Abstract
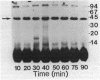
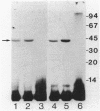
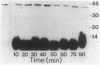
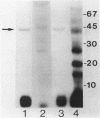
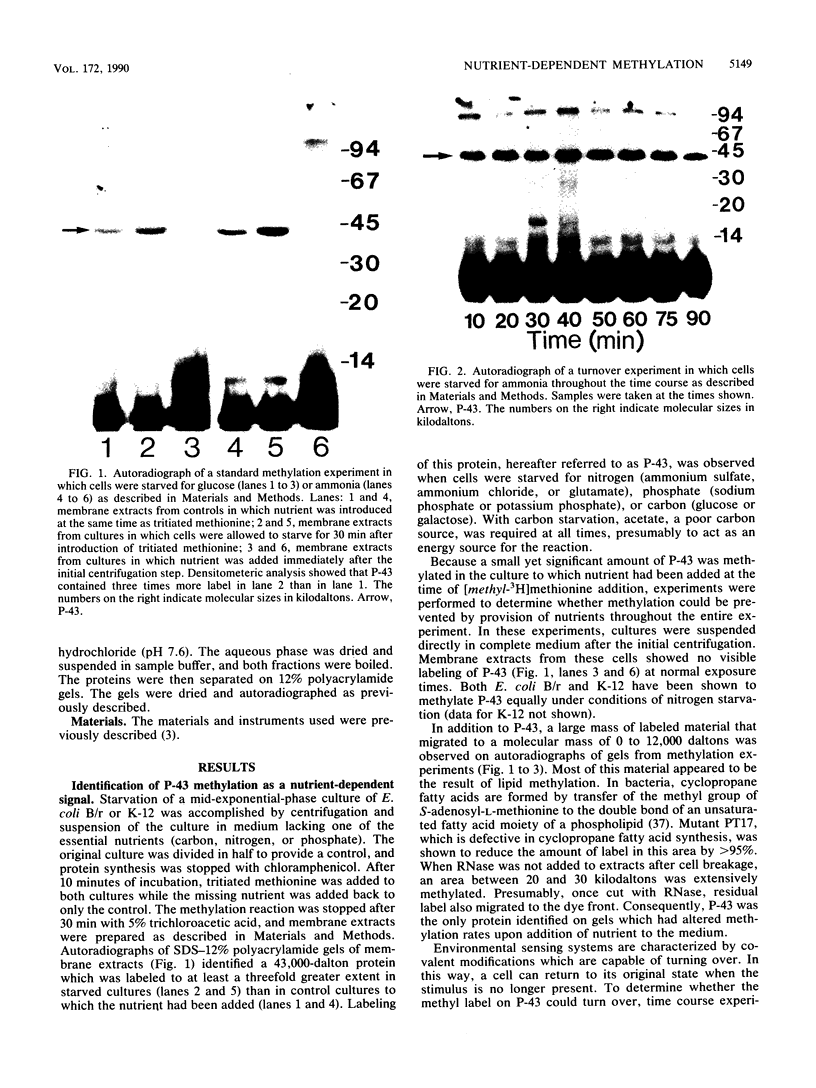
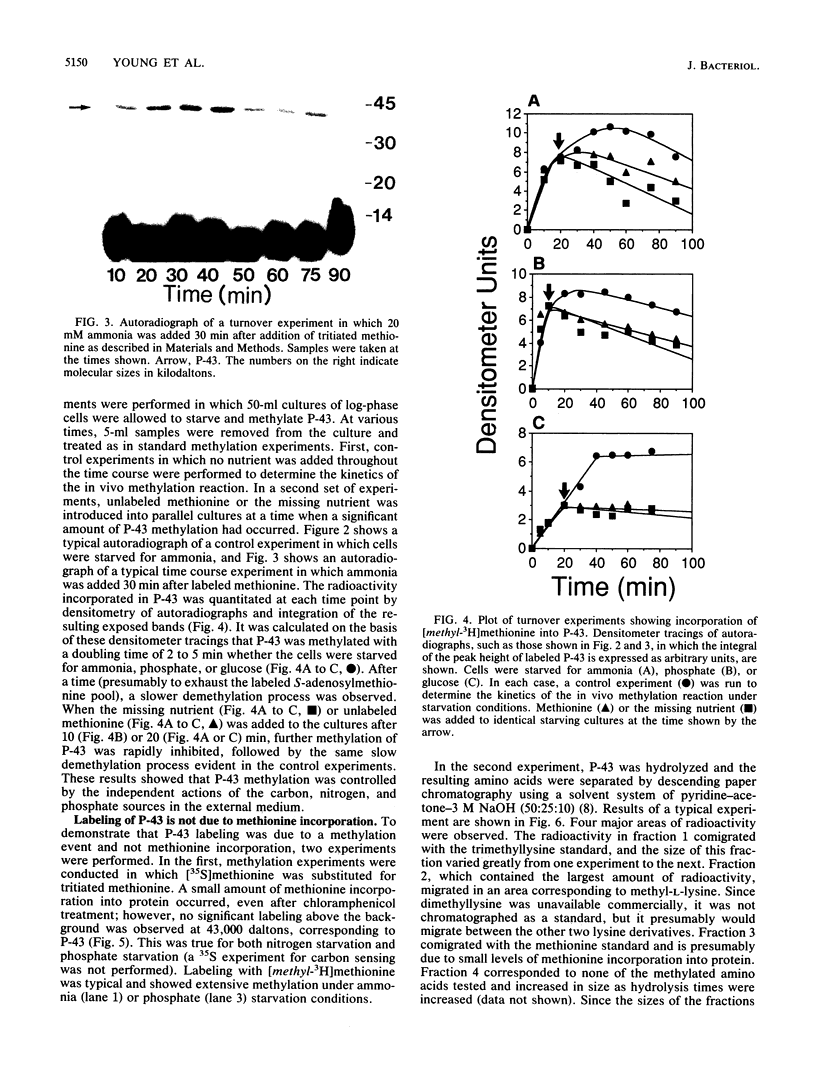
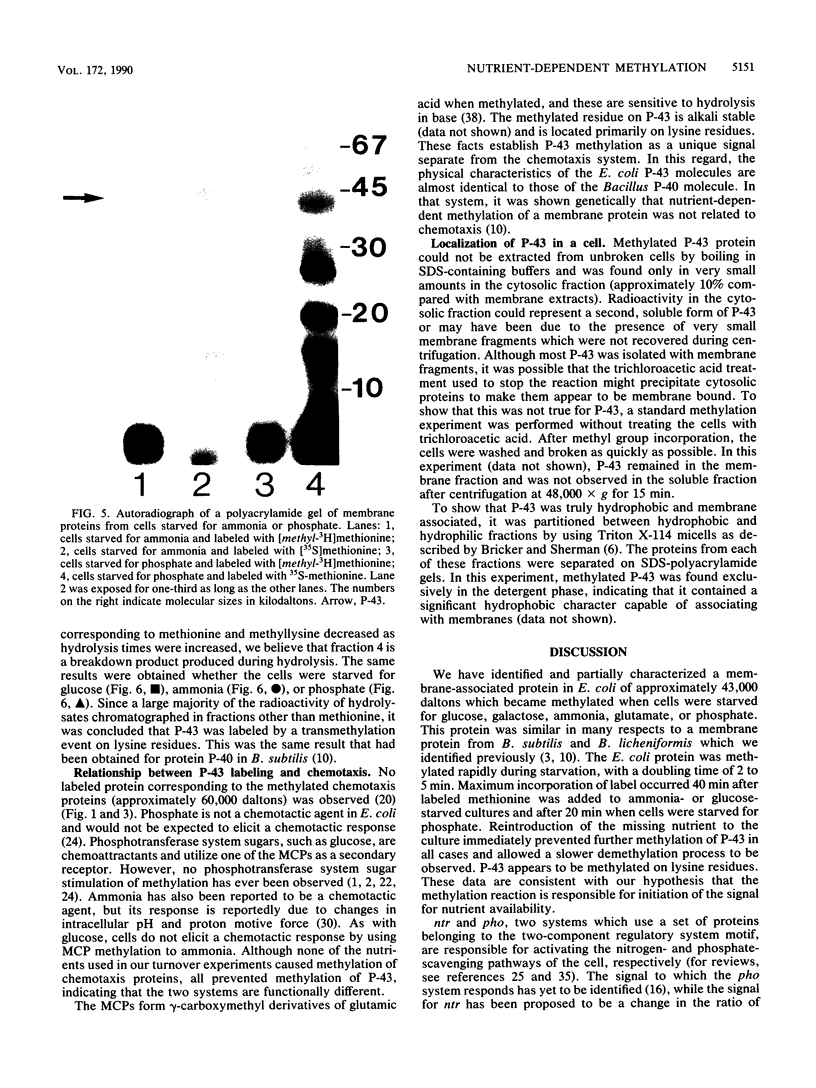
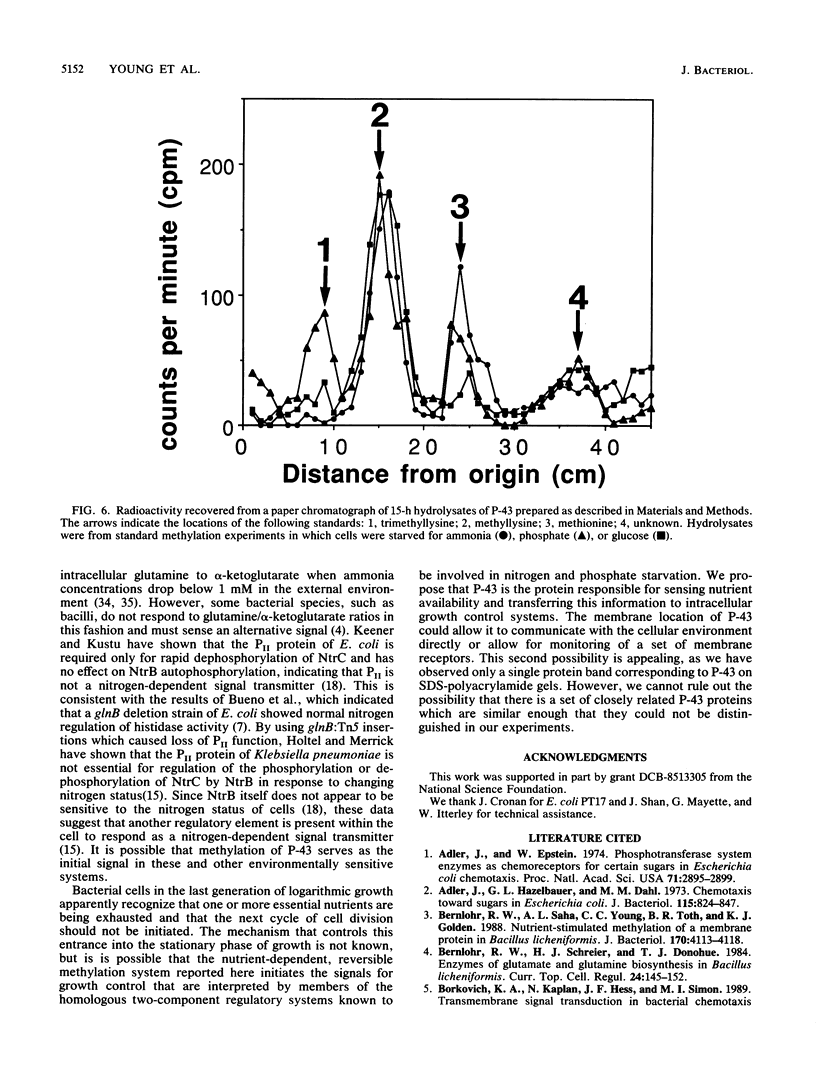
Starvation of a mid-log-phase culture of Escherichia coli B/r for nitrogen, phosphate, or carbon resulted in methylation of a membrane-associated protein of about 43,000 daltons (P-43) in the presence of chloramphenicol and [methyl-3H]methionine. The in vivo methylation reaction occurred with a doubling time of 2 to 5 min and was followed by a slower demethylation process. Addition of the missing nutrient to a starving culture immediately prevented further methylation of P-43. P-43 methylation is not related to the methylated chemotaxis proteins because P-43 is methylated in response to a different spectrum of nutrients and because P-43 is methylated on lysine residues. The characteristics of P-43 are similar to those of a methylated protein previously described in Bacillus subtilis and B. licheniformis (R. W. Bernlohr, A. L. Saha, C. C. Young, B. R. Toth, and K. J. Golden, J. Bacteriol. 170:4113-4118, 1988; K. J. Golden and R. W. Bernlohr, Mol. Gen. Genet. 220:1-7, 1989) and are consistent with the proposal that methylation of this protein functions in nutrient sensing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Saha A. L., Young C. C., Toth B. R., Golden K. J. Nutrient-stimulated methylation of a membrane protein in Bacillus licheniformis. J Bacteriol. 1988 Sep;170(9):4113–4118. doi: 10.1128/jb.170.9.4113-4118.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Schreier H. J., Donohue T. J. Enzymes of glutamate and glutamine biosynthesis in Bacillus licheniformis. Curr Top Cell Regul. 1984;24:145–152. doi: 10.1016/b978-0-12-152824-9.50020-4. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Kaplan N., Hess J. F., Simon M. I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno R., Pahel G., Magasanik B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J Bacteriol. 1985 Nov;164(2):816–822. doi: 10.1128/jb.164.2.816-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Chang N. Methylation of the ribosomal proteins in Escherichia coli. Nature and stoichiometry of the methylated amino acids in 50S ribosomal proteins. Biochemistry. 1975 Feb 11;14(3):468–477. doi: 10.1021/bi00674a002. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Bernlohr R. W. Properties of the Bacillus licheniformis A5 glutamine synthetase purified from cells grown in the presence of ammonia or nitrate. J Bacteriol. 1981 Aug;147(2):589–601. doi: 10.1128/jb.147.2.589-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden K. J., Bernlohr R. W. Defects in the nutrient-dependent methylation of a membrane-associated protein in spo mutants of Bacillus subtilis. Mol Gen Genet. 1989 Dec;220(1):1–7. doi: 10.1007/BF00260847. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Failure of sensory adaptation in bacterial mutants that are defective in a protein methylation reaction. Cell. 1978 Dec;15(4):1231–1240. doi: 10.1016/0092-8674(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Park C., Nowlin D. M. Adaptational "crosstalk" and the crucial role of methylation in chemotactic migration by Escherichia coli. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1448–1452. doi: 10.1073/pnas.86.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Holtel A., Merrick M. J. The Klebsiella pneumoniae PII protein (glnB gene product) is not absolutely required for nitrogen regulation and is not involved in NifL-mediated nif gene regulation. Mol Gen Genet. 1989 Jun;217(2-3):474–480. doi: 10.1007/BF02464920. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Keener J., Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M. R., Doak T. G., Dahlquist F. W. Sensory adaptation in bacterial chemotaxis: regulation of demethylation. J Bacteriol. 1985 Sep;163(3):983–990. doi: 10.1128/jb.163.3.983-990.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Mutoh N., Simon M. I. Cloning of the C-terminal cytoplasmic fragment of the tar protein and effects of the fragment on chemotaxis of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2521–2526. doi: 10.1128/jb.170.6.2521-2526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Chock P. B. Interconvertible enzyme cascades in metabolic regulation. Curr Top Cell Regul. 1978;13:53–95. doi: 10.1016/b978-0-12-152813-3.50007-0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F., Cronan J. E., Jr Selection and properties of Escherichia coli mutants defective in the synthesis of cyclopropane fatty acids. J Bacteriol. 1976 Feb;125(2):518–523. doi: 10.1128/jb.125.2.518-523.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Brissette R. E., Rampersaud A., Forst S. A., Oosawa K., Inouye M. Activation of bacterial porin gene expression by a chimeric signal transducer in response to aspartate. Science. 1989 Sep 15;245(4923):1246–1249. doi: 10.1126/science.2476847. [DOI] [PubMed] [Google Scholar]