CD4+CD38+HLA-DR+ | 0.5 (0.3–2.7) | 1.9 (1.6–3.2) |
| CD4+CD38+HLA-DR− | 3.8 (2.6–5.6) | 15.4 (9.1–27.0)a |
| CD4+CD38−HLA-DR− | 94.2 (89.9–96.1) | 77.6 (67.0–88.1)a |
| CD8+CD38−HLA-DR+ | 0.3 (0.2–1.9) | 2.6 (0.5–5.0) |
| CD8+CD38+HLA-DR+ | 0.5 (0.4–8.9) | 2.1 (0.8–3.8) |
| CD8+CD38+HLA-DR− | 3.5 (1.7–13.9) | 9.2 (4.9–27.8) |
| CD8+CD38−HLA-DR− | 95.6 (75.5–98.0) | 80.8 (60.1–91.0) |
NOTE. Data are median percentage (25th–75th percentile).
P < .05, patients with secondary syphilis vs. healthy control subjects (Mann-Whitney U test).
DISCUSSION
Over the past 3 decades, evidence has emerged about the importance of innate and adaptive cellular immune responses in the immunopathogenesis of syphilis [1]. The adaptive component was first documented in the late 1970s and early 1980s with the publication of a series of experiments in the rabbit model showing that the replication of treponemes at the inoculation site elicited an intense inflammatory response resembling a classic delayed-type hypersensitivity reaction [34, 35]. Investigators also showed that the production of opsonic antibodies is essential for clearance of the bacterium from infected tissues [34, 36]. Immunohistochemical analysis revealed that syphilitic lesions in humans also contained cellular elements associated with adaptive immunity; including Th1 cytokine–producing T cells [16, 17]. More recently, the contribution of innate responses to lesion development and resolution during syphilis was studied using both in vitro and in vivo model systems [1]. Several groups have established that spirochetal lipoproteins and/or synthetic lipopeptides are capable of activating macrophages and DCs via CD14 [37–40] and Toll-like receptor (TLR) 1/TLR2–dependent signaling pathways [38, 39, 41–43]; consequently, these pathogen-associated molecular patterns are now believed to be the major proinflammatory agonists during spirochetal infection [44, 45]. In the present study, we used our previously described suction-blister model to characterize the complex interplay of innate and adaptive cellular responses elicited by T. pallidum in skin and blood from patients with secondary syphilis, the stage of the disease with the most exuberant local and systemic clinical manifestations. Because primary and secondary syphilis lesions are very similar histopathologically [16], it is reasonable to infer that findings in secondary skin can be extrapolated to the inflammatory processes elicited by spirochetes in genital ulcers.
We have previously shown that lipopeptides administered intradermally have the capacity to recruit to skin a complex mixture of cellular elements associated with innate and adaptive immunity, including neutrophils, monocytes/macrophages, DCs, and CD4+ and CD8+ T cells with memory and/or memory effector immunophenotypes [4, 7]. Here, we have shown that syphilitic dermal infiltrates have an overall similar cellular composition to those in lipoprotein injected tissues, thereby further supporting our contention that the inflammatory milieu established by spirochetal lipoproteins is a principal driving force for immune cell recruitment to T. pallidum–infected tissues. From the standpoint of innate immunity, we have confirmed previous findings that macrophages are indeed activated within syphilitic lesions [1] and, in conjunction with opsonic antibodies, are likely to be the effectors of spirochetal clearance [45]. As was previously demonstrated for erythema migrans lesions in patients with Lyme disease (LD) [22] and lipopeptideinjected skin [4, 7], both mDCs and pDCs were also present in syphilitic skin lesions. The presence of pDCs is noteworthy, because this DC subset has generally been associated primarily with antiviral immunity [30, 46], and it is not well recognized for its capability to traffic into inflamed peripheral sites, including skin [31]. Our finding that pDCs did not up-regulate HLA-DR at the site of infection is consistent with in vitro studies demonstrating that they are less able to function as APCs [30] than are mDCs, and it implies that their primary function in syphilitic lesions is not antigen presentation. On the other hand, pDCs did express higher levels of CCR5 and DC-SIGN than their counterparts in PB, indicating that they were responding to the inflammatory milieu either directly, as has been previously shown for HIV infection [46], or as a bystander effect. Their precise function has yet to be determined and needs further investigation. Superimposed on the innate response is the presence of large numbers of CCR5+, memory and memory/effector, CD4+, and CD8+ T cells that have entered a local environment replete with macrophages and mDCs. This inflammatory setting is highly favorable for secondary activation of T. pallidum–reactive T cells.
One of the most significant findings in our study is the extent of the immunological alterations seen in PB from patients with secondary syphilis. Similarly to the skin, although not as intensely, approximately one-third of circulating mDCs and pDCs expressed CCR5 and DC-SIGN. These findings suggest that the “danger signal” resulting from spirochetal dissemination triggers a systemic up-regulation of innate immune cells. With respect to adaptive immunity, immunophenotype modifications were elicited primarily for circulating CD4+ T cell subsets (see tables 2 and 3), which are associated with exogenous antigen presentation—a finding that is not surprising, given that T. pallidum is found almost exclusively extracellularly. Our results are also consistent with a model of linear T cell demarcation in which newly sensitized T cells differentiate into memory, memory/effector, and effector subsets as they circulate and traffic to sites of infection (i.e., skin) where they can interact with their cognate treponemal antigens. According to this notion, as the disease evolves from primary to secondary syphilis, it is likely that there will be progressive antigen sensitization of T cells that eventually gain the upper hand against the invading spirochete. This process in conjunction with a broadening humoral response has the potential to confer increasing degrees of resistance against reinfection. Nevertheless, additional experiments need to be done to show that CD4+ T cells are in fact specific for T. pallidum during early syphilis. Interestingly, many of our patients had lymphopenia without demonstrable modifications in the percentages of CD4+ and CD8+ T cells. In a previous report, similar degrees of lymphopenia were thought to be the result of Fas-mediated apoptosis [21]. Alternatively, lymphopenia may be the result of massive T cell recruitment into inflamed organs and tissues, such as the skin. This finding deserves confirmation and further analysis.
Finally, our results also help us to identify a number of points at which syphilis and HIV could interact at the cellular level in tissues and blood. As previously mentioned, resident and recruited mDCs and pDCs in skin were shown to express high levels of 2 key HIV coreceptors, CCR5 [24] and DC-SIGN [25, 47]. Activated DCs, which express high levels of CCR5 and DC-SIGN, could become infected or more effectively capture HIV in tissue via gp-120, respectively, and then migrate to regional lymph nodes where they may transmit the virus to CD4+ naive T cells [25]. Likewise, the abundance of CCR5+ CD4+ T cells in tissues could facilitate the direct infection by HIV without needing DCs or macrophages [48]. Additionally, increased expression of CCR5 and DC-SIGN in circulating DCs and the striking shift of CD4+ T cells expressing CCR5 within the memory (CD45RO+) immunophenotype has the potential to generate an environment in PB that is highly conducive to HIV transmission [49]. Finally, CD4+ T cell activation, may contribute to a more rapid CD4+ T cell decline, as has been seen in the context of other HIV coinfections [50].
In conclusion, our findings provide strong evidence that, during the course of secondary syphilis, T. pallidum induces a potent innate and adaptive cellular immune response in both skin and PB. Although this inflammatory response promotes clearance of the spirochete and may ultimately lead to some degree of resistance, at the same time it has the potential to create an environment optimal for the bidirectional transmission of HIV.
Acknowledgments
We thank Ana M. Obando, Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), for her assistance during all stages of the research project, including establishment of the syphilis network, patient recruitment, clinical care, and follow-up; Alisson Sombredero, CIDEIM, for her help with patient enrollment and follow-up; and Jorge Duque, Nancy Landeazabal, Gloria Aristizabal, and the late Marlene Bahos, Cali Public Health Department, for their support and advice. We are grateful to all the nurses and physicians affiliated with the Public Health Network, especially Patricia Cubillos, Giselle N. Agudelo, Lida A. Jojoa, and Carmen R. Silva. We also thank Carlos H. Quiceno, CIDEIM, for his assistance with data management and Ken Bourell, University of Connecticut Health Center, for his assistance with the preparation of the manuscript. Finally, we are indebted to Drs. Georgine Burke and Kathleen McKay, for their expert statistical advice and consultation.
Financial support: Public Health Service (grants 1 K23 AI62439-01A1 to J.C.S. and AI-38894 to J.D.R.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 16th biennial meeting of the International Society for STD Research, Amsterdam, 10–13 July 2005 (poster 791).
References
- 1.Radolf JD, Lukehart SA. Immunology of syphilis. In: Radolf JD, Lukehart SA, editors. Pathogenic treponemes: cellular and molecular biology. Norfolk, UK: Caister Academic Press; 2006. pp. 285–322. [Google Scholar]
- 2.Rompalo AM, Lawlor J, Seaman P, Quinn TC, Zenilman JM, Hook EW., III Modification of syphilitic genital ulcer manifestations by coexistent HIV infection. Sex Transm Dis. 2001;28:448–54. doi: 10.1097/00007435-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TC, Glasser D, Cannon RO, et al. Human immunodeficiency virus infection among patients attending clinics for sexually transmitted diseases. N Engl J Med. 1988;318:197–203. doi: 10.1056/NEJM198801283180401. [DOI] [PubMed] [Google Scholar]
- 4.Sellati TJ, Waldrop SL, Salazar JC, Bergstresser PR, Picker LJ, Radolf JD. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J Immunol. 2001;166:4131–40. doi: 10.4049/jimmunol.166.6.4131. [DOI] [PubMed] [Google Scholar]
- 5.Theus SA, Harrich DA, Gaynor R, Radolf JD, Norgard MV. Treponema pallidum, treponemal lipoproteins, and synthetic lipoprotein analogs (lipopeptides) induce human immunodeficiency virus-1 gene expression in monocyte/macrophages via nuclear translocation of NF-κB. J Infect Dis. 1998;177:941–50. doi: 10.1086/515240. [DOI] [PubMed] [Google Scholar]
- 6.Sellati TJ, Wilkinson DA, Sheffield JS, Koup RA, Radolf JD, Norgard MV. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J Infect Dis. 2000;181:283–93. doi: 10.1086/315209. [DOI] [PubMed] [Google Scholar]
- 7.Salazar JC, Pope CD, Moore MW, Pope J, Kiely TG, Radolf JD. Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin Diagn Lab Immunol. 2005;12:949–58. doi: 10.1128/CDLI.12.8.949-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavia CS, Folds JD, Baseman JB. Cell-mediated immunity during syphilis: a review. Br J Vener Dis. 1978;54:144–50. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner DR, Wright DJM. Lymphadenopathy in early syphilis. J Pathol. 1973;110:305–8. [Google Scholar]
- 10.Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205–16. doi: 10.1128/CMR.18.1.205-216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker-Zander SA, Fohn MJ, Lukehart SA. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J Immunol. 1988;141:4363–9. [PubMed] [Google Scholar]
- 12.Arroll TW, Centurion-Lara A, Lukehart SA, Van Voorhis WC. T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect Immun. 1999;67:4757–63. doi: 10.1128/iai.67.9.4757-4763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukehart SA, Baker-Zander SA, Cheri Lloyd RM, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980;124:461–7. [PubMed] [Google Scholar]
- 14.Sell S, Baker-Zander SA, Lloyd RM. T-cell hyperplasia of lymphoid tissues of rabbits infected with Treponema pallidum: evidence for a vigorous immune response. Sex Transm Dis. 1980;7:74–84. doi: 10.1097/00007435-198004000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Sell S, Baker-Zander S, Powell HC. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982;46:355–64. [PubMed] [Google Scholar]
- 16.Van Voorhis WC, Barrett LK, Nasio JM, Plummer FA, Lukehart SA. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect Immun. 1996;64:1048–50. doi: 10.1128/iai.64.3.1048-1050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Voorhis WC, Barrett LK, Koelle DM, Nasio JM, Plummer FA, Lukehart SA. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J Infect Dis. 1996;173:491–5. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- 18.McBroom RL, Styles AR, Chiu MJ, Clegg C, Cockerell CJ, Radolf JD. Secondary syphilis in persons infected with and not infected with HIV-1: a comparative immunohistologic study. Am J Dermatopathol. 1999;21:432–41. doi: 10.1097/00000372-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Tosca A, Lehou J, Hatjivasiliou M, Varelzidis A, Stratigos JD. Infiltrate of syphilitic lesions before and after treatment. Genitourin Med. 1988;64:289–93. doi: 10.1136/sti.64.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope V, Larsen SA, Rolfs R, Brady W, Multisite Cohort Study Group Effect of syphilis and HIV coinfection on expression of peripheral blood lymphocyte immunophenotypes [abstract L8]. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy (Orlando); Washington, DC. 1994. p. 180. [Google Scholar]
- 21.Fan YM, Zeng WJ, Wu ZH, Li SF. Immunophenotypes, apoptosis, and expression of Fas and Bcl-2 from peripheral blood lymphocytes in patients with secondary early syphilis. Sex Transm Dis. 2004;31:221–4. doi: 10.1097/01.olq.0000119172.42652.51. [DOI] [PubMed] [Google Scholar]
- 22.Salazar JC, Pope CD, Sellati TJ, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–70. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 23.Koga T, Duan H, Moroi Y, Urabe K, Furue M. Activated and mature CD83-positive dendritic cells and interferon-gamma-positive cells in skin eruptions of secondary syphilis. Acta Derm Venereol. 2003;83:214–7. doi: 10.1080/00015550310007247. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Morb Mortal Wkly Rep. 2002;51(RR6):1–78. [Google Scholar]
- 27.Picker LJ, Martin RJ, Trumble A, et al. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol. 1994;24:1269–77. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 28.Jensen JR, From E. Alterations in T lymphocytes and T-lymphocyte subpopulations in patients with syphilis. Br J Vener Dis. 1982;58:18–22. doi: 10.1136/sti.58.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 30.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 31.Narbutt J, Lesiak A, Sysa-Jedrzejowska A, Smolewski P, Robak T, Zalewska A. The number and distribution of blood dendritic cells in the epidermis and dermis of healthy human subjects. Folia Histochem Cytobiol. 2006;44:61–3. [PubMed] [Google Scholar]
- 32.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–12. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Lukehart SA, Miller JN. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978;121:2014–24. [PubMed] [Google Scholar]
- 35.Baker-Zander S, Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit: demonstration of long-lasting cellular immunity. Am J Pathol. 1980;101:387–413. [PMC free article] [PubMed] [Google Scholar]
- 36.Baker-Zander SA, Shaffer JM, Lukehart SA. VDRL antibodies enhance phagocytosis of Treponema pallidum by macrophages. J Infect Dis. 1993;167:1100–5. doi: 10.1093/infdis/167.5.1100. [DOI] [PubMed] [Google Scholar]
- 37.Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, Radolf JD. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect Immun. 1996;64:3845–52. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellati TJ, Bouis DA, Kitchens RL, et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–64. [PubMed] [Google Scholar]
- 39.Lien E, Sellati TJ, Yoshimura A, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–25. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 40.Wooten RM, Morrison TB, Weis JH, Wright SD, Thieringer R, Weis JJ. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–92. [PubMed] [Google Scholar]
- 41.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 42.Wooten RM, Ma Y, Yoder RA, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–55. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulou L, Thomas V, Schnare M, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–84. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 44.Wooten RM, Weis JJ. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr Opin Microbiol. 2001;4:274–9. doi: 10.1016/s1369-5274(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 45.Salazar J, Hazlett KRO, Radolf J. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002;4:1133. doi: 10.1016/s1286-4579(02)01638-6. [DOI] [PubMed] [Google Scholar]
- 46.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geijtenbeek TB, van Vliet SJ, van Duijnhoven GC, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1 receptor present in placenta that infects T cells in trans-a review. Placenta. 2001;22 A:S19–23. doi: 10.1053/plac.2001.0674. [DOI] [PubMed] [Google Scholar]
- 48.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 49.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–9. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]


