Abstract
Lymphodepletion with chemotherapeutic agents or total body irradiation (TBI) before adoptive transfer of tumor-specific T cells is a critical advancement in the treatment of patients with melanoma. More than 50% of patients that are refractory to other treatments experience an objective or curative response with this approach. Emerging data indicate that the key mechanisms underlying how TBI augments the functions of adoptively transferred T cells include (a) the depletion of regulatory Tcells (Treg) and myeloid-derived suppressor cells that limit the function and proliferation of adoptively transferred cells; (b) the removal of immune cells that act as “sinks” for homeostatic cytokines, whose levels increase after lymphodepletion; and (c) the activation of the innate immune system via Toll-like receptor 4 signaling, which is engaged by microbial lipopolysaccharide that translocated across the radiation-injured gut. Here, we review these mechanisms and focus on the effect of Toll-like receptor agonists in adoptive immunotherapy. We also discuss alternate regimens to chemotherapy or TBI, which might be used to safely treat patients with advanced disease and promote tumor regression.
It is the information carried by the bacteria that we cannot abide. The gram-negative bacteria are the best examples of this. They display lipopolysaccharide endotoxin in their walls, and these macromolecules are read by our tissues as the very worst of bad news. When we sense lipopolysaccaride, we are likely to turn on every defense at our disposal. . . Lewis Thomas, New England Journal of Medicine, 1971
Historical Perspective on Exploiting Bacteria to Treat Cancer
Over a century ago, William B. Coley observed spontaneous tumor regression in some of his patients with bacterial infection (1). He hypothesized that the tumor regression was caused by infection and designed a mixture of bacteria consisting of heat-killed cultures of Streptococci and Serratia marcescens known as Coley’s toxins. He reported success using these mixtures in patients with soft tissue sarcoma. Subsequent investigators, however, failed to reproduce these findings (2). Nonetheless, Coley’s notion that bacteria can activate immune responses to cancer cells persists to the present day.
Bacterial mixtures as activators of immune responses could be understood in the danger model formulated by Polly Matzinger in the early 1990s (3, 4). According to this model of immune activation versus tolerance, tumor antigens would be viewed as dangerous to the immune system in the presence of bacteria. A molecular understanding of how host-derived bacteria stimulate the immune system was later elucidated by Charles Janeway and colleagues (5–7) who found that bacteria contain conserved pathogen-associated molecular patterns such as lipopolysaccharide (LPS) and bacterial DNA that can ligate pattern recognition receptors, such as the Toll-like receptors (TLR) of the innate immune system (8–11). Engagement of TLRs on antigen presenting cells (APC; such as dendritic cells) of the innate immune system results in their maturation and migration to lymph nodes where they initiate adaptive immune responses (12, 13).
TLRs in Intestinal Immune Health and Disease
It is now clear that recognition of host-derived bacteria by TLRs is vital for intestinal health, exerting a variety of structural, metabolic, and protective effects on the intestinal mucosal barrier (14–17). Medzhitov and colleagues first found that TLR detection of gut microbes could protect mice from life-threatening colitis. They found that mice genetically deficient in My D88 (a molecule important for TLR recognition of gut microbes) succumbed to increased disease pathogenesis as evidenced by pronounced gut injury and related mortality compared with wild-type mice (18). Conversely, gut injury and mortality was increased in wild-type mice with colitis upon removal of gut microbes using broad-spectrum antibiotics. Interestingly, administrating LPS to their drinking water rescued antibiotic-treated wild-type mice from disease pathogenesis, indicating that TLR recognition of microbial products was surprisingly important for maintaining intestinal immune health (18).
TLR recognition of gut microflora can also be deleterious to the host, especially if the host is rendered lymphopenic by a chronic infection or by lymphodepleting preparative regimens (19–21). Translocated colonic microbes have been implicated in exacerbating graft-versus-host disease in patients receiving allogeneic stem cell transplant (22–27) and have recently been reported to cause systemic immune activation in patients with chronic HIV infection (28, 29). Thus, it is clear that commensal gut microflora can exacerbate unwanted immune responses, but the potential for exploitation in T cell–based antitumor immunotherapies remains to be fully explored.
Recent Efforts in Exploiting TLRs for the Treatment of Cancer
Given the notion that bacteria might increase antitumor immunity, the question arises “Can bacterial TLR agonists augment cancer immunotherapy?” Early studies indicated that inserting model tumor-associated antigens into viruses, which contain TLR agonists, can augment their immunogenicity and function as tumor vaccines (30–32), but these studies largely used very early treatment models in which tumors were not yet vascularized. In humans, cancer vaccines have not proven to be consistently therapeutic even when used under circumstances in which recombinant and synthetic vaccines are capable of activating the innate immune system (33).
Recently, Speiser and colleagues have made efforts to use TLR agonists in conjunction with vaccination in patients with melanoma (34, 35). They found that combining TLR9 agonist CpG ODN 7909 (a 24-mer oligodeoxynucleotide containing 3CpG motifs) with a Melan A26–35/MART1 peptide and incomplete Freund’s adjuvant increased the number of MART1-specific T cells by >10-fold compared with vaccination without CpG. This heightened immune response, however, did not promote tumor regression (35). These findings might imply that the MART1 T cells induced by CpG ODN 7909 and vaccination are functionally tolerized. The tumor-reactive T cells might be tolerized by regulatory T cells (Treg) cells, as hypothesized by the same investigators, who reported that Treg cells were elevated in the tumors of vaccinated patient’s receiving CpG ODN 7909 (36).
Adoptive Immunotherapy
Adoptive transfer of autologous tumor-specific T cells, as initially reported in 1988, has been shown to reproducibly mediate the destruction of bulky tumors in ~30% of the patients treated (37, 38). This approach involves growing tumor-infiltrating lymphocytes ex vivo from the resected tumor nodules of patients and then adoptively transferring them into the patient in conjunction with bolus high-dose interleukin-2 (IL-2; refs. 37, 38). Although this approach is cumbersome, it has a number of advantages. One can administer large numbers of naturally occurring or genetically engineered cells with high avidity for tumor antigens (39, 40). Both CD4+ and CD8+ T cells have been shown to be capable of recognizing tumor antigens and to potentially play a role in the antitumor immune response (41–45). These cells can be selected for particular functions (46), they can be activated from their poorly functional state in vivo (47), and they can be programmed for optimal function (48). After adoptive transfer into a tumor-bearing host, they are capable of massive expansion in both mice and humans (49, 50). Adoptively transferred T cells are capable of trafficking to virtually every somatic site (51). Despite all of these advantages, the adoptive transfer of cells triggered objective immune responses in only a small minority of patients until investigators began to use lymphodepleting preparative regimens to provide an altered environment for transferred cells (49, 52–56).
Lymphodepletion Augments Adoptive Immunotherapy
Adoptive transfer of ex vivo expanded antitumor T cells after a nonmyeloablative lymphodepleting preparative regimen with chemotherapeutic reagents is the most effective treatment for patients with metastatic melanoma. When antitumor T cells are given after lymphodepletion, ~50% of patients refractory to other treatments will experience objective clinical responses with this treatment (Fig. 1A and B; refs. 49, 55, 57). Not all patients’ tumors yield antitumor T cells, however, and only about a third of the patients experience durable tumor regressions. Thus, there are significant efforts to continue to improve the current therapies. Preclinical data show that increasing the intensity of lymphodepletion to a myeloablative preparative regimen that requires hematopoietic stem cell (HSC) transplant might further improve the effectiveness of adoptive cell transfer. This approach is currently being examined in clinical trials at the NIH, National Cancer Institute, Surgery Branch (58–60). Preliminary findings indicate long-term responses in patients pretreated with chemotherapeutics agents in conjunction with 200 cGy total body irradiation (TBI; Fig. 1C) or 1,200 cGy TBI (six fractionated doses of 200 cGy TBI, twice a day for 3 days) plus HSC transplant (Fig. 1D).
Fig. 1.
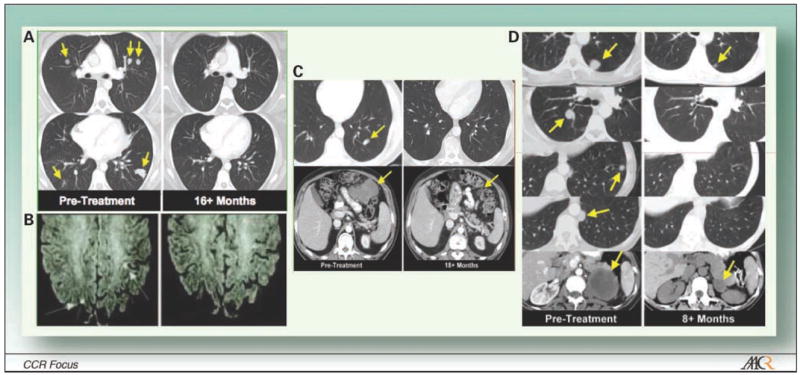
Tumor destruction of metastatic disease in various anatomic sites by adoptively transferred tumor-specific T cells in the setting of lymphodepletion with chemotherapeutic agents and/or TBI. Computed tomography scan of tumors in patients with stage IV melanoma. A, multiple large metastatic liver lesions existed before the treatment (pretreatment) and then regressed dramatically after adoptive T-cell transfer (16+ mo). B, patients with brain and recurrent axillary metastases experience tumor regression. C, multiple large metastatic liver lesions existed before the treatment (pretreatment) and then regressed dramatically after adoptive T-cell transfer (18+ mo) in patients receiving chemotherapeutic reagents in conjunction with 200 cGy TBI plus HSC transplant. D, multiple large metastatic liver lesions existed before the treatment (pretreatment) and then regressed dramatically after adoptive T-cell transfer (8+ mo) in patients receiving chemotherapeutic reagents in conjunction with six fractionated doses of 200 cGy TBI (twice a week for 3 d; 12 Gy TBI total) plus HSC transplant.
The concept that lymphopenia-induced homeostatic expansion of T cells intensifies immune responses to tumor antigens and facilitates potent antitumor immune responses was first reported by Fefer and colleagues in the late 1960s (61). They found that i.p. administration of lymphocytes in conjunction with chemotherapy could treat mice bearing virally induced lymphomas (61). Likewise, other groups in the early 1980s found that this approach could mediate the regression of established sarcomas in mice and rats (62, 63). More recently, Dummer and colleagues found that homeostatic expansion of autologous CD8+ T cells can inhibit tumor growth. Importantly, CD8+ T cells from these mice mediated tumor-specific cytotoxicity and IFN-γ production associated with long-term tumor-specific memory (64). In addition, tumor growth was profoundly inhibited in irradiated animals vaccinated with tumor lysate–pulsed dendritic cells (65). This work clearly showed that lymphodepletion can enhance T cell–mediated antitumor immune responses.
We have explored the use of lymphodepletion with a nonmyeloablative preparative conditioning regimen using 5 Gy TBI in a model of adoptive immunotherapy using CD8+ pmel-1 T cells that are specific for the self/tumor antigen gp100 (66–68). The administration of 5 Gy TBI before a tripartite regimen consisting of adoptive transfer of transgenic pmel-1 cells, vaccination encoding gp100, and IL-2 significantly enhanced the destruction of large, established B16 tumors (69). The mechanisms underlying this improvement are multifold and have been substantially elucidated using varieties of knockout and transgenic animals (Fig. 2). These mechanisms include the removal of inhibitory lymphocytes such as regulatory T cell and cytokines sinks, elevation of homeostatic cytokines, and activation of the innate immune system (Fig. 3).
Fig. 2.
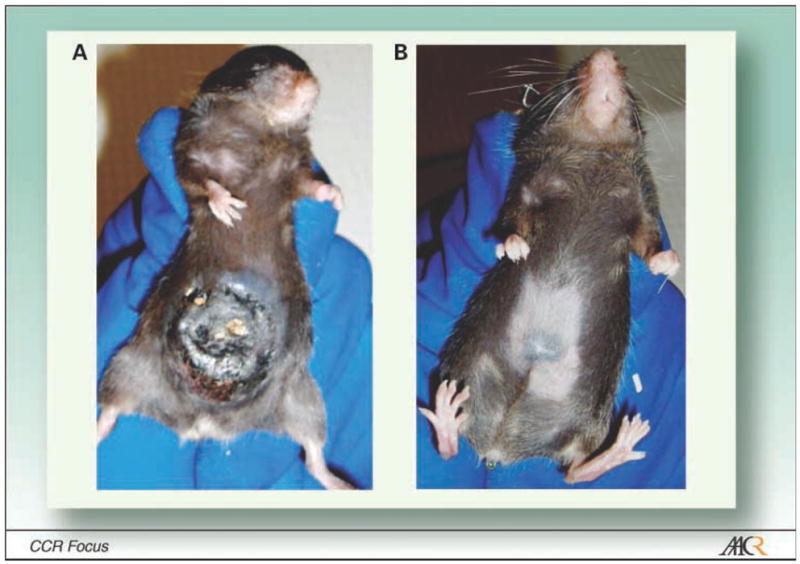
Tumor regression can be triggered by transgenic pmel-1Tcells. A, a mouse bearing a large, established, vascularized B16 mouse melanoma tumor shown after the start of treatment (19 d after tumor implantation). B, the same mouse (day 67).
Fig. 3.
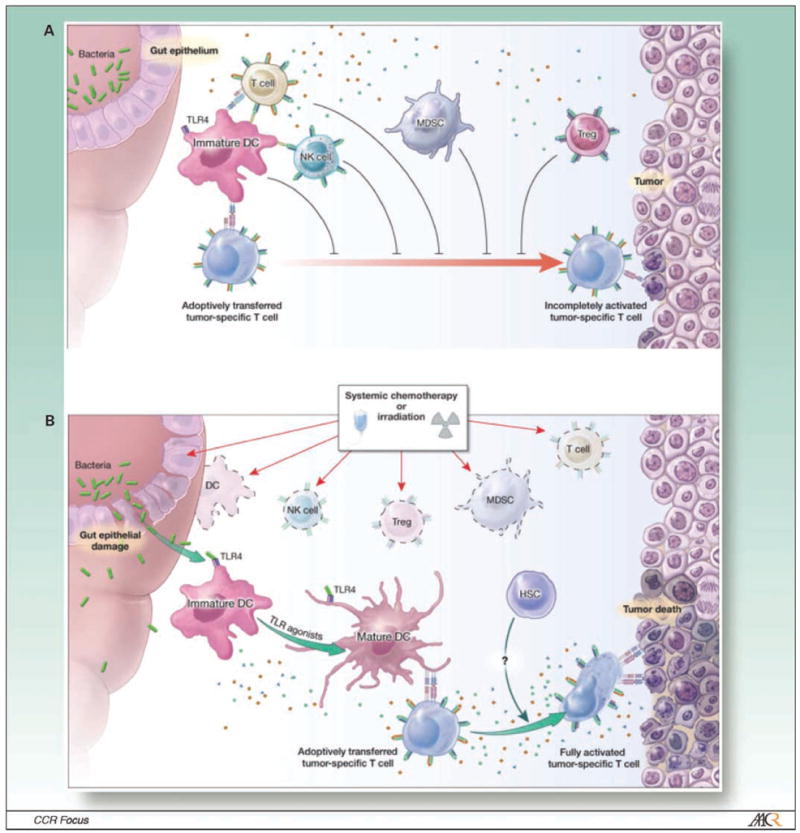
Adoptive cell therapy in a lymphoreplete host. A, in a lymphoreplete environment, antitumor responses induced by adoptively transferred T cells are impaired because of their reduced availability of homeostatic cytokines (including IL-2, IL-7, and IL-15) by immune cells that consume them (cytokine sinks, which might include B cells,Tcells, and natural killer cells); and the suppressive activities of Treg cells, MDSCs, quiescent monocytes and dendritic cells (DC), and possibly natural killer cells. B, systemic chemotherapy or radiation before adoptive cell transfer alters the milieu of the tumor-bearing host. APCs are reduced in number by direct killing but there might be a net increase in lymphocyte activation because of reduced competition for antigen at the APC surfaces. At the same time, as a result of the translocation of microbial LPS after chemotherapeutic or radiation-induced injury to the gut, dendritic cells mature and migrate to the lymph node via TLR4 signaling. This TBI action increases the functionality of the adoptively transferred lymphocyte. Activating γ-chain cytokines, such as IL-2, IL-7, and IL-15, as well as inflammatory cytokines, such as IL-6, IL-12, and tumor necrosis factor-α, are increased because of the removal of cellular sinks, Treg cells, MDSCs, and natural killer cells, and concomitant innate immune activation. Collectively, these modifications promote the full activation of adoptively transferred T cells and tumor destruction.
Mechanisms Underlying the Effectiveness of TBI in Adoptive Immunotherapy
Depletion of Treg cells
Lymphodepletion before the adoptive transfer of T cells can also reduce the numbers of CD4+ CD25+FoxP3+ Treg cells whose generation and function are reviewed elsewhere (70, 71). Treg cells maintain tolerance to self-antigens by blocking the functionality of effector T cells (72–80), as reported by North and colleagues more than two decades ago (81). Emerging data indicate that the CD39 and CD73 are surface markers for Treg cells that impart a specific biochemical signature characterized by adenosine generation (82). These phenotypic markers might have more functional relevance than the cell surface marker CD25 for cellular immunoregulation. It is now clear that removal of Treg cells by 5 Gy TBI improve the function of adoptively transferred cells (Fig. 3; refs. 58, 78). Another category of immune cells that have been reported to induce lymphocyte dysfunction and promote tumor growth are designated myeloid-derived suppressor cells (MDSC; refs. 83–89). Removal of these cells might also contribute to the effectiveness of TBI.
Removal of cytokine sinks
Lymphodepletion with nonmyeloablative 5 Gy TBI augment the effectiveness of adoptively transferred cells in part by depleting the lymphoid compartment that consumes homeostatic cytokines (cytokine sinks such as host natural killer, CD4+ and CD8+ T cells; refs. 69, 78). Removal of this compartment increase the availability of homeostatic γ-chain cytokines such as IL-7, IL-15, and possibly IL-21. An increased availability of these cytokines for the adoptively transferred cells may enhance their function and enable them to destroy bulky nonimmunogenic tumors (58, 69).
Activation of innate immunity via translocated microbes
Interestingly, removal of Treg cells and cytokine sinks do not fully account for the dramatically improved tumor regression resulting from TBI. We recently found that Rag2−/ − γc−/ − mice, which lack both Treg cells and sinks for homeostatic cytokines, benefited from TBI preconditioning, an unexpected finding given that these mice are deficient in all lymphocyte subsets. These data indicate the existence of another mechanism by which TBI enhances adoptive immunotherapy (90).
TBI has recently been reported to increase antigens on the cell surface of the tumor stroma (91, 92). Increased expression of antigens was reported to contribute to elimination of the immunogenic tumors by T cells. We found that local irradiation at the tumor site of up to 10 Gy before adoptive cell transfer, however, had virtually no effect on the growth rate of established B16 melanoma (69). Conversely, TBI with 5 Gy before adoptive cell transfer enhanced tumor destruction even when the tumor was shielded from radiation. This indicated that the observed enhancement of CD8+ T-cell function after TBI did not directly result from its influence on the tumor, but rather resulted from its effect on the host cells. This finding is in accordance with earlier studies by Hellstrom in 1978, who first reported the irradiation might be beneficial for the treatment of MCA-1315 tumors in mice because of the effect of radiation on host but not tumor cells (93).
Additional investigation conducted in our laboratory revealed that radiation-induced injury to the gut permitted translocation of microbes to the mesenteric lymph nodes and systemic liberation of bacterial-derived LPS (90). Bacterial translocation resulted in activation of the innate immune system, as indicated by an increase in the splenic CD11c+ CD86hi dendritic cells, which are capable of activating adoptively transferred T cells (94–96). In addition, higher levels of pro-inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α were found in the serum of irradiated mice (90). Furthermore, IL-12, a well-described enhancer of CD8+ T-cell function (97, 98), was found in the serum as well (90).
Neutralization of gut microbes with ciprofloxacin reduced the absolute numbers of activated host dendritic cells and impaired tumor destruction by the adoptively transferred cells in irradiated mice (90). These data have shown that innate activation by the translocated microbes is surprisingly important for improving T cell–based immunotherapy. As cancer immunotherapy develops, it is important to understand the effect of these treatments on host-microbe homeostasis and the role of gut microbes in antitumor immunity. A better understanding of this mechanism might allow us to optimize our therapies to enhance clinical responses.
Translocation of Microbial LPS Augments Adoptive Immunotherapy via TLR4 Signaling
Microbes that translocate across the perturbed gut contain a plethora of TLR agonists (i.e., lipoproteins, lipoteichoic acid, peptidoglycan, flagellin, bacterial DNA, and LPS; refs. 11, 13, 99). Thus, any of these agonists might have been responsible for enhancing adoptive immunotherapy. We found, however, that LPS is principally responsible for the effectiveness of TBI because neutralizing it with polymyxin B, a cyclic cationic polypeptide antibiotic that blocks the biological effect of Gram-negative LPS (100), also impaired tumor destruction mediated by the adoptively transferred cells.
LPS, which binds to the soluble LPS binding protein, activates innate immunity primarily through engagement of TLR4, which acts in conjunction with accessory CD14 and MD2 molecules (refs. 101, 102; Fig. 4). These accessory molecules are important for stabilizing TLR4. TLR4 engagement induces nuclear factor-κB activation via multiple pathways (i.e., MyD88, TRIF, TIRAP, and TRAM pathways; refs. 103, 104), leading to the production of pro-inflammatory cytokines, up-regulation of costimulatory molecules, and greater expression of MHC class II on phagocytes (Fig. 4). Innate activation via TLR4 engagement of microbial LPS was important for the effectiveness of TBI because removal of LPS signaling components using mice genetically deficient in CD14 or TLR4 reduced destruction of large tumors by the adoptively transferred cells (90).
Fig. 4.
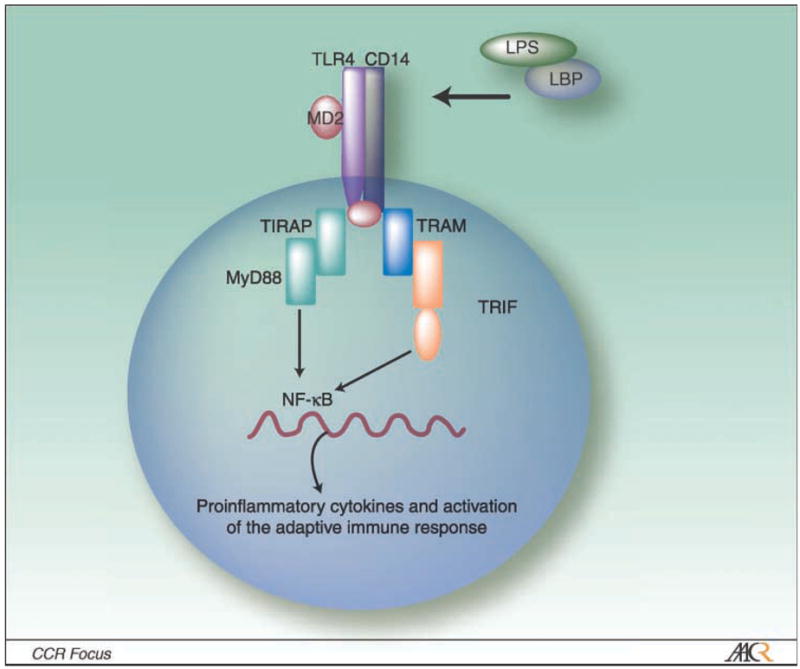
LPS signals the TLR4 pathway on an APC (i.e., monocyte or dendritic cell). TLR4 is a pathogen-associated receptor for Gram-negative bacterial product LPS.TLR4 requires MD-2, a secreted molecule, to functionally interact with the LPS:LPS binding protein complex (LPB). CD14 stabilizes and enhances LPS signaling. TLR4 engagement of LPS leads to nuclear factor-κB translocation via MyD88-dependent and MyD88-independent pathway that involve the TIR domain–ontaining adapter protein TIRAP. Adapted by permission from MacMillan Publisher Ltd: Nature Immunology 4; 1144–1150; Copyright 2003.
Depletion of Sinks and Suppressors: Requirements for TLR Agonists to Enhance Adoptive Immunotherapy
Investigators have previously reported that TLR agonists can inhibit tumor growth in prevention models of adoptive immunotherapy (105–108). Because of this finding and because endogenous microbial LPS plays a role in the effectiveness of TBI, we hypothesized that exogenous administration of LPS to nonirradiated mice might replace preconditioning animals with TBI. We found, however, that exogenous LPS alone does not mediate regression of large, established, nonimmunogenic B16 tumors in nonirradiated mice receiving adoptive immunotherapy in comparison with mice irradiated with 5 Gy TBI. Furthermore, administration of other TLR agonists [i.e., imiquimod, zymosan, poly(I:C), or CpG] alone were likewise ineffective.1 Thus, in contrast to prevention models, TLR agonist cannot replace the effectiveness of TBI in adoptive cell transfer models with large, established tumors.
What is required for TLR agonists to mediate destruction of large tumors by adoptively transferred cells? The answer to this question is clear from mechanisms underlying the effectiveness of TBI (58, 59, 69, 78, 90, 109). TBI enhances adoptive immunotherapy via removal of cytokine sinks, depletion of Treg cells, and activation of innate immunity via TLR ligation. In fact, we found that depletion of cytokine sinks alone (with a single administration of natural killer–depleting antibody), removal of Treg cells alone (with a single administration of CD4-depleting antibody), activation of the innate immune system via TLR4 signaling alone (with systemic administration of LPS at 1 day after adoptive cell transfer), or any two combinations of the three mechanisms underlying the effectiveness of TBI induces nominal antitumor immune responses by the adoptively transferred cells in nonirradiated mice with bulky tumors (90). Destruction of bulky tumors achieved in irradiated mice is only achieved in nonirradiated mice by mimicking all three mechanisms underlying the effectiveness of TBI. These findings are important because they define the variables required for TBI to improve adoptive immunotherapy for cancer.
Administration of LPS to Irradiated Animals Enhances Adoptive Immunotherapy
We recently reported that exogenous administration of LPS to irradiated animals further enhanced the proliferation and function of adoptively transferred cells, resulting in long-term cures and enhanced autoimmune vitiligo in these animals. (90). These data indicated that administration of a clinical adjuvant that signals TLR4 might treat patients with advanced disease receiving T cell–based immunotherapy. It is important to find an alternate agonist to LPS, however, because LPS cannot be realistically used in clinical trials of adoptive immunotherapy because of its inherent toxicity (110).
It is important to keep in mind that the expression and function of TLRs on immune cells in mice are different than humans (111). Thus, a variety of clinically available TLR agonists, such as virally derived imiquimod, that were ineffective in our hands (data not shown) might be effective in treating human patients, especially considering that imiquimod has been reported to treat some patients with superficial early-stage disease (112, 113).
Future Directions: Alternate Reagents to Chemotherapy or TBI
Activation of the innate immune system via TLR4 signaling, removal of cytokine sinks, and depletion of MDSC and Treg cells are key mechanisms underlying the effectiveness of TBI (Fig. 3). Based on these mechanisms, alternate reagents could be used in combination to enhance tumor destruction by adoptively transferred cells in humans. Alternate reagents to mimic these mechanisms are outlined in Table 1.
Table 1.
Alternative clinical reagents that might mimic the effectiveness of lymphodepleting preparative regimens
| Reagent | Mechanism of action |
|---|---|
| Depletion of regulatory elements and elimination of lymphocytes that consume homeostatic cytokines | |
| ONTAK | Denileukin diftitoxin |
| Humax-CD4 | Human monoclonal antibody to CD4 molecule |
| RFT5-dgA | Recombinant immunotoxin to IL-2 receptor |
| LMB-2 | Recombinant immunotoxin to Fv fragment of the anti-TAC anti-CD25 mAb |
| Rituximab | Depletes human B cells |
| Activation of the innate immune system | |
| MPL | TLR2/4 |
| Imiquimod | TLR7 agonist |
| CpG ODN 7909 | TLR9 agonist |
| Other reagents that might boost the expansion and/or function of adoptively transferred cells | |
| HSC | |
| Exogenous administration of IL-7, IL-12, IL-15, and/or IL-21 | |
| CD40 ligation | Signal 2: T cells expressing CD40L engage CD40 molecule on APCs |
| CD28 | Signal 2: T cells expressing CD28 engage B7 molecule on APCs |
The patient’s innate immune system might be activated with clinically relevant TLR agonists, including monophosphoryl lipid A, imiquimod, or CpG ODN 7909 (34, 35, 105–108, 114–117). The CD40 costimulatory molecule, which is up-regulated on innate immune cells, such as dendritic cells, upon their activation might also enhance adoptive immunotherapy through engaging the CD40L molecule on adoptively transferred tumor-specific T cells. A recombinant human CD40 ligand could be used to mimic the activated innate immune cell, thereby enhancing the tumor-specific transferred T cells (118–123). Because combining TLR agonists have been reported to further activate the innate immune system, coadministration of these adjuvants might greatly enhance adoptive immunotherapy (124, 125).
A number of approaches to eliminate human Treg cells and cytokines sinks have been done with varying degrees of success, including ONTAK, Hu Max-CD4 (Zanolimumab), and RFT5 (126–133). Furthermore, B cells might act as sinks and/ or suppressor lymphocytes and thus their removal with rituximab, a chimeric anti-CD20 monoclonal antibody, might also improve adoptive immunotherapy (134, 135). Alternatively, homeostatic cytokines IL-7, IL-15, or IL-21 could be administered systemically to support the adoptively transferred cells because they are of low basal level in patient (136–143). Importantly, combining these reagents with clinical TLR agonists might greatly improve adoptive immunotherapy.
TLR Ligation May Foster the Development of Th17-like Cells
TLR agonists have been reported to reverse Treg-mediated suppression on effector CD8 T cells, thereby enhancing their effector function (105, 106, 144). This blockade was partly dependent on IL-6 (produced by TLR-ligated APCs; ref. 144). However, emerging data indicate that IL-6 (together with transforming growth factor-β produced by activated APCs) also supports the development of CD4 IL-17 producing cells known as Th17 cells (145, 146). Given the finding that Th17-like cells exacerbate autoimmune responses (ranging from uveitis, scleritis, arthritis to myocarditis) and are abundant in intestinal disease models (i.e., inflammatory bowel diseases such as colitis and Crohn’s disease; refs. 147, 148), it is possible that TLR ligation of translocated microbes might also fuel surviving and reconstituting CD4 cells to develop into Th17-like cells (149, 150). Although the net effect of lymphodepletion-induced TLR4 ligation on surviving and reconstituting CD4 cells is less clear than its effect on Treg cells and APCs, ablation might ultimately increase the antitumor reactivity of transferred T cells by increasing the activation and availability of Th17-like cells.
Conclusion
Lymphodepletion with chemotherapy or TBI enhances adoptive immunotherapy via several mechanisms. Beyond the removal of cytokine sinks and Treg cells, translocation of gut microflora and especially of microbial-derived LPS by TBI can clearly affect the outcome of adoptive immunotherapy, a finding reminiscent of Coley’s findings published >100 years ago.
Importantly, beyond a greater innate activation by gut microbes and more complete removal of inhibitory host cells (i.e., Foxp3+CD4+ cells, CD8+ cells, and NK1.1+ cells) with a myeloablative preparative regimen, the HSC transplant promoted superior destruction of tumor by adoptively transferred CD8+ T cells (58). The efficacy of the transplant was attributed to the ability of the HSC to support the enhanced expansion and function of tumor-specific CD8+ T cells. Interestingly, however, little is known about the specific factors produced by HSC that improved the efficacy of the adoptively transferred cells. It is likely that the factors that influence HSC will be important for current T cell–based immunotherapy.
Although the tolerated doses of TBI and chemotherapy are well known, these systemic approaches are not devoid of toxicities. More targeted approaches that recapitulate the benefits of lymphodepletion may become valuable in the development of adoptive immunotherapy. The improved understanding of the mechanisms of action of TBI generates potential alternatives in the nonirradiated host, such as antibody-mediated lymphodepletion, administration of homeostatic cytokines, exogenous delivery of vaccine and/or TLR agonists, as well as HSC transplant in combination with adoptive immunotherapy (Table 1). The findings described in this review may therefore initiate novel treatment modalities for adoptive immunotherapy.
It is important to recognize the potential limitations of T cell–based immunotherapies. The extent to which tumor histologies other than melanoma will be susceptible to adoptive immunotherapy–based approaches is not yet known (151) and tumor cells are capable of escaping from T cell – based immunotherapies through a variety of mechanisms (152, 153), included those described elsewhere in this Clinical Cancer Research Focus issue (154–157).
The ability to genetically engineer T cells promises to expand the histologies that will be susceptible to tumor destruction (40). The engagement of the innate immune system as a trigger of the adaptive immune system represents a powerful new approach as the field of the adoptive immunotherapy of cancer moves forward (90).
Acknowledgments
We thank Bianca Heemskerk, Julie Hong, Jennifer Wargo, and John Wunderlich for critically reading the manuscript; and the clinical team and the patients at the National Cancer Institute in Bethesda, Maryland, for help and guidance in the development of new cancer immunotherapies.
Footnotes
C. Paulos, unpublished data.
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases.1893. Clin Orthop Relat Res. 1991:3–11. [PubMed] [Google Scholar]
- 2.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–2. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–6. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA, Jr, Goodnow CC, Medzhitov R. Danger pathogen on the premises! Immunological tolerance Curr Biol. 1996;6:519–22. doi: 10.1016/s0960-9822(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 8.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–11. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 9.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;(54 Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 13.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–5. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 16.McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–4. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 17.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–14. [PubMed] [Google Scholar]
- 20.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9. [PubMed] [Google Scholar]
- 21.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–69. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 22.Copelan EA. Hematopoietic stem-cell transplantation. N Engl JMed. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 23.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 24.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor- α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–91. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–8. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 26.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Fowler DH, Foley J, Whit-Shan Hou J, et al. Clinical “cytokine storm”as revealed by monocyte intracellular flow cytometry: correlation of tumor necrosis factor αwith severe gut graft-versus-host disease. Clin Gastroenterol Hepatol. 2004;2:237–45. doi: 10.1016/s1542-3565(04)00011-4. [DOI] [PubMed] [Google Scholar]
- 28.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 29.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–9. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 30.Chen PW, Wang M, Bronte V, Zhai Y, Rosenberg SA, Restifo NP. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J Immunol. 1996;156:224–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SA, Restifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. JNatl Cancer Inst. 1997;89:1595–601. doi: 10.1093/jnci/89.21.1595. [DOI] [PubMed] [Google Scholar]
- 32.Restifo NP. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol. 2000;12:597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lienard D, Rimoldi D, Marchand M, et al. Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA. Cancer Immun. 2004;4:4. [PubMed] [Google Scholar]
- 35.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–46. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appay V, Jandus C, Voelter V, et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–8. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14639–45. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 39.deWitte MA, Coccoris M, Wolkers MC, et al. Targeting self-antigens through allogeneic TCR gene transfer. Blood. 2006;108:870–7. doi: 10.1182/blood-2005-08-009357. [DOI] [PubMed] [Google Scholar]
- 40.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg PD. Therapy of murine leukemia with cyclophosphamide and immune Lyt-2+ cells: cytolytic Tcells can mediate eradication of disseminated leukemia. J Immunol. 1986;136:1917–22. [PubMed] [Google Scholar]
- 42.Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- Tcells. Tumor eradication does not require participation of cytotoxic T cells. JExp Med. 1985;161:1122–34. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ Tcell control of CD8+ Tcell reactivity to a model tumor antigen. J Immunol. 2000;164:562–5. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–94. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 45.Touloukian CE, Leitner WW, Topalian SL, et al. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J Immunol. 2000;164:3535–42. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–70. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang LN, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ Tcells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–8. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer DC, Balasubramaniam S, Hanada K, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 53.Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2- lymphocytes. JExp Med. 1981;154:952–63. doi: 10.1084/jem.154.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenberg PD, Cheever MA. Treatment of disseminated leukemia with cyclophosphamide and immune cells: tumor immunity reflects long-term persistence of tumor-specific donor T cells. J Immunol. 1984;133:3401–7. [PubMed] [Google Scholar]
- 55.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–7. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 57.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrzesinski C, Paulos CM, Gattinoni L, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy-how far can we go? Nat Clin Pract Oncol. 2006;3:668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fefer A. Immunotherapy and chemotherapy of Moloney sarcoma virus-induced tumors in mice. Cancer Res. 1969;29:2177–83. [PubMed] [Google Scholar]
- 62.Berendt MJ, North RJ. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980;151:69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Cruz E, Woda BA, Feldman JD. Elimination of syngeneic sarcomas in rats by a subset of T lymphocytes. JExp Med. 1980;152:823–41. doi: 10.1084/jem.152.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dummer W, Niethammer AG, Baccala R, et al. Tcell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–92. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002;99:931–6. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Overwijk WW, Tsung A, Irvine KR, et al. gp100/ pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhai Y, Yang JC, Spiess P, et al. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1and gp100. J Immunother. 1997;20:15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ Tcells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 71.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Immunosuppression in melanoma immunotherapy: potential opportunities for intervention. Clin Cancer Res. 2006;12:2359–65s. doi: 10.1158/1078-0432.CCR-05-2537. [DOI] [PubMed] [Google Scholar]
- 73.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12:4794–803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 74.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 76.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 77.Antony PA, Paulos CM, Ahmadzadeh M, et al. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–66. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antony PA, Restifo NP. CD4+CD25+ Tregulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–8. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antony PA, Restifo NP. Do CD4+ CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother. 2002;25:202–6. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. JExp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 84.Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ Tcell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 85.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. JClin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 87.Bronte V, Wang M, Overwijk WW, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–20. [PMC free article] [PubMed] [Google Scholar]
- 88.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 89.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–51. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 90.Paulos CM, Wrzesinskil C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ Tcells via TLR4 signaling. J Clin invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hellstrom KE, Hellstrom I, Kant JA, Tamerius JD. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978;148:799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–44. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Specht JM, Wang G, Do MT, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–21. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lou Y, Wang G, Lizee G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–90. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 98.Rao JB, Chamberlain RS, Bronte V, et al. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7 – 1 expression. J Immunol. 1996;156:3357–65. [PMC free article] [PubMed] [Google Scholar]
- 99.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–7. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 100.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–8. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 101.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 102.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/He J and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 103.Takeda K, Akira S. Microbial recognition by Toll-like receptors. J Dermatol Sci. 2004;34:73–82. doi: 10.1016/j.jdermsci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 106.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 107.Garbi N, Arnold B, Gordon S, Hammerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–9. doi: 10.4049/jimmunol.172.10.5861. [DOI] [PubMed] [Google Scholar]
- 108.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–64. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 109.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances Tcell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 111.Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–8. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki H, Wang B, Shivji GM, et al. Imiquimod,atopical immune response modifier, induces migration of Langerhans cells. JInvest Dermatol. 2000;114:135–41. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 113.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 114.Wolf IH, Richtig E, Kopera D, Kerl H. Locoregional cutaneous metastases of malignant melanoma and their management. Dermatol Surg. 2004;30:244–7. doi: 10.1111/j.1524-4725.2004.30091.x. [DOI] [PubMed] [Google Scholar]
- 115.Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139:273–6. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- 116.Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995;155:5038–45. [PubMed] [Google Scholar]
- 117.Kochenderfer JN, Simpson JL, Chien CD, Gress RE. Vaccination regimens incorporating CpG-containing oligodeoxynucleotides and IL-2 generate antigen-specific anti-tumor immunity from T cell populations undergoing homeostatic peripheral expansion after BMT. Blood. 2007;110:450–60. doi: 10.1182/blood-2006-11-057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vonderheide RH, Dutcher JP, Anderson JE, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–7. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 119.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 120.Gurunathan S, Irvine KR, Wu CY, et al. CD40 ligand/trimer DNA enhances bothhumoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–71. [PMC free article] [PubMed] [Google Scholar]
- 121.Blair PJ, Riley JL, Harlan DM, et al. CD40 ligand (CD154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J Exp Med. 2000;191:651–60. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vonderheide RH, June CH. A translational bridge to cancer immunotherapy: exploiting costimulation and target antigens for active and passive T cell immunotherapy. Immunol Res. 2003;27:341–56. doi: 10.1385/IR:27:2-3:341. [DOI] [PubMed] [Google Scholar]
- 123.Liebowitz DN, Lee KP, June CH. Costimulatory approaches to adoptive immunotherapy. Curr Opin Oncol. 1998;10:533–41. doi: 10.1097/00001622-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 125.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dang NH, Hagemeister FB, Pro B, et al. Phase II study of denileukin diftitox for relapsed/refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4095–102. doi: 10.1200/JCO.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 127.Hagberg H, Pettersson M, Bjerner T, Enblad G. Treatment of a patient with a nodal peripheral T-cell lymphoma (angioimmunoblastic T-Cell lymphoma) with a human monoclonal antibody against the CD4 antigen (HuMax-CD4) Med Oncol. 2005;22:191–4. doi: 10.1385/MO:22:2:191. [DOI] [PubMed] [Google Scholar]
- 128.Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655–62. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 129.Skov L, Kragballe K, Zachariae C, et al. HuMax-CD4: a fully human monoclonal anti-CD4 antibody for the treatment of psoriasis vulgaris. Arch Dermatol. 2003;139:1433–9. doi: 10.1001/archderm.139.11.1433. [DOI] [PubMed] [Google Scholar]
- 130.Attia P, Powell DJ, Jr, Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother. 2006;29:208–14. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Powell DJ, Jr, de Vries CR, Allen T, Ahmadzadeh M, Rosenberg SA. Inability to mediate prolonged reduction of regulatory T cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy. J Immunother. 2007;30:438–47. doi: 10.1097/CJI.0b013e3180600ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–39. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Powell DJ, Jr, Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. J Immunother. 2005;28:403–11. doi: 10.1097/01.cji.0000170363.22585.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 135.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 136.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ Tcells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang LX, Li R, Yang G, et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–77. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120:121–8. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 141.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–71. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 142.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 143.Guimond M, Fry TJ, Mackall CL. Cytokine signals in T-cell homeostasis. J Immunother. 2005;28:289–94. doi: 10.1097/01.cji.0000165356.03924.e7. [DOI] [PubMed] [Google Scholar]
- 144.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ Tcell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 145.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type1and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 146.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 147.Amadi-Obi A, Yu CR, Liu X, et al. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 148.Hirota K, Hashimoto M, Yoshitomi H, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. JExp Med. 2007;204:41–7. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marsland BJ, Nembrini C, Grun K, et al. TLR ligands act directly upon T cells to restore proliferation in the absence of protein kinase C-θ signaling and promote autoimmune myocarditis. J Immunol. 2007;178:3466–73. doi: 10.4049/jimmunol.178.6.3466. [DOI] [PubMed] [Google Scholar]
- 150.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Restifo NP, Marincola FM, Kawakami Y, Tauben-berger J, Yannelli JR, Rosenberg SA. Loss offunctional β2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–8. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lizee GA, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer: immunotherapy. Clin Cancer Res. 2007;13:5250–6. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 155.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5280–90. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 156.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 157.Gajewski T. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]


