Abstract
Background
Hemorrhage-induced activation of endothelial cell Na+/H+-exchanger results in cellular swelling, which physically impedes capillary filling and compromises gut perfusion. We hypothesized that correction of the vascular volume deficit by conventional resuscitation does not improve capillary filling unless cellular swelling is prevented. Also, we hypothesized that adjunctive direct peritoneal resuscitation (DPR) with topical peritoneal dialysis solution (Delflex; Fresenius USA, Inc., Ogden, Ut) enhances capillary filling and gut perfusion by mechanisms that are independent of the Na+/H+ function.
Methods
In vivo intravital videomicroscopy and Doppler velocimeter were used by us to measure microvascular diameter and flow, capillary filling (index of functional capillary density, FCD), and endothelial cell function in the terminal ileum of anesthetized rats. Rats were bled to 50% mean arterial pressure for 60 min and resuscitated with the shed blood plus 2 volumes of saline (conventional resuscitation). Prevention of endothelial cell swelling was achieved with topical amiloride (specific Na+/H+ inhibitor) in the tissue bath before hemorrhage or simultaneously with conventional resuscitation. DPR was simulated by instillation of Delflex in the tissue bath as adjunctive to conventional resuscitation. Sham no hemorrhage group and a simulated DPR group that received topical amiloride treatment served as controls.
Results
Conventional resuscitation from hemorrhagic shock restored and maintained central hemodynamics but caused progressive and persistent intestinal vasoconstriction and hypoperfusion associated with low FCD and endothelial cell dysfunction. Prevention of endothelial cell swelling when combined with conventional resuscitation, preserved endothelial cell function, and restored local intestinal microvascular variables to near-prehemorrhage levels. Simulated adjunctive DPR produced rapid, sustained, and generalized vasodilation associated with restoration of endothelial cell function, and maximum recruitment of FCD independent of the Na+/H+-exchanger function.
Conclusions
Paradoxical endothelial cell swelling occurs early during hemorrhagic shock because of activation of the Na+/H+ exchanger. This cellular edema, which is not resolved by correction of the vascular volume deficit, explains the persistent postresuscitation endothelial cell dysfunction and gut hypoperfusion. Simulated adjunctive DPR in this study reversed endothelial cell swelling and enhanced gut perfusion by mechanisms that are independent of the Na+/H+ exchanger activity.
High morbidity and mortality from trauma and hemorrhagic shock remains a significant and costly clinical problem.1 Hemorrhagic shock results in systemic endothelial cell activation associated with redistribution of the cardiac output, profound microvascular derangements, and inadequate end-organ perfusion. Conventional resuscitation from hemorrhagic shock rapidly corrects the intravascular volume deficit and restores and maintains central hemodynamics, but it fails to restore fully splanchnic end-organ perfusion to meet local metabolic demand. Central to the shock-induced peripheral microvascular derangement is compromised capillary filling and decreased functional capillary density (FCD). Previous studies have demonstrated that these capillary events are caused by a paradoxical vascular endothelium swelling prompted by hemorrhage-induced activation of the membrane-bound Na+/H+ exchanger.2 The Na+/H+ exchanger is an electro-neutral ion channel that plays a pivotal role in the regulation of intracellular volume and pH. Maintenance of intracellular volume is critical to maintaining cellular morphology and cell membrane integrity. In addition to endothelial cell swelling, studies show that hemorrhagic shock prevents high-yield ATP generation from oxidative phosphorylation, which leads to a profound decrease in cellular levels of high-energy phosphates.3-5 Thus, all energy-dependent processes, including active and passive membrane transport at the capillary level, are impaired severely. Similar abnormalities occur in the membrane-bound organelles such as lysosomes and mitochondria. Alternative energy production by anaerobic glycolysis is thus stimulated from a low-energy charge with the resultant lactic acidemia and low intracellular pH.
Hemorrhage-induced activation of the Na+/H+ exchanger exchanges accumulated intracellular H+ for extracellular Na+. This process is paralleled with Cl−/HCO3− exchange across the cytoplasm to reduce cellular swelling and correct intracellular pH. Recent studies based on this mechanistic premise, and conducted with systemic inhibition of the Na+/H+ exchanger during resuscitation from hemorrhagic shock, demonstrated a modest attenuation of end-organ damage by unknown mechanisms.6-8 Better end-organ protection was obtained with combined Na+/H+ exchanger inhibition and hypertonic saline resuscitation.6,7 it is important, however, to emphasize that hemorrhage alters the ability of the cell membrane to regulate the interchange of ions between the cell and its immediate microenvironment. This phenomenon is global and not restricted to the vascular endothelium. Sentinel studies by Shires et al9-11 demonstrated doubling of skeletal muscle intracellular Na+ ions after hemorrhagic shock by ion exchange mechanisms other than the Na+/H+ exchanger. Given the large volume of distribution of the body's intracellular compartment, the sizable Na+ influx into this compartment during shock could be a plausible explanation for the Na+ deficit observed in shock and in trauma and operative patients who require fluid therapy.9-11
Adjunctive direct peritoneal resuscitation (DPR) is a newly developed resuscitation technique aimed at restoring microvascular function and tissue perfusion. DPR is accomplished by intraperitoneal instillation of a glucose-based clinical peritoneal dialysis as an adjunct to conventional resuscitation from hemorrhagic shock. DPR enhances splanchnic and distant organ perfusion,12-14 downregulates the exaggerated systemic inflammatory response, prevents fluid sequestration, promotes early fluid mobilization, and results in improved survival.15 The mechanistic objectives of the current study required that the physiologic conditions of the tissue, a small segment of the terminal ileum, be carefully controlled in a tissue bath. Therefore DPR was simulated by the addition of Delflex solution (Fresenius USA, Inc., Ogden, Ut) to the tissue bath, which allowed for direct, real-time observation of the microvasculature under specific experimental interventions.
Methods
Animal preparation
Male Sprague-Dawley rats (Harlan, Ind) (200-215 g) were used for this study. Animals were maintained in a facility approved by the American Association for the Accreditation of Laboratory Animal Care. Animals were acclimated for 2 weeks before the experimental use and received a standard rat show (15 g/day) and water ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee and Biohazard Safety Committee at the Veteran's Administration Hospital, Louisville, Ky. Anesthesia was induced with intraperitoneal pentobarbital (50 mg/kg) and supplemented hourly by subcutaneous injections equal to 25% of the initial dose to maintain a surgical plane of anesthesia throughout the experimental protocol. Before the surgical preparation, each animal received 2 mL of normal saline subcutaneously to compensate for body fluid loss during surgical preparation and equilibration periods. Body temperature was maintained throughout the experiment at 37 ± 0.5°C with a rectal probe and a servo-controlled heating pad. Surgery was performed after loss of blink and withdrawal reflexes. Tracheostomy was performed to reduce airway resistance, and the animals were allowed to breathe spontaneously. PE-50 catheters were inserted into the right femoral artery and vein for blood withdrawal and administration of the resuscitation fluids, respectively. The right carotid artery was cannulated to allow for a continuous monitoring and recording of blood pressure on a pressure measurement system (Digi-Med, Louisville, Ky).
In vivo intestinal microvascular preparation
The peritoneal cavity was exposed through a midline abdominal incision, and a segment of the terminal ileum was selected for the study. The selected segment was exteriorized with its neurovascular supply intact. Two ligatures were placed on the selected segment at a distance of 2 cm apart to exclude collateral circulation, and the segment was opened along the anti-mesenteric border with electrocautery. Enteric contents were washed gently from the mucosal surface, and the segment was suspended with 4-0 silk sutures, serosal side up, over an optical port of a Plexiglas tissue bath. During this surgical procedure, the tissue was bathed continually in a modified nonvasoactive (no-glucose Krebs) solution and bubbled with CO2 to regulate bath pH at 7.40 ± 0.05 and N2 to maintain a physiologic level of O2 within the tissue bath. Tissue temperature was maintained at 37.0 ± 0.5°C by thermistor and a feedback-controlled heating coil. Isoproterenol was added to the bath solution at a very low concentration (0.01 μg/mL) to retard the peristalsis. This dose of isoproterenol is below the threshold affecting the vascular smooth muscle tone.16 The rat and tissue bath were positioned on the stage of a trinocular Zeiss microscope, and 45 min was allowed for the animal to recover from the surgical stress and for hemodynamics and microvascular equilibration. Animals were considered to be equilibrated when hemodynamic parameters remained within 5% of each other over a 10-min period. Centerline red blood cell velocity in the intestinal A1 inflow arteriole was measured online by an optical Doppler velocimeter (Microcirculation Research Institute, Texas A & M University, College Station, Tex). Intestinal microvascular images displayed in real time on a high-resolution computer monitor were recorded via a closed-circuit charge-coupled device camera (KP-D50 Color Digital Camera; Hitachi Denshi, Ltd., Tokyo, Japan), desktop computer (Gateway Inc., North Sioux City, Sd) and digital video recorder (D-R4SU DVD video recorder; Toshiba, Tokyo, Japan) for off-line measurements of vascular diameter using calipers. Criteria for an intestinal preparation acceptable for intravital videomicroscopy included a baseline mean arterial pressure (MAP) >90 mm Hg, red blood cell velocity in first-order arterioles >20 mm/s, and an active vasomotion in the arteriolar system. Animals that do not meet all these criteria during a 60-min equilibration period were scarified and not used in the study.
Definition of intestinal microvessels and perfused capillaries
The microvascular anatomy was identified according to the nomenclature of Bohlen and Gore.16 Briefly, first-order arteriole (A1) originates from a mesenteric arcade artery and traverses the mesenteric border of the bowel wall, penetrating through the muscle layers to the submucosal layer. In the submucosa, second-order arterioles (A2) originate from A1 and run along the longitudinal axis of the bowel. First- and second-order venules parallel the A1 and A2 arterioles. Third-order arterioles (A3) branch at right angle from A2 arterioles and continue on to terminate in the mucosa as central villus arterioles. Along their course, the A3 arterioles also give rise to smaller arterioles that supply the seromuscular layers of the bowel wall. Centerline red blood cell velocity in A1 arterioles was measured with optical Doppler velocimetry. The maximal velocity signal, displayed digitally, was used to calculate A1 blood flow according to the formula: (V/1.6) × (R2 × 0.001), where V is the centerline flow velocity, 1.6 is a correction factor that converts centerline velocity to average cross-sectional velocity, R is the intraluminal microvascular radius in micrometers, and 0.001 is a conversion factor to express flow in nL/s. This equation assumes a parabolic flow velocity and a circular conduit. Studies have identified 1.58-1.60 as the ideal correction factor for a wide range of microvessels.
The number of continuously perfused capillaries (index of functional capillary density, FCD) was counted directly in 5 microscopic fields, and the average was obtained at each time-point.17,18 To directly measure FCD, the microscopic stage was driven manually through a meander consisting of 2 steps of 0.9 mm in the x-direction and 2 steps of 0.55 mm in the y-direction. The microscopic image was recorded at each of these 5 positions. FCD was determined for each microscopic image, and the average was calculated. Only capillaries with active and sustained flow were included in the analysis. Endothelial cell function and maximal dilation capacity were assessed at the conclusion of the experiment, from the topical application of the endothelium-dependent, receptor-dependent, and the endothelium-independent, receptor-independent acetylcholine (ACh, 10−5 M) and sodium nitroprusside (SNP, 10−4 M), respectively.
Experimental protocol
The timeline for the experimental protocol is shown in Fig 1. After surgical preparation, 45 min was allowed for systemic equilibration and recovery of the animal from surgical stress. Throughout the experiment, the exteriorized ileum in the tissue bath was bathed continuously in Krebs solution. Blood pressure, heart rate, rectal and bath temperatures, and bath pH were monitored continuously and recorded every 5 min (Digi-Med, Louisville, KY). Baseline microvascular measurements were measured continuously during the equilibration period, and they were considered valid when the variability in the measurements within a 10-min interval was <5%. Measurements included mean arterial pressure (MAP), heart rate, rectal and bath temperatures, bath pH, and diameters of the intestinal inflow A1 arteriole, A2 arteriole, A3 pre-mucosal arteriole proximally (pA3) and distally (dA3), and centerline red cell velocity in the A1 arteriole. Hemorrhagic shock was initiated and maintained by blood withdrawal from the femoral artery into a syringe containing 1 mL of normal saline and 8 units of heparin (minimal amount to prevent blood clotting) at a rate of 1 mL/min. Blood withdrawal continued until 50% of baseline MAP was attained. The nominal 50% MAP was maintained for 60 minutes with further blood withdrawal or reinfusion as required. On average, the total volume of the withdrawn blood was 5.3 ± 0.6 mL. After 60 min of hemorrhagic shock, animals were resuscitated with the return of shed blood over 5 min, followed by normal saline (2X the shed blood volume) infused intravenously over the subsequent 25 min. To simulate DPR, the Krebs solution was withdrawn from the tissue bath and replaced with 60 mL of prewarmed, glucose-based, clinical peritoneal dialysis solution (Delflex). Microvascular and hemodynamic data acquisition was continued at 20-min intervals during shock and a subsequent 120 min postresuscitation. Na+/H+ exchanger inhibition was achieved with amiloride (10 μmol/L) applied topically in the tissue bath. This amiloride dose was chosen from previous studies that found no difference between intravenous or topical application of the drug.2 Topical amiloride application was initiated simultaneously with resuscitation (Groups II and V) or preemptively at the beginning of hemorrhagic shock in Group III. At 120 min after resuscitation, a single dose of acetylcholine (ACh, 10−5 M), an endothelium-dependent, receptor-dependent agonist was added to the tissue bath to determine endothelial cell function.
Fig 1.
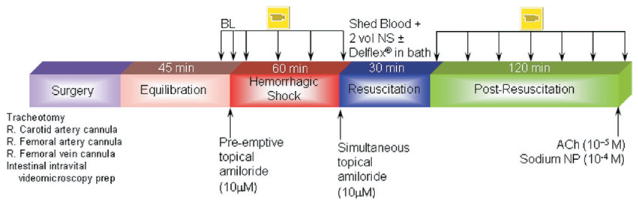
Timeline protocol. Ach, acetylcholine; Amiloride preemptively, drug administration preemptively at the beginning of hemorrhagic shock; Amiloride simultaneously, drug administration simultaneously with resuscitation; BL, baseline; NP, sodium nitroprusside.
Chemicals and solutions
All chemicals were purchased from the Sigma Chemical Company (St. Louis, Mo). The nonvasoactive, modified Krebs solution that was used to bath continuously the intestinal segment during tissue preparation and equilibration and during conventional resuscitation contained 6.92 g/L of sodium chloride, 0.44 g/L of potassium chloride, 0.37 g/L of calcium chloride, and 2.1 g/L of sodium bicarbonate at a pH of 7.4 and osmolality of 285 mOsm/L. Simulated DPR was achieved with a clinical 2.25% dextrose-based peritoneal dialysis solution (Delflex) that contained 5.67 g/L of sodium chloride, 3.92 g/L of sodium lactate, 0.257 g/L of calcium chloride, and 0.152 g/L of magnesium chloride at a pH of 5.5 and an initial osmolality of 398 mOsm/L. A selective Na+/H+ inhibitor, 5-(N-ethyl-N-isopropyl) amiloride, was used topically with a final concentration at 10 μmol/L in tissue bath.
Experimental groups
Animals were assigned randomly to one of the following groups (n = 7 animals/group):
Group I: Hemorrhagic shock + Conventional resuscitation.
Group II: Hemorrhagic shock + Conventional resuscitation + Amiloride simultaneously with resuscitation.
Group III: Hemorrhagic shock + Conventional resuscitation + Amiloride preemptively at the beginning of Hemorrhagic shock.
Group IV: Hemorrhagic shock + Conventional resuscitation + DPR.
Group V: Hemorrhagic shock + Conventional resuscitation + DPR + Amiloride simultaneously with resuscitation.
Group VI: Sham control + Amiloride.
Data reduction and statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Microvascular diameter, intestinal A1 blood flow, and FCD data were normalized and presented as percentage change from corresponding prehemorrhage baseline for each variable. Differences in percentage change from baseline for each measured variable were assessed with repeated-measures, 1-way analysis of variance (ANOVA) followed by Dunnett's multiple-range test to evaluate changes of the measured variable from the corresponding baseline within the same animal. Differences between groups in microvascular response, A1 blood flow, or FCD were determined by 2-way ANOVA followed by Bonferroni multiple comparison post-tests when the ANOVA indicated significant differences in time-points, resuscitation type, or groups. Intestinal microvascular response to acetylcholine (ACh, 10−5 M) for each microvascular level was normalized to the vessel's maximum dilation capacity as determined from the response to sodium nitroprusside (SNP, 10−4 M). Differences between groups in microvascular response to acetylcholine were determined by 2-way ANOVA followed by Bonferroni multiple comparison post-tests when the ANOVA indicated significant differences in vascular level response, resuscitation type, or groups. Statistical significance was set a priori for the probability of a type 1 error at P < .05.
Results
No significant difference was observed among experimental groups in measured variables (Table). No difference was found in the maximal dilation capacity at each microvascular level between groups. As described, mean arterial pressure dropped by 50% from baseline in all hemorrhage groups and was restored fully to prehemorrhage baseline level after intravascular volume replacement with conventional resuscitation. Heart rate was decreased slightly during the shock period but returned to baseline levels after resuscitation. No significant differences were found among groups with hemorrhage in terms of mean arterial pressure.
Table.
Baseline parameters
| Experimental group | A1 (μm) | A2 (μm) | pA3 (μm) | dA3 (μm) | A1 flow(nL/s) | FCD
capillary/field |
|---|---|---|---|---|---|---|
| Hemorrhagic shock + conventional resuscitation | 86 ± 3 | 40 ± 5 | 14 ± 1 | 11 ± 1 | 147 ± 6 | 5.5 ± 0.54 |
| Hemorrhagic shock + conventional resuscitation + amiloride simultaneously with resuscitation | 89 ± 5 | 28 ± 4 | 14 ± 1 | 10 ± 1 | 145 ± 6 | 5.3 ± 0.19 |
| Hemorrhagic shock + conventional resuscitation + amiloride preemptively at the beginning of hemorrhagic shock | 87 ± 3 | 38 ± 3 | 13 ± 1 | 9 ± 0.3 | 130 ± 11 | 5.2 ± 0.40 |
| Hemorrhagic shock + conventional resuscitation + DPR | 92 ± 3 | 37 ± 5 | 12 ± 1 | 10 ± 0.4 | 160 ± 11 | 5.2 ± 0.34 |
| Hemorrhagic shock + conventional resuscitation + DPR + amiloride simultaneously with resuscitation | 90 ± 3 | 35 ± 5 | 14 ± 1 | 10 ± 0.3 | 144 ± 12 | 4.5 ± 0.24 |
| Sham control + amiloride | 87 ± 3 | 30 ± 4 | 13 ± 1 | 11 ± 1 | 161 ± 13 | 4.7 ± 0.24 |
Intestinal microvascular diameter
Figure 2 shows the intestinal microvascular changes during the experimental protocol. After hemorrhage, A1 arterioles constricted progressively and reached approximately −25% from baseline in all experimental groups. In contrast, no significant change in the microvascular diameter of the smaller A3 premucosal arterioles was observed during shock. There was, however, a progressive constriction of all arteriolar levels after conventional resuscitation alone.
Fig 2.
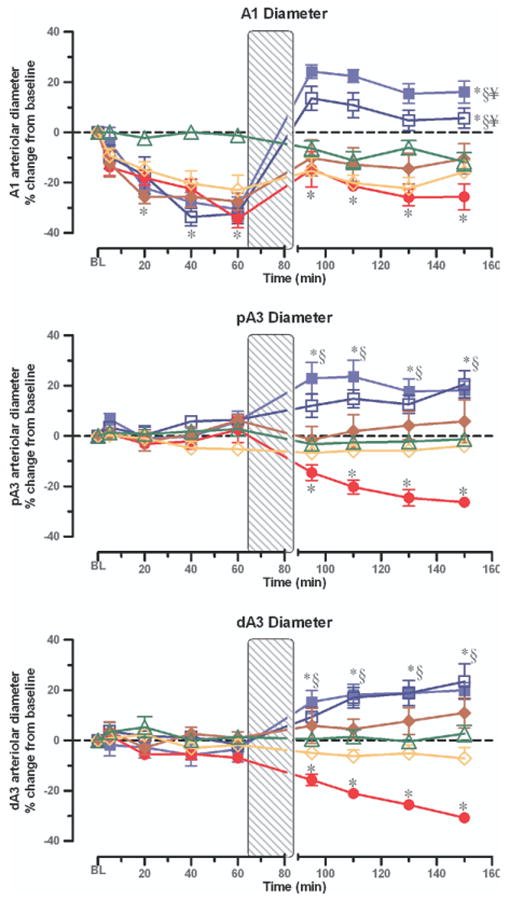
Intestinal microvascular response expressed as percentage change from corresponding baseline (BL). Vessel diameter of inflow distributing arteriole (A1), and the smaller premucosa's proximal precapillary (pA3) and distal (dA3) precapillary arterioles after hemorrhagic shock + conventional resuscitation (solid circles); hemorrhagic shock + conventional resuscitation + Amiloride simultaneously with resuscitation (open diamonds); hemorrhagic shock + conventional resuscitation + Amiloride preemptively at the beginning of hemorrhagic shock (solid diamonds); hemorrhagic shock + conventional resuscitation + DPR (solid squares); hemorrhagic shock + conventional resuscitation + DPR + Amiloride simultaneously with resuscitation (open squares); and after instrumentation, time-matched and Amiloride administration but no hemorrhage controls (open triangles). *P < .01 versus corresponding baseline by repeated-measures 1-way ANOVA followed by Dunnett's multiple-range test, §P < .01 for the simulated DPR group versus the conventional resuscitation group by 2-way ANOVA followed by Bonferroni multiple comparison post-tests, ¥P <.05 for the simulated DPR group versus the sham no hemorrhage group by 2-way ANOVA followed by Bonferroni multiple comparison post-tests.
Simulated adjunctive DPR with Delflex solution in the tissue bath caused a rapid and sustained generalized vasodilation of the intestinal microvasculature at all microvascular levels. Topical application of amiloride did not affect the microvascular dilator response to DPR. Similarly, the conventional resuscitation-related vasoconstriction during the postresuscitation period was not prevented by amiloride in the A1 arteriole whether the inhibitor was applied preemptively (before hemorrhage) or simultaneously with conventional resuscitation. The post-resuscitation vascular constriction in the smaller premucosal, precapillary A3 arterioles, however, did not occur when amiloride was added to conventional resuscitation (P < .05).
Intestinal A1 blood flow
As depicted in the upper panel of Fig 3, blood flow in the A1 arteriole was remarkably reduced by −75% from baseline during the hemorrhagic shock period in all groups. Blood flow was only restored partially to baseline by conventional resuscitation and remained below the prehemorrhage baseline level during the postresuscitation period in the conventional resuscitation group. Topical application of amiloride did not improve the conventional resuscitation-related decrease in A1 blood flow no matter when the inhibitor was used before hemorrhage or at the time of resuscitation. In contrast, simulated adjunctive DPR alone reversed the conventional resuscitation-related decrease in A1 blood flow, which remained about 25% above prehemorrhage baseline level during the entire postresuscitation period. Addition of amiloride to adjunctive DPR was not associated with additional improvement in A1 blood flow than that observed with adjunctive DPR alone. A1 arteriolar blood flow was decreased slightly when amiloride was applied topically in the tissue bath of the sham group.
Fig 3.
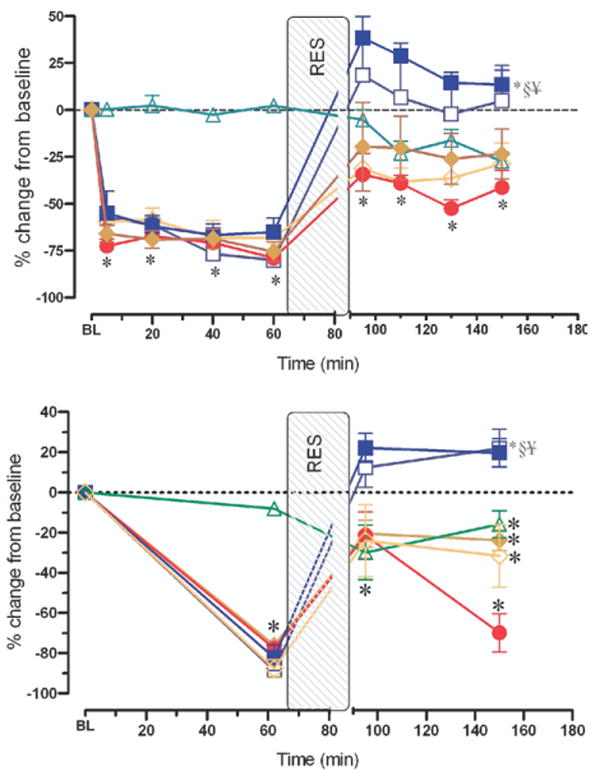
Intestinal A1 blood flow (upper panel) and functional capillary density (lower panel), each expressed as a percentage change from corresponding baseline after hemorrhagic shock + conventional resuscitation (solid circles); hemorrhagic shock + conventional resuscitation + Amiloride simultaneously with resuscitation (open diamonds); hemorrhagic shock + conventional resuscitation + Amiloride preemptively at the beginning of hemorrhagic shock (solid diamonds); hemorrhagic shock + conventional resuscitation + DPR (solid squares); hemorrhagic shock + conventional resuscitation + DPR + Amiloride simultaneously with resuscitation (open squares); and after instrumentation, time-matched and Amiloride administration but no hemorrhage controls (open triangles). *P < .01 versus corresponding baseline by repeated-measures 1-way ANOVA followed by Dunnett's multiple-range test, §P < .01 for the simulated DPR group versus the conventional resuscitation group by 2-way ANOVA followed by Bonferroni multiple comparison post-tests, ¥P < .05 for the simulated DPR group versus the sham no hemorrhage group by 2-way ANOVA followed by Bonferroni multiple comparison post-tests.
Capillary filling and FCD
Continuously perfused intestinal capillaries on the serosal side were counted as an estimate of FCD. This method generally underestimates the absolute FCD in the observed segment of the intestine from the 3-dimensional arrangement of the capillary network. Therefore, our current FCD measured in 1 optical plane qualifies as the best estimate of FCD. In the current studies, FCD was assessed in each animal at baseline, at 60 min after shock, immediately on completion of resuscitation, and at 120 min postresuscitation. As shown in the lower panel of Fig 3, no significant difference was found in baseline FCD among all groups. Hemorrhagic shock markedly reduced FCD by −80% from the baseline prehemorrhage level. Conventional resuscitation alone caused an initial transient restoration of FCD toward baseline level. This restoration was followed by a remarkable decrease in FCD to an average −70 ± 10% from baseline at 2 h postresuscitation (P < .01). Topical amiloride before hemorrhagic shock or simultaneously with conventional resuscitation completely abolished the conventional resuscitation-related decrease in FCD. On average, topical amiloride application in the tissue bath improved the FCD from −88 ± 5% during shock to −24 ± 18% at 2 h after resuscitation (P < .01). In contrast, simulated adjunctive DPR alone increased and maintained FCD at +22 ± 9% above prehemorrhage baseline level during the entire postresuscitation period. However, addition of amiloride to adjunctive DPR did not produce any more increase in FCD than DPR alone.
Endothelial cell function
The endothelial-dependent, receptor-dependent dilator function was assessed from the microvascular response to a single dose of acetylcholine (ACh, 10−5M) applied topically in the tissue bath. No difference was found in A1 response to ACh between the groups (data not show); however, hemorrhage and conventional resuscitation significantly impaired the premucosal A3 arteriolar dilation response to ACh as shown for the dA3 arteriole in Fig 4. Combined Na+/H+ exchanger inhibition with conventional resuscitation, or in conjunction with DPR, equally restored the dA3 dilation response to ACh. Whereas DPR alone without the Na+/H+ exchanger inhibition restored the dA3 arteriolar response to ACh. The maximal dilation capacity of all intestinal microvessels, assessed from the response to the endothelium-independent, receptor-independent nitric oxide donor sodium nitroprusside (SNP), was similar among groups to indicate that the vascular smooth-muscle functions remained intact after hemorrhage and resuscitation.
Fig 4.
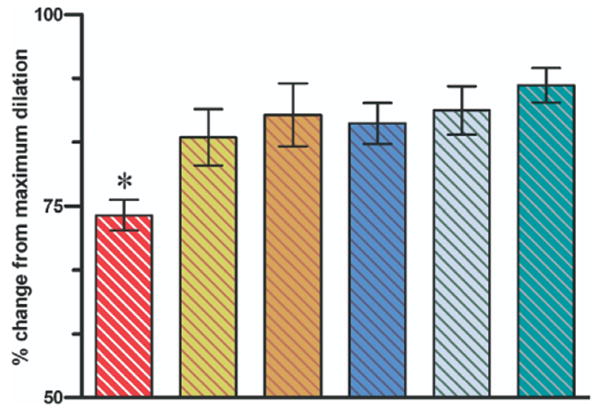
Endothelial cell function in the distal dA3 arteriole assessed from the response to acetylcholine and expressed as a percentage change from maximum dilation capacity (response to sodium nitroprusside). Bar from left to right are the dA3 response after hemorrhagic shock + conventional resuscitation; hemorrhagic shock + conventional resuscitation + Amiloride simultaneously with resuscitation; hemorrhagic shock + conventional resuscitation + Amiloride preemptively at the beginning of hemorrhagic shock; hemorrhagic shock + conventional resuscitation + DPR; hemorrhagic shock + conventional resuscitation + DPR + Amiloride simultaneously with resuscitation; and after instrumentation, time-matched and Amiloride administration but no hemorrhage controls. dA3 response to acetylcholine was impaired significantly in the hemorrhagic shock + conventional resuscitation group. *P < .01 by 2-way ANOVA followed by Bonferroni multiple comparison post-tests.
Discussion
The salient findings of the current studies are as follows: 1) conventional resuscitation from hemorrhagic shock that restores and maintains hemodynamics is associated with a progressive and persistent intestinal arteriolar vasoconstriction and hypoperfusion, endothelial cell dysfunction, and poor capillary filling. 2) The vascular endothelium's Na+/H+ exchanger was activated early during hemorrhagic shock. This activation contributes to the intestinal microvascular derangements noted with conventional intravascular volume resuscitation from hemorrhagic shock. 3) Combined Na+/H+ exchanger inhibition with conventional resuscitation restored local microvascular variables to near-prehemorrhage baseline levels. 4) Adjunctive DPR enhanced intestinal microvascular functions to levels above prehemorrhage baseline. 5) The DPR effects are independent from the Na+/H+ exchanger.
The current model and techniques
The current fixed-pressure hemorrhage model is nonlethal and designed specifically to study the intestinal microvasculature during hypovolemic shock. The severity of trauma or shock, however, can be adjusted easily to allow for the evaluation of end-points of resuscitation and outcomes.15 The mechanistic objectives of the current studies require that experiments be conducted acutely in a nonlethal model and essentially under anesthesia. Intravital microscopy required that the small intestinal segment be opened along the anti-mesenteric border to allow for direct visualization of the microvasculature.19 Anesthesia produces significant changes in the microcirculation, including alteration of the microvascular diameter and the response to vasoactive drugs (catecholamines) and stressors (hypovolemia caused by hemorrhagic shock).20,21 In recent studies, we demonstrated that the intestinal microvascular reactivity to Delflex solution in pentobarbital anesthesia is similar in both pattern and magnitude to the reactivity observed in decerebrate, anesthetic-free conscious rats.22 As in our previous and current studies, to obviate any local effects of anesthesia on the microvessel, we only studied microvessels exhibiting spontaneous vasomotion, which is characteristic of normal tissue microcirculation. Since all animals used in this study were subjected to the same experimental conditions of anesthesia and surgical stress, statistical comparison between groups is justified.
In the current study, the simulated DPR with Delflex solution in the tissue bath exposes both the serosa and the mucosa of the intestinal segment to the solution. This experimental design makes the microvascular reactivity and the local changes in hemodynamics liable to potentially different interpretations. In previous studies, we examined the effect of adjunctive DPR (injection of 30 mL of the Delflex solution intraperitoneally) on whole-organ blood flow distribution as measured with colored microspheres. Adjunctive DPR increased splanchnic organs blood flow by 30% to 50% and muscle blood flow by >100%. Although the intestinal mucosa was not exposed to the intraperitoneal Delflex, significant systemic glucose absorption was observed in these studies.13 Similarly rat cremaster muscle topically exposed to glucose-based peritoneal dialysis solutions exhibited microvascular vasodilation that could not be explained by mucosal absorption.23 Exposure of a small segment of intestine to a relatively large volume of Delflex solution in the tissue bath does not result in measurable systemic glucose absorption nor does osmolality of bath Delflex change during the experimental period. Studies have demonstrated intestinal endothelium impairment within 30 min of transient hyperglycemia because of systemic glucose absorption.24 Therefore, the intestinal microvascular effects of DPR in our previous, and in particular our current, studies are unlikely to be explained by mucosal or remarkable systemic glucose absorption. Instead, DPR-mediated intestinal microvascular changes are attributed primarily to endothelium-dependent mechanisms mediated by local hyperosmolality-induced activations of glibenclamide-sensitive K+ channels and secondary nitric oxide release.25
Significance of capillary filling in tissue perfusion
The capillary network of the end-organ tissue is the unit for O2 and nutrient blood exchanges between the cellular and the vascular compartments. For these exchange mechanisms to transpire, capillary filling must occur. As reviewed elsewhere, capillary filling and the basic capillary function of fluid exchange are compromised during hypovolemic shock.26 In the normal physiologic state, the number of perfused capillaries in any vascular bed is determined primarily by the pre-to-post capillary resistance ratio. In hemorrhagic shock and resuscitation, numerous compounding variables potentially can influence capillary filling and thus drastically reduce the effective capillary surface area available for exchange. Among these variables are factors that influence the pre-to-post capillary resistance such as vasomotion and vascular tone, vascular endothelium status, blood rheology, and a pressure drop. Other factors that can affect directly capillary filling include capillary vascular endothelium swelling, leukocyte plugging, and interstitial edema. Morphometric studies by Mazzoni et al2,27,28 have shown that hemorrhagic shock reduces the capillary cross-sectional area by more than 20% because of capillary vascular endothelium swelling, which is mediated by activation of the Na+/H+ exchanger. This remarkable capillary lumen narrowing drastically impedes capillary filling, since capillary blood flow directly relates to the fourth power of the capillary radius (Poiseuille's law). The Na+/H+ exchanger plays an active role in the regulation of intracellular volume and pH during low-flow states. Accumulated intracellular H+ from the hypoxia-induced anaerobic metabolism activates the Na+/H+ exchanger. This activation results in cellular H+ efflux and Na+ influx associated with an osmotically driven solvent-drag (water shift), which causes endothelial cell swelling. Other factors prevalent during the low-flow state of hemorrhagic shock and resuscitation, such as endothelin-1, angiotensin II, autocrine and paracrine factors, thrombin, α1-adrenergic agonists, and H2O2 are also known to activate the Na+/H+ exchanger.29,30 The direct consequences of capillary narrowing caused by endothelium swelling are the loss of the basic capillary function of fluid exchange and a profound effect on O2 delivery and CO2 removal. Therefore, restoration of the capillary filling remains a potential therapeutic target in low-flow states.
Resuscitation strategies directed toward offsetting the activation of the Na+/H+ exchanger during shock include the systemic administration of amiloride and the use of hypertonic saline/colloid resuscitation.2,27,28 Systemic amiloride seems to improve cardiac function, attenuate hemorrhage-induced metabolic acidosis, and improve O2 delivery and consumption presumably by mechanisms related to prevention of capillary endothelium swelling and restoration of capillary filling.31 Systemic amiloride does not protect against gut or lung injures associated with hemorrhagic shock and resuscitation.6,7 In addition, the effect of systemic amiloride administration on resuscitation survival outcome remains to be determined. Studies by Mazzoni et al provided a link between hypertonic saline/colloid resuscitation and the restoration of capillary diameter and filling, which was observed 30 min after hypertonic saline/colloid resuscitation.28 Interestingly, hypertonic saline resuscitation alone does not restore or maintain hemodynamics unless the shed blood is returned, and yet, it causes a preferential vasodilation and improvement of endothelial cell function of the intestinal precapillary arterioles.32 Taken together, these data suggest that the improved capillary filling after hypertonic saline resuscitation is in part mediated by modulation of the pre-to-post capillary resistance as evidenced by the preferential vasodilation of the precapillary arterioles. Similarly, adjunctive DPR prevents endothelial cell swelling and improves capillary filling by mechanisms that do not involve the Na+/H+ exchanger. The clinical Delflex solution used for adjunctive DPR is vasoactive and produces a rapid and sustained near-maximal vasodilation at all intestinal microvascular levels.33,34 This Delflex-mediated vasodilation is a strong modulator of the pre-to-post capillary resistance ratio, which determines the number of perfused capillaries. It should be emphasized that for the Delflex-mediated intestinal microvascular vasodilation to occur, a contact between the Delflex solution and the intestine must be established. This concept was demonstrated in control studies in which 20 mL of the Delflex was instilled and allowed to dwell in the peritoneal cavity, whereas the small segment of the terminal ileum continued to be bathed with a nonvasoactive isotonic Krebs solution in the tissue bath. No change in any ileal microvascular diameters at any level was observed. These experiments confirm that the vasoactivity of the Delflex solution is attributed to local effects and not from systemic glucose absorption as defined pharmacologically in our recent studies.25
In conclusion, paradoxical endothelial cell swelling occurs early during hemorrhagic shock because of activation of the Na+/H+ exchanger. This cellular edema, which is not resolved by correction of the vascular volume deficit, explains the persistent postresuscitation endothelial cell dysfunction and gut hypoperfusion. Simulated adjunctive DPR in this study, reversed endothelial cell swelling, and enhanced gut perfusion by mechanisms that are independent of the Na+/H+ exchanger activity. Restoration of capillary filling and the basic capillary functions are critical for adequate tissue perfusion during hemorrhagic shock and resuscitation.
Acknowledgments
Supported by a VA Merit Review grant, an NIH Research Grant R01 HL076160-03, the National Heart, Lung, and Blood Institute, and the United States Army Medical Resources and Material Command.
References
- 1.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–73. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 3.Chaudry IH, Baue A. Alterations in adenosine 3′,5′-monophosphate levels in hemorrhagic shock. Surg Forum. 1976;27:51–3. [PubMed] [Google Scholar]
- 4.Chaudry IH, Sayeed MM, Baue AE. Alterations in high-energy phosphates in hemorrhagic shock as related to tissue and organ function. Surgery. 1976;79:666–8. [PubMed] [Google Scholar]
- 5.Van WC, III, Dhar A, Morrison DC, Longorio MA, Maxfield DM. Cellular energetics in hemorrhagic shock: restoring adenosine triphosphate to the cells. J Trauma. 2003;54:S169–76. doi: 10.1097/01.TA.0000047226.36678.EE. [DOI] [PubMed] [Google Scholar]
- 6.Fujiyoshi N, Deitch EA, Feketeova E, Lu Q, Berezina TL, Zaets SB, et al. Amiloride combined with small-volume resuscitation with hypertonic saline is superior in ameliorating trauma-hemorrhagic shock-induced lung injury in rats to the administration of either agent alone. Crit Care Med. 2005;33:2592–8. doi: 10.1097/01.ccm.0000186770.59312.44. [DOI] [PubMed] [Google Scholar]
- 7.Fujiyoshi N, Feketeova E, Lu Q, Xu DZ, Hasko G, Deitch EA. Amiloride moderates increased gut permeability and diminishes mesenteric lymph-mediated priming of neutrophils in trauma/hemorrhagic shock. Surgery. 2006;140:810–7. doi: 10.1016/j.surg.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth ZH, Deitch EA, Szabo C, Mabley JG, Pacher P, Fekete Z, et al. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G122–32. doi: 10.1152/ajpgi.00015.2002. [DOI] [PubMed] [Google Scholar]
- 9.Carrico CJ, Canizaro PC, Shires GT. Fluid resuscitation following injury: rationale for the use of balanced salt solutions. Crit Care Med. 1976;4:46–54. [PubMed] [Google Scholar]
- 10.Shires GT, Cunningham JN, Backer CR, Reeder SF, Illner H, Wagner IY, et al. Alterations in cellular membrane function during hemorrhagic shock in primates. Ann Surg. 1972;176:288–95. doi: 10.1097/00000658-197209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shires GT. Pathophysiology and fluid replacement in hypovolemic shock. Ann Clin Res. 1977;9:144–50. [PubMed] [Google Scholar]
- 12.Zakaria ER, Garrison RN, Spain DA, Matheson PJ, Harris PD, Richardson JD. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–11. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakaria ER, Hurt RT, Matheson PJ, Garrison RN. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–8. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Zakaria ER, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrison RN, Conn AA, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–8. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–10. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, et al. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int. 2001;60:202–10. doi: 10.1046/j.1523-1755.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 18.De Vriese AS, Lameire NH. Intravital microscopy: an integrated evaluation of peritoneal function and structure. Nephrol Dial Transplant. 2001;16:657–60. doi: 10.1093/ndt/16.3.657. [DOI] [PubMed] [Google Scholar]
- 19.Matheson PJ, Garrison RN. Intravital intestinal videomicroscopy: techniques and experiences. Microsurgery. 2005;25:247–57. doi: 10.1002/micr.20120. [DOI] [PubMed] [Google Scholar]
- 20.Longnecker DE, McCoy S, Drucker WR. Anesthetic influence on response to hemorrhage in rats. Circ Shock. 1979;6:55–60. [PubMed] [Google Scholar]
- 21.Longnecker DE, Harris PD. Microcirculatory actions of general anesthetics. Fed Proc. 1980;39:1580–3. [PubMed] [Google Scholar]
- 22.Zakaria ER, Patel A, Li N, Matheson PJ, Garrison RN. Vasoacive components of the dialysis solution. Perit Dial Int. 2007;27 1:S12. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller FN, Nolph KD, Joshua IG, Wiegman DL, Harris PD, Andersen DB. Hyperosmolality, acetate, and lactate: dilatory factors during peritoneal dialysis. Kidney Int. 1981;20:397–402. doi: 10.1038/ki.1981.152. [DOI] [PubMed] [Google Scholar]
- 24.Bohlen HG, Nase GP, Jin JS. Multiple mechanisms of early hyperglycaemic injury of the rat intestinal microcirculation. Clin Exp Pharmacol Physiol. 2002;29:138–42. doi: 10.1046/j.1440-1681.2002.03617.x. [DOI] [PubMed] [Google Scholar]
- 25.Zakaria ER, Li N, Garrison RN. Mechanisms of direct peritoneal resuscitation-mediated splanchnic hyperperfusion following hemorrhagic shock. Shock. 2007;27:436–42. doi: 10.1097/01.shk.0000245017.86117.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974;41:157–68. doi: 10.1097/00000542-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ Shock. 1989;29:27–39. [PubMed] [Google Scholar]
- 28.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Capillary narrowing in hemorrhagic shock is rectified by hyperosmotic saline-dextran reinfusion. Circ Shock. 1990;31:407–18. [PubMed] [Google Scholar]
- 29.Avkiran M, Snabaitis AK. Regulation of cardiac sarcolemmal Na+/H+ exchanger activity: potential pathophysiological significance of endogenous mediators and oxidant stress. J Thromb Thrombol. 1999;8:25–32. doi: 10.1023/a:1008938513337. [DOI] [PubMed] [Google Scholar]
- 30.Karmazyn M. Mechanisms of protection of the ischemic and reperfused myocardium by sodium-hydrogen exchange inhibition. J Thromb Thrombol. 1999;8:33–8. doi: 10.1023/a:1008990530176. [DOI] [PubMed] [Google Scholar]
- 31.Wu D, Bassuk J, Arias J, Doods H, Adams JA. Cardiovascular effects of NA+/H+ exchanger inhibition with BIIB513 following hypovolemic circulatory shock. Shock. 2005;23:269–74. [PubMed] [Google Scholar]
- 32.Zakaria ER, Tsakadze NL, Garrison RN. Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery. 2006;140:579–87. doi: 10.1016/j.surg.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakaria ER, Spain DA, Harris PD, Garrison RN. Generalized dilation of the visceral microvasculature by peritoneal dialysis solutions. Perit Dial Int. 2002;22:593–601. [PubMed] [Google Scholar]
- 34.Zakaria ER, Hunt CM, Li N, Harris PD, Garrison RN. Disparity in osmolarity-induced vascular reactivity. J Am Soc Nephrol. 2005;16:2931–40. doi: 10.1681/ASN.2004090764. [DOI] [PMC free article] [PubMed] [Google Scholar]


