Abstract
Background:
Hemorrhagic Shock (HS) with conventional resuscitation (CR) primes circulating neutrophils and activates vascular endothelium for increased systemic inflammation, superoxide release and end-organ damage. Adjunctive direct peritoneal resuscitation (DPR) with intraperitoneal instillation of a clinical peritoneal dialysis solution decreases systemic inflammation and edema formation by enhancing tissue perfusion. The aim of this study is to determine the effect of adjunctive DPR on neutrophil and fluid sequestration.
Methods:
Anesthetized rats were hemorrhaged to 40% mean arterial pressure for 60 min. Animals were randomized for CR with the return of the shed blood plus two volumes of saline, or CR plus adjunctive DPR with 30 ml of intraperitoneal injection of a clinical peritoneal dialysis solution. Tissue myeloperoxidase (MPO) level, a marker of neutrophil sequestration, and total water content were assessed in the gut, lung, and liver in sham animals and at time-points 1, 2, 4 and 24 hours post-resuscitation.
Results:
Resuscitation from HS increases MPO level in all tissues in a near-linear fashion during the first 4 hours following resuscitation. This occurs irrespective of the resuscitation regimen used. Tissue MPO level returned to baseline at 24 hours following resuscitation except in the liver where CR and not adjunctive DPR caused a significant rebound increase. Adjunctive DPR prevented the CR-mediated obligatory fluid sequestration in the gut and lung and maintained a relative normal tissue water in these organs compared to CR alone (n = 7, F = 10.1, p < 0.01).
Conclusion:
Hemorrhagic shock and resuscitation produces time-dependent organ-specific trends of neutrophil sequestration as measured with tissue levels of myeloperoxidase, a marker of neutrophil infiltration. Modulation of the splanchnic blood flow by direct peritoneal resuscitation did not alter the time-dependent neutrophil infiltration in end-organs suggesting a subordinate role of blood rheology in the hemorrhage-induced neutrophil sequestration. Vulnerable window for neutrophil-mediated tissue damage exists during the first 4 hours following resuscitation from hemorrhagic shock in rats. Direct peritoneal resuscitation prevents the early obligatory fluid sequestration and promotes early fluid mobilization.
Keywords: hemorrhagic shock, peritoneal dialysis solution, neutrophils, endothelium, myeloperoxidase, peritoneal resuscitation
Introduction
High morbidity and mortality from conventionally resuscitated hemorrhagic shock and the subsequent multiple organ failure remains a very significant and a costly clinical problem [1;2]. The essence of the pathogenesis of circulatory collapse due to blood loss stems from three major events 1) a persistent and progressive splanchnic vasoconstriction and hypoperfusion [3;4], 2) a gut-derived systemic inflammatory response [5;6], and 3) an obligatory fluid sequestration and failure of early mobilization [7-10]. It is possible that an integral and cause-effect relationship exists between these three events to cause tissue injury and multiple organ failure. Studies on ligation of the mesenteric lymphatics suggested that nonbacterial soluble factors, generated in the gut and drained by the local lymphatics appear to be the mediator of distant organ injury [11-16]. However, neither the mechanism nor the mediators of such lymph's cytotoxic effect are currently known. Recent studies have shown that gut lymph-induced lung injury can be ameliorated by low-dose albumin [17], sphingosine inhibition [18], hypertonic or hyper-oncotic resuscitation [19;20], specific neutrophil elastase inhibition [21] or by specific inhibition of the Na+/H+ exchanger [22;23] presumably by mechanisms related to the detoxifying ability of the resuscitation solution, inhibition of the inflammatory signaling pathways or by modulation of white cell functions and their interaction with vascular endothelial cell. However, none of the studies cited reported an improved outcome.
Direct peritoneal resuscitation (DPR) is a resuscitation technique recently described. DPR utilizes intraperitoneal instillation of a glucose-based clinical peritoneal dialysis solution into the peritoneal cavity as an adjunct to conventional intravascular fluid resuscitation. DPR improves splanchnic and distal organ blood flow [24-26], promotes early fluid mobilization and markedly decreases the gut-derived exaggerated systemic inflammatory response; and therefore results in a remarkable improvement in survival [27]. However, in a recent intravital microscopy study of the terminal ileum we have confirmed that topical intraperitoneal application of a glucose-based clinical peritoneal dialysis solution in naive rats produced an instantaneous and generalized vasodilation at all intestinal microvascular levels. This vasodilation was associated with a marked increase in local blood flow and a significant decrease in the white blood cell-endothelium interaction. Conversely, no decrease in white blood cell-endothelium interaction was observed in rats subjected to hemorrhagic shock and resuscitated with conventional resuscitation alone or with conventional resuscitation plus adjunctive DPR [28]. In addition, regardless of the resuscitation regimen utilized, no white blood cell migration and extravasations were observed in our model. Thus, it is likely that intravital microscopy of the intestinal wall (terminal ileum) is not sensitive to visualize neutrophil extravasations because of the relatively thick tissue for Trans-illumination. For this reason, the present study was conducted to characterize the potential time dependency of neutrophil extravasations and sequestration and the possible modulation of neutrophil kinetics by adjunctive DPR from HS in rats.
Methods
Animals were maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee and Biohazard Safety Committee at the Louisville Veterans Affairs Medical Center approved the research protocol. Male Sprague–Dawley rats (Harlan, Inc. Indianapolis) were used in the experiments. Animals were acclimated for 1 to 2 weeks before experimental use, during which time each animal received 15 g of rat food per diem and water ad libitum, except for the night before the experiment. All animal and experimental interventions were performed under standard aseptic conditions. Anesthesia was induced with intraperitoneal pentobarbital (60 mg/kg), and supplemental subcutaneous injections (20% the original dose) were given as needed to maintain a surgical plan of anesthesia. Body temperature was maintained at 37 ± 0.5°C with a rectal probe and a servo-controlled heating pad. Surgery was performed after loss of blink and withdrawal reflexes. The right carotid was cannulated with PE-50 tubing for blood pressure monitoring with a pressure transducer (Digi-Med Signal Analyzers, Louisville, KY). The femoral artery was cannulated with PE-50 tubing for blood withdrawal and the femoral vein for resuscitation.
Hemorrhagic shock and resuscitation model
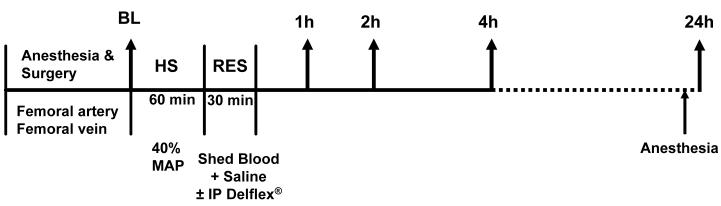
The time-line for the experimental protocol is depicted in figure 1. Hemorrhagic shock (HS) was achieved with blood withdrawal at a maximum rate of 1 ml/min, from the femoral artery in a syringe pretreated with 8 units of heparin in 0.8 ml saline. This was continued until achievement of 40 mmHg mean arterial blood pressure. This nominal mean arterial pressure was maintained for 60 minute by blood withdrawal/reinfusion as needed. On average, the blood volume withdrawn was of the order of 5.8 m1. Conventional resuscitation (CR) was achieved with the return of the shed blood over 5 minute, followed by intravenous infusion of normal saline (×2 of the shed blood volume) from an infusion pump over the subsequent 25 minutes. A conventional 2.25% dextrose-based dialysis solution (Delflex®, Fresenius USA, INC. Ogden, UT) that contained 5.67 g/L sodium chloride, 3.92 g/L sodium lactate, 0.257 g/L calcium chloride, 0.152 g/L magnesium chloride at a pH of 5.5 and an initial osmolality of 398 mOsm/L served as the DPR solution. Adjunctive DPR was achieved with 30 ml of the Delflex® solution injected intraperitoneally simultaneously with the start of CR. Tissue harvest for measurements of MPO level was achieved according to the protocol depicted in figure 1. MPO levels determined from tissues harvested immediately after anesthesia induction in naïve animals are considered reference basal levels in the present studies. At the conclusion of the experiment exsanguinations' of the animal was achieved via cardiac puncture at varying time points according to protocol at one, two, four and twenty-four hours following resuscitation and tissue samples from the lung, liver, duodenum, jejunum, and ileum were harvested. Tissues were sectioned and weighed immediately. Weights of 50 mg for the lung section and 100 mg for all other samples were selected and recorded. Samples were placed in 50 ml Corning tubes containing 2 ml chilled homogenization buffer on ice. All samples were then pureed via a polytron for 10 to 30 seconds. Sample homogenates were placed in eppendorf tubes and centrifuged at 10000× g for 10 minutes. Pellets were re-suspended in 10 volumes of re-suspension buffer using tissue weights that were previously recorded. Samples were then vortexed and stored in a −20 °C freezer. Tissue samples were collected from the gut, liver and lung. Their wet weight was determined and the tissues were then dried till constant weight.
Figure 1.
Time-line protocol. HS = Hemorrhagic shock; RES = Resuscitation; BL = Baseline for tissue harvest for MPO level; MAP = mean arterial pressure; IP = Intraperitoneal; tissue was harvested at 1, 2, 4 and 24 hour post-resuscitation for MPO level assessment.
Experimental groups
animals were randomized for a resuscitation regimen and time of tissue harvest according to the following groups (n = 7/group)
Group I: sham operation control
Group II: HS + CR harvested 1 hour post CR
Group III: HS + CR+ adjunct DPR harvested 1 hour post resuscitation
Group IV: HS + CR harvested 2 hours post resuscitation Group V: HS + CR + adjunct DPR harvested 2 hours post resuscitation
Group VI: HS + CR harvested 4 hours post resuscitation
Group VII: HS + CR + adjunct DPR harvested 4 hours post resuscitation
Group VIII: HS + CR harvested 24 hours post resuscitation
Group IX: HS + CR + adjunct DPR harvested 4 hours resuscitation
Myeloperoxidase assay
Frozen samples were allowed to thaw in a room temperature water bath. A myeloperoxidase (MPO) cocktail was prepared at pH adjusted to 5.4. A tube rack was placed in ice and filled with varying numbers borosilicate tubes depending upon the number of samples. Samples were spun briefly to repellet tissue. The supernatant then contains the myeloperoxidase. The liver contains very little MPO compared to tissue of lung and intestine, so 100 μl of liver sample were added undiluted to its appropriate representative tube. Lung tissue was diluted using 10 μl sample plus 90 μl re-suspension buffer. All intestinal samples were diluted twice with the 10:90 ratios to give 100 μl 1:100 sample. After addition of 100 μl of each sample to the appropriate tubes, 500 μl of MPO Cocktail was then added. 50 μl hydrogen peroxide was then added to begin the color change reaction. A standard curve of known values was also mixed for comparative analysis of concentrations. The tubes were shaken to accelerate mixing and placed in a 37 °C water bath. The rack was returned to the ice bath and quickly mixed with 1.0 ml of chilled acetate buffer. Colorimetry then quantified the myeloperoxidase level through use of a spectrophotometer set at 655 nm.
Total tissue water
Total tissue water and fluid sequestration were assessed from the tissue wet weight (WW) and tissue dry weight (DW) ratio. Tissues were collected at the conclusion of the experiment and their WW immediately determined. Tissue DW was determined by allowing the tissue to dry in an oven (70 °C) to a constant weight.
Data analysis and Statistics
All data is presented as mean ± SD unless stated otherwise. Differences from baseline sham control were assessed by One-way ANOVA and Dunnet post-test. Differences between groups in time points and resuscitation modality were assessed with two-way ANOVA and Bonferroni post-test. A result was considered to be significant if the probability of a type-one error was p < 0.05.
Results
There were no differences in baseline hemodynamics between the nine experimental groups (data not shown). In all experimental groups the greatest MPO level at baseline was elicited in the gut, with intermediate values in the lung and minimal MPO level detected in the liver. Tissue MPO levels (units/g tissue) assessed in animals immediately after induction of anesthesia averaged 647.8 ± 71.6 in the gut (range 218 – 1647), 169.5 ± 36.6 in the lung (range 112 – 205) and 0.737 ± 0.282 in the liver (range 0.469 – 1.23). These values are significantly higher (n = 6, p < 0.05) than the 217.6 ± 29.4 and 47.3 ± 4.4 (units/g tissue) assessed in the gut and lung respectively, at four hours following induction of anesthesia and sham operation.
MPO level
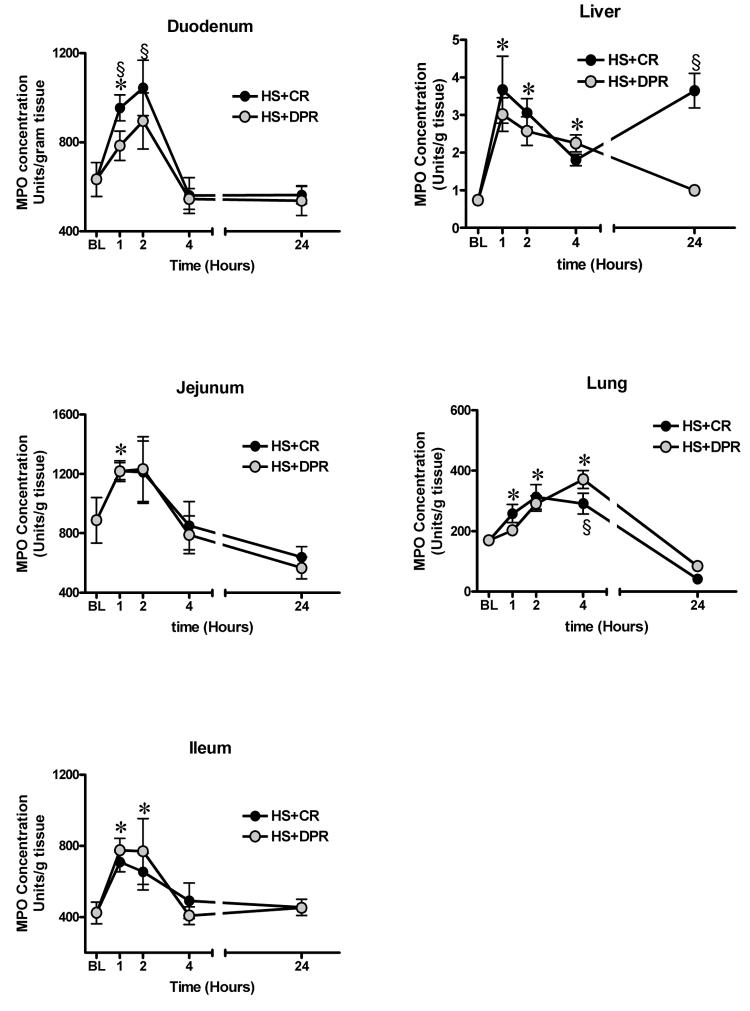
Hemorrhagic shock regardless of the resuscitation regimen caused a near linear increase over the first two hours in MPO level in all segments of the gut following resuscitation (figure 2). At 4 hour post resuscitation, gut's MPO level returned to baseline level and remained at baseline level at 24 hour following hemorrhagic shock and resuscitation (n = 7, F = 21.2, p < 0.01). This trend of time-dependency of MPO level was similar in all segments of the small intestine. Baseline MPO level was preferentially greater in the jejunum (887 ± 407 units/g tissue, n = 7, p < 0.05), compared to (423 ± 160 units/g tissue) in the terminal ileum. This preferential MPO level between the two intestinal segments was maintained within the first 4 hours following resuscitation ( n = 7, p < 0.05) regardless of the resuscitation regimen. However, at 24 hours post-resuscitation, no difference in MPO level between the jejunum and terminal ileum was observed.
Figure 2.
Tissue levels of myeloperoxidase, a marker of neutrophil sequestration. BL = Baseline; HS = Hemorrhagic shock; CR = Conventional resuscitation; DPR = Adjunctive direct peritoneal resuscitation. * p < 0.01 versus baseline sham control, § p < 0.01 versus DPR by two-way ANOVA and Bonferoni post-test.
Hemorrhagic shock regardless of the resuscitation regiment caused a linear increase in lung MPO level over the first 4 hours following resuscitation. However, at 24 hours post-resuscitation, MPO level in the lungs decreased to levels significantly below the pre-hemorrhage baseline level.
Liver MPO level demonstrated a different trend of time-dependency, with a rapid maximum increase within the first hour following resuscitation. Adjunctive DPR caused a progressive decrease in liver MPO level. However, conventional resuscitation caused a rebound increase in liver MPO level at 24 hour following resuscitation.
Total tissue water
Total tissue water was assessed from the tissue wet weight to dry weight ratio. As shown in figure 3, conventional resuscitation from hemorrhagic shock caused a significant obligatory fluid sequestration in the gut and lung, which persist during the first 2 hours following resuscitation (n = 7, F = 4.7, p < 0.01). Adjunctive DPR prevented the conventional resuscitation-mediated obligatory fluid sequestration in both organs and maintained a relative normal tissue water in these organs compared to conventional resuscitation (n = 7, F = 10.1, p < 0.01). Conventional resuscitation slightly increased liver total tissue water from sham control; however the difference between the means did not reach a statistical significance. In contrast, adjunctive DPR caused a delayed but significant decrease in total liver water between the 2 and 4 hours following resuscitation (n = 7, p < 0.01).
Figure 3.
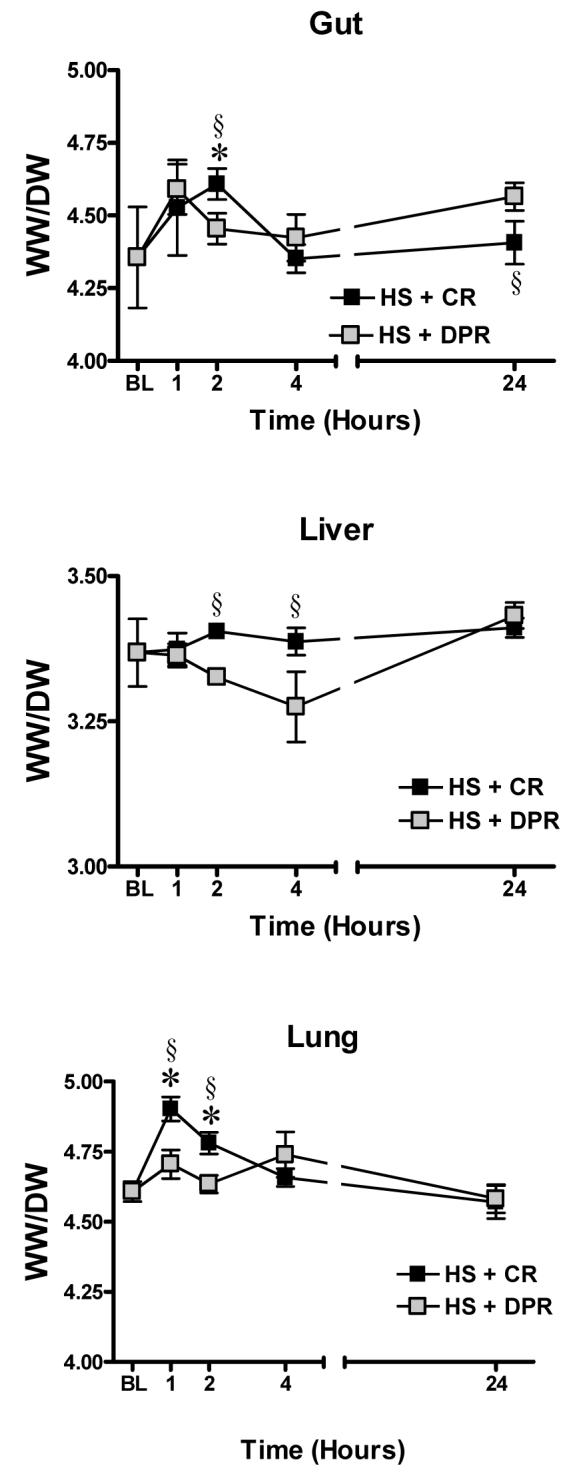
Total tissue water, a measure of fluid sequestration. BL = Baseline; WW/DW = tissue wet to dry weight ratio; HS = Hemorrhagic shock; CR = Conventional resuscitation; DPR = Adjunctive direct peritoneal resuscitation. * p < 0.01 versus baseline sham control, § p < 0.01 versus DPR by two-way ANOVA and Bonferoni post-test.
Discussion
The present studies demonstrate a time-dependency and organ-specific trends of hemorrhagic shock-induced neutrophil sequestration as measured with MPO level. This explains the desperate literature results on neutrophil sequestration studies in animal models of hemorrhagic shock and resuscitation. Neutrophil-endothelium interaction is characterized by defined steps starting with margination followed by rolling, firm adhesion, emigration, and finally migration into the interstitial space. This neutrophil-endothelium interaction is mediated by families of adhesion molecules and immunoglobulin-like receptors. Theoretically, shear forces exerted by blood flow should influence rolling neutrophil concentration and explain the predominance of this interaction with the venular rather than the arteriolar endothelium. However, our recent intravital microscopy studies of the terminal ileum have demonstrated no change in neutrophil-endothelium interaction after adjunctive DPR from hemorrhagic shock [28]. Unlike conventional resuscitation, adjunctive DPR modulates shear force by markedly increasing intestinal arteriolar blood flow and venous outflow, without the anticipated concomitant decrease in neutrophil-endothelium interaction [28]. These findings are supported by the present studies since no differences in neutrophil sequestration were found between conventional resuscitation and adjunctive DPR. Thus, our present and previous studies [28], support the notion that blood rheology is not a major determinant of neutrophil-endothelium interaction in hemorrhage with resuscitation. However, other variables such as time on anesthesia, hemorrhagic shock with resuscitation and tissue type appear to affect trends of neutrophil infiltration and tissue levels of MPO. In naïve animals there seems to be a negative correlation between the time on anesthesia and tissue levels of MPO, which are also organ specific. The mechanism of this anesthetic-induced anti-inflammatory action is not clear and can not be discerned from the present studies. Literature data suggest that application of anesthetics locally or systemically exerts anti-inflammatory effects such as attenuation of tissue MPO levels and protein extravasation in a model of chemical-induced colitis, possibly by mechanisms related to modulation of neuropeptides liberated from enteric neurons [29-31]. Following resuscitation from hemorrhagic shock, there was a near linear time-dependent and transient increase in tissue MPO levels independently from the resuscitation regimen. This MPO increase contrasts with the decrease seen in naïve animals.
It is important to note that for neutrophil-mediated tissue injury and end-organ failure to occur, neutrophils must adhere tightly to the microvascular endothelium and migrate into surrounding parenchyma, where they release toxic oxygen metabolites [32;33]. Hemorrhagic shock with resuscitation results in priming and activation of both the vascular endothelium and the circulating neutrophils. This priming results in multiple phenotype changes of both cells, such as up-regulation of cell surface related adhesion molecules, induction of pro-coagulant state and cytokines production. Evidence from trauma and hemorrhagic shock patients suggest that the severity of shock as assessed from the degree of lactic acidemia correlates directly with the degree of neutrophil priming and expression of adhesion molecules [34]. Therefore, rapid correction of the hemorrhage-induced lactic acidemia should remain the therapeutic target for a better outcome in trauma patients with severe hemorrhagic shock. Theoretically, replacement of the fluid deficit with conventional resuscitation from hemorrhagic shock, should improve cardiac filling, cardiac output, and lessen the need for increasing the peripheral resistance to sustain and maintain an effective arterial pressure. Two separate clinical studies document that despite normalization of blood pressure, heart rate, and urine output, tissue hypoperfusion persists in 80% to 85% of patients, as evidenced by lactic acidemia and decreased mixed venous oxygen saturation [35;36]. Other clinical studies have shown that the level and rate of normalization of serum lactate (index of tissue oxygen utilization) correlated with mortality both in degree of elevation and in the time-dependent rate of normalization [37;38], which support the notion that correction of post-traumatic acidemia should be the ultimate end-point of resuscitation. Systemic base deficit (index of tissue perfusion) also shows a similar predictive pattern of mortality [39]. However, interventions that focus on correction of this oxygen debt by driving oxygen transport variables, such as cardiac index or oxygen delivery index following conventional resuscitation, to supernormal levels fails to reduce mortality in severely injured patients [40;41]. Alternatively, research efforts directed at limiting neutrophils adhesion and sequestration with monoclonal antibodies against adhesion molecules yielded only limited improvements in end-organ injury without improvement of the overall outcome [42]. This data suggest that the mechanism of tissue injury and end-organ damage following resuscitation from hemorrhagic shock is complex, multi-factorial and involves mechanisms other than neutophil-mediated injury. Unfortunately, the MPO level in tissues tells us only about relative neutrophil levels but does not reflect the issue of neutrophil function within the tissue space. Similarly, Regular histology (also called thick sections) can not resolve the nuclear morphology necessary to differentiate PMNs from regular cell nuclei. Also histology is not a sensitive test to demonstrate neutrophil activity but specific to cell presence. This is the same thing that the MPO assay shows.
Previous studies in animal models of shock/resuscitation in which splanchnic vasculature and blood flow were directly monitored, have confirmed a progressive and persistent vasoconstriction and hypoperfusion despite aggressive fluid resuscitation that restored and maintained central hemodynamics [3;4;43]. This state of splanchnic and distant hypoperfusion can be promptly reversed to a state of instantaneous and continuous hyperperfusion by adjunctive DPR [24-26]. Thus, the improved overall resuscitation outcome observed with adjunctive DPR in our previous studies must be due to DPR effects other than modulation of neutrophil function. Adjunctive DPR improves the splanchnic and distant tissue perfusion, down-regulates the exaggerated systemic inflammatory response, prevents the early obligatory fluid sequestration and promotes early fluid mobilization as demonstrated in our present and previous studies [24-27]. It is important to note that in addition to conventional resuscitation (return of shed blood plus two volumes of saline), the DPR animals in the present studies received intraperitoneal instillation of 30 ml of clinical peritoneal dialysis solution (Delflex®), yet the overall organ total tissue water in the DPR animals is remarkably similar to the sham control. However, tissue edema in the present studies was assessed from total tissue water. The pattern of portioning of this total tissue water between the local intravascular, interstitial and intracellular compartments of the tissue, can not be discerned from the present data. Therefore, our measurements of total tissue water in the present studies qualify as best estimate.
In conclusion, hemorrhagic shock and resuscitation produces time-dependent organ-specific trends of neutrophil sequestration as measured with tissue levels of myeloperoxidase, a marker of neutrophil infiltration. Modulation of splanchnic blood flow by direct peritoneal resuscitation did not alter the time-dependent neutrophil infiltration in end-organs suggesting a subordinate role of blood rheology in the hemorrhage-induced neutrophil sequestration. Vulnerable window for neutrophil-mediated tissue damage exists during the first 4 hours following resuscitation from hemorrhagic shock in rats. Direct peritoneal resuscitation prevents the early obligatory fluid sequestration and promotes early fluid mobilization.
Acknowledgements
This project was supported by a VA Merit Review grant and by NIH research Grant # 5R01 HL076160-03, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann. Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Zakaria ER, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–5. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr. Opin. Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Lucas CE. The water of life: a century of confusion. J Am Coll Surg. 2001;192:86–93. doi: 10.1016/s1072-7515(00)00761-4. [DOI] [PubMed] [Google Scholar]
- 8.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ. Shock. 1989;29:27–39. [PubMed] [Google Scholar]
- 9.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr., Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl. Physiol. 1992;73:1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 10.Moon PF, Hollyfield-Gilbert MA, Myers TL, Kramer GC. Effects of isotonic crystalloid resuscitation on fluid compartments in hemorrhaged rats. Shock. 1994;2:355–361. doi: 10.1097/00024382-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Adams CA, Jr., Sambol JT, Xu DZ, Lu Q, Granger DN, Deitch EA. Hemorrhagic shock induced up-regulation of P-selectin expression is mediated by factors in mesenteric lymph and blunted by mesenteric lymph duct interruption. J. Trauma. 2001;51:625–631. doi: 10.1097/00005373-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Adams JM, Hauser CJ, Adams CA, Jr., Xu DZ, Livingston DH, Deitch EA. Entry of gut lymph into the circulation primes rat neutrophil respiratory burst in hemorrhagic shock. Crit Care Med. 2001;29:2194–2198. doi: 10.1097/00003246-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 14.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann. Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore EE. Mesenteric lymph: the critical bridge between dysfunctional gut and multiple organ failure. Shock. 1998;10:415–416. [PubMed] [Google Scholar]
- 16.Moore E, Moore FA, Franciose RJ, Kim FJ, Biffl WI, Banerjee A. Postischemic gut serves as a priming bed for circulation neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881–887. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Osband AJ, Deitch EA, Hauser CJ, Lu Q, Zaets S, Berezina T, Machiedo GW, Rajwani KK, Xu DZ. Albumin protects against gut-induced lung injury in vitro and in vivo. Ann. Surg. 2004;240:331–339. doi: 10.1097/01.sla.0000133359.12284.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Xu DZ, Feketeova E, Kannan KB, Yun JK, Deitch EA, Fekete Z, Livingston DH, Hauser CJ. Attenuation of shock-induced acute lung injury by sphingosine kinase inhibition. J. Trauma. 2004;57:955–960. doi: 10.1097/01.ta.0000149495.44582.76. [DOI] [PubMed] [Google Scholar]
- 19.Powers KA, Zurawska J, Szaszi K, Khadaroo RG, Kapus A, Rotstein OD. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66–74. doi: 10.1016/j.surg.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 20.Powers KA, Kapus A, Khadaroo RG, Papia G, Rotstein OD. 25% Albumin modulates adhesive interactions between neutrophils and the endothelium following shock/resuscitation. Surgery. 2002;132:391–398. doi: 10.1067/msy.2002.126508. [DOI] [PubMed] [Google Scholar]
- 21.Toda Y, Takahashi T, Maeshima K, Shimizu H, Inoue K, Morimatsu H, Omori E, Takeuchi M, Akagi R, Morita K. A neutrophil elastase inhibitor, sivelestat, ameliorates lung injury after hemorrhagic shock in rats. Int J Mol. Med. 2007;19:237–243. [PubMed] [Google Scholar]
- 22.Fujiyoshi N, Deitch EA, Feketeova E, Lu Q, Berezina TL, Zaets SB, Machiedo GW, Xu DZ, Hasko G. Amiloride combined with small-volume resuscitation with hypertonic saline is superior in ameliorating trauma-hemorrhagic shock-induced lung injury in rats to the administration of either agent alone. Crit Care Med. 2005;33:2592–2598. doi: 10.1097/01.ccm.0000186770.59312.44. [DOI] [PubMed] [Google Scholar]
- 23.Fujiyoshi N, Feketeova E, Lu Q, Xu DZ, Hasko G, Deitch EA. Amiloride moderates increased gut permeability and diminishes mesenteric lymph-mediated priming of neutrophils in trauma/hemorrhagic shock. Surgery. 2006;140:810–817. doi: 10.1016/j.surg.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria ER, Garrison RN, Spain DA, Matheson PJ, Harris PD, Richardson JD. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–713. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakaria ER, Hurt RT, Matheson PJ, Garrison RN. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am. J. Surg. 2003;186:443–448. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Zakaria ER, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J. Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrison RN, Conn AA, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–908. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Campbell JE, Garrison RN, Zakaria ER. Clinical peritoneal dialysis solutions modulate white blood cell-intestinal vascular endothelium interaction. Am J Surg. 2006;192:610–616. doi: 10.1016/j.amjsurg.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chevalier E, Petoux F, Chovet M, Langlois A. Beneficial effect of trimebutine and N-monodesmethyl trimebutine on trinitrobenzene sulfonic acid-induced colitis in rats. Life Sci. 2004;76:319–329. doi: 10.1016/j.lfs.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 30.McCafferty DM, Sharkey KA, Wallace JL. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol. 1994;266:G560–G567. doi: 10.1152/ajpgi.1994.266.4.G560. [DOI] [PubMed] [Google Scholar]
- 31.McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol. 1997;272:G272–G280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- 32.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc. Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 33.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 34.Botha AJ, Moore FA, Moore EE, Peterson VM, Goode AW. Base deficit after major trauma directly relates to neutrophil CD11b expression: a proposed mechanism of shock-induced organ injury. Intensive Care Med. 1997;23:504–509. doi: 10.1007/s001340050365. [DOI] [PubMed] [Google Scholar]
- 35.Scalea TM, Maltz S, Yelon J, Trooskin SZ, Duncan AO, Sclafani SJ. Resuscitation of multiple trauma and head injury: role of crystalloid fluids and inotropes. Crit Care Med. 1994;22:1610–1615. [PubMed] [Google Scholar]
- 36.Abou-Khalil B, Scalea TM, Trooskin SZ, Henry SM, Hitchcock R. Hemodynamic responses to shock in young trauma patients: need for invasive monitoring. Crit Care Med. 1994;22:633–639. doi: 10.1097/00003246-199404000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35:584–588. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Broder G, Weil MH. Excess lactate: An index of reversibility of shock in human patients. Science. 1964;143:1457–1459. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 39.Rutherford EJ, Morris JA, Jr., Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Dunham CM, Siegel JH, Weireter L, Fabian M, Goodarzi S, Guadalupi P, Gettings L, Linberg SE, Vary TC. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit Care Med. 1991;19:231–243. doi: 10.1097/00003246-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24:517–524. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 42.Koike K, Moore EE, Moore FA, Franciose RJ, Fontes B, Kim FJ. CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion. J Trauma. 1995;39:23–27. doi: 10.1097/00005373-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Scalia SV, Taheri PA, Force S, Ozmen V, Lui D, Fish J, Hansen D, Chambers R, Flint L, Steinberg S. Mesenteric microcirculatory changes in nonlethal hemorrhagic shock: the role of resuscitation with balanced electrolyte or hypertonic saline/dextran. J Trauma. 1992;33:321–5. [PubMed] [Google Scholar]