Abstract
Objective
Coupling of glucose oxidation to glycolysis is lower in hypertrophied than in non-hypertrophied hearts, contributing to the compromised mechanical performance of hypertrophied hearts. Here, we describe studies to test the hypothesis that low coupling of glucose oxidation to glycolysis in hypertrophied hearts is due to reduced activity and/or expression of the pyruvate dehydrogenase complex (PDC).
Methods
We examined the effects of dichloroacetate (DCA), an inhibitor of PDC kinase, and of alterations in exogenous palmitate supply on coupling of glucose oxidation to glycolysis in isolated working hypertrophied and control hearts from aortic-constricted and sham-operated male Sprague–Dawley rats. It was anticipated that the addition of DCA or the absence of palmitate would promote PDC activation and consequently normalize coupling between glycolysis and glucose oxidation in hypertrophied hearts if our hypothesis was correct.
Results
Addition of DCA or removal of palmitate improved coupling of glucose oxidation to glycolysis in control and hypertrophied hearts. However, coupling remained substantially lower in hypertrophied hearts. PDC activity in extracts of hypertrophied hearts was similar to or higher than in extracts of control hearts under all perfusion conditions. No differences were observed between hypertrophied and control hearts with respect to expression of PDC, PDC kinase, or PDC phosphatase.
Conclusions
Low coupling of glucose oxidation to glycolysis in hypertrophied hearts is not due to a reduction in PDC activity or subunit expression indicating that other mechanism(s) are responsible.
Keywords: Energy metabolism, Gene expression, Glycolysis, Hypertrophy, Protein phosphorylation
1. Introduction
Glucose utilization is enhanced in hearts exposed to a prolonged pressure or volume overload [1–5]. In myocardium, glucose is catabolized predominantly by the glycolytic pathway which is linked (or coupled) to a varying degree to oxidative degradation to CO2 in the mitochondria [6–8]. Glucose that is not oxidized is, instead, converted to lactate and alanine [6–8]. Flux through these different catabolic pathways is not uniformly enhanced in the hypertrophied heart [4,5].
Rates of glycolysis are accelerated in non-ischemic, ischemic, and reperfused hypertrophied hearts compared to non-hypertrophied hearts [4,5,9–12]. However, there is no corresponding increase in rates of glucose oxidation [4,5,9–12]. In fact, glucose oxidation rates can actually be lower in hypertrophied hearts than non-hypertrophied hearts, despite the enhanced rates of pyruvate generation [9,13]. As a consequence, coupling of glucose oxidation to glycolysis is lower in hypertrophied hearts (10–11% glucose oxidized) than in non-hypertrophied hearts (20– 25% glucose oxidized) [9,13,14]. These alterations in glucose catabolism have functional relevance because low coupling of glucose oxidation to glycolysis contributes to contractile dysfunction of hypertrophied hearts, especially during reperfusion after ischemia [9,11,12].
Low coupling of glucose oxidation to glycolysis in hypertrophied hearts is surprising because fatty acid oxidation rates are also low in these hearts [4,5]. Low rates of fatty acid oxidation would normally be expected to cause a compensatory stimulation of myocardial glucose oxidation and increased coupling [14]. Moreover, the increased production of pyruvate accompanying accelerated rates of glycolysis is expected to favour higher rates of glucose oxidation by way of pyruvate-induced activation of the pyruvate dehydrogenase complex [15–19]. The mechanism(s) responsible for low coupling of glucose oxidation to glycolysis in hypertrophied hearts are not yet known.
The multi-enzyme pyruvate dehydrogenase complex (PDC) catalyzes the oxidative decarboxylation of pyruvate and contributes strongly to flux control of myocardial glucose oxidation [15–17,20]. The activity of PDC and, therefore, the rate of pyruvate decarboxylation are regulated, in concert, by reversible phosphorylation of the α-subunit of the E1 (pyruvate dehydrogenase) component of PDC and inhibition by its end products, acetyl CoA and NADH2 [15–17,20]. The proportion of active dephosphorylated PDC in the heart is determined by the opposing actions of pyruvate dehydrogenase kinase isoforms (PDK- 1, -2, and -4) and pyruvate dehydrogenase phosphatase (PDP) [15–17,20–22].
Alterations in PDC activity are observed in a number of pathological states. For example, in diabetes mellitus, starvation, and hyperthyroidism, the activation state of PDC is reduced without changes in the expression of the complex itself [17,23]. The reduced activation state of myocardial PDC in these (patho)physiological states can be accounted for by stable increases in the activity of PDK [17,22–24], in turn explained by dramatic increases in PDK mRNA and protein levels [22,23]. As in hypertrophy, hearts from diabetic rats show lower glucose oxidation rates and lower coupling of glucose oxidation to glycolysis than normal hearts [25].
The studies reported here were carried out to test the hypothesis that coupling of glucose oxidation to glycolysis is low in hypertrophied hearts because the activation state of PDC is reduced by increased expression of PDK in hypertrophied hearts compared to non-hypertrophied hearts.
2. Experimental methods
2.1. Animal model
Pressure-overload left ventricular hypertrophy was produced in 3-week-old male Sprague–Dawley rats (50–75 g) by constriction of the suprarenal abdominal aorta with a metallic clip (0.4 mm diameter) [4]. In control rats, the aorta was isolated but not constricted. Experiments were performed 8 weeks after surgery. This model of mild cardiac hypertrophy was used because it exposes the effects of hypertrophy without overt cardiac failure [26]. The model is also characterized by lack of obvious fibrosis, as documented previously [27,28] and confirmed by the similar recovery of protein from hearts (Control, 792±43 vs. Hypertrophy, 746±22 mg protein/g dry weight, n=15–16 per group, P=NS).
Food and water were available ad libitum. These experiments were carried out in accordance with guidelines set out by the Canadian Council on Animal Care and the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
2.2. Heart preparation and perfusion protocol
As described [4,9,10,29], spontaneously beating hearts from halothane (3–4%)-anaesthetized rats were perfused as isolated working preparations at a preload of 11.5 mmHg and an afterload of 80 mmHg with 100 ml of modified Krebs-Henseleit solution. The solution contained 1.2 mM palmitate pre-bound to defatted albumin (final concentration 3%, w/v), 5.5 mM [U-14C]/[5-3H]glucose, 0.5 mM lactate, 2.5 mM calcium chloride, and 100 mU/l insulin, except where indicated, and was continuously circulated through the closed perfusion system. Calcium concentration above the physiological level (1.25 mM) was used to offset binding to albumin and to obtain optimum and sustained cardiac performance [30]. High concentrations of palmitate were utilized to minimize differences in rates of fatty acid oxidation, evident at 0.4 mM palmitate, between control and hypertrophied hearts [4,11]. Insulin was included in the perfusate to ensure that the uptake and phosphorylation of glucose were not limiting. The solution was oxygenated with 95% O2/5% CO2, filtered in-line with each recirculation cycle, and maintained at 37 °C throughout the perfusion.
A pressure transducer (Viggo-Spectramed, Oxmard, CA, USA) inserted in the afterload line was used to measure heart rate and peak systolic pressure. Cardiac output and aortic flow were measured by means of external flow probes (Transonic Systems, Ithaca, NY, USA) on the preload and afterload lines, respectively. Coronary flow was calculated as the difference between cardiac output and aortic flow. Rate-pressure product, calculated as the product of heart rate and peak systolic pressure, and hydraulic work, calculated as the product of cardiac output and peak systolic pressure, were used to measure external work performed by the heart. All perfusions were 30 min in duration. Measurements of heart function were taken every 10 min.
Hearts were studied under four different conditions in each group, being perfused with either the perfusate described above (Basal), with the addition of dichloroacetate (DCA, 1 or 3 mM), or with palmitate omitted from the perfusate (No palmitate). When present, DCA was added at the beginning of the working heart perfusion. According to our working hypothesis, activation of PDC by DCA or in the absence of palmitate should normalize coupling of glucose oxidation to glycolysis in hypertrophied hearts.
An additional series of experiments was performed to determine if accumulation of lactate over the duration of the perfusion could contribute to the changes in glucose catabolism. Hearts were perfused as above, except that the perfusate included 3 mM DCA and 3 mM lactate, a lactate concentration greater than that observed at the end of 30-min perfusion (Control, 1.15±0.09 mM, n=3; Hypertrophy, 1.53±0.20 mM, n=4, P<0.05).
At the end of the perfusion period, the ventricles were quickly frozen using tongs cooled to the temperature of liquid nitrogen. Selected hearts were also rapidly frozen after removal from anaesthetized rats. The frozen tissue was weighed to determine ventricular weight, powdered, and then stored at −70 °C. Remaining atrial tissue and portions of ventricular tissue were dried, weighed, and used in the calculation of total heart weight.
2.3. Measurement of glucose oxidation and glycolysis
Glucose oxidation was measured by quantitatively determining the rate of 14CO2 production from [U-14C]glucose [4,9,10]. Rates of glycolysis were determined by measuring the rate of 3H2O production from [5-3H]glucose [4,9,10]. Samples of perfusate were taken every 10 min throughout the perfusion and radioisotope content determined by standard techniques.
2.4. Determination of PDC and PDK activity
2.4.1. PDC activity
PDC activity was determined in homogenates of frozen ventricular tissue by measuring [14C]citrate synthesis from [14C]oxaloacetate and PDC-derived acetyl CoA [31–33].
2.4.1.1. Total PDC
Samples of frozen ventricular muscle (100 mg) were extracted in 2.4 ml of ice-cold buffer using a Polytron homogenizer (setting #6, for two bursts of 2 s). The extraction buffer, pH 7.2, contained Tris–HCl (50 mM), sucrose (200 mM), KCl (50 mM), EGTA (5 mM), dichloroacetate (5 mM), glutathione (2.5 mM) and Triton X-100 (0.1%, w/v). The homogenate was centrifuged (10,000×g for 2 min) and supernatant fractions kept on ice prior to the PDC assays, usually carried out within 1 h. Samples of 10,000×g supernatant (100 μl) were incubated with pre-activated purified recombinant pyruvate dehydrogenase phosphatase-1 (PDP-1) [34] for 20 min at 37 °C to allow PDC dephosphorylation. Pre-activated PDP-1 (10 μg recombinant protein per PDC incubation) was prepared by incubating purified PDP-1 with Mg2+ (10 mM) and EGTA-buffered Ca2+ (0.1 mM) in MOPS (25 mM) buffer at 30 °C for 10 min. PDC reactions were then initiated by the addition of pre-warmed buffer (pH 7.8) giving final concentrations of Tris–HCl (120 mM), EDTA (0.6 mM), MgCl2 (1.2 mM), NAD+ (0.6 mM), Coenzyme A (0.6 mM), thiamine pyrophosphate (1.2 mM), and sodium pyruvate (1.2 mM). Incubations were allowed to continue for 10 min and were then stopped with removal of aliquots (210 μl) into ice-cold perchloric acid (40 μl, 0.5 M). After incubation on ice for at least 5 min, samples were neutralized with 10 μl KHCO3 (2.2 M) and centrifuged (10,000×g for 3 min). Clarified, neutralized samples were stored at −20 °C for later assay of the acetyl-CoA produced by the PDC reaction. This was accomplished by allowing reaction of the PDC-derived acetyl-CoA with [14C]oxaloacetate to produce [14C]citrate [31–33]. Using the conditions described, rates of acetyl-CoA production by PDC in tissue homogenates were linear for at least 20 min.
2.4.1.2. Active PDC
Tissue homogenates were prepared as for total PDC except that homogenization buffer also contained NaF (50 mmol/l) to inhibit PDP. Assay incubations, quenching by acidification, and then neutralization were all as described for total PDC activity, except that no incubation with PDP was included prior to initiation of the PDC reaction. Activities were expressed as international units (U, μmol product produced/min). To avoid bias in estimating recovery of PDC, total and active PDC were expressed in relation to frozen dry tissue weight, recovered total protein [35], and recovered glutamate dehydrogenase (GDH) activity [36]. GDH was used as an index of the recovery of protein from the mitochondrial matrix. The activation state of PDC (% active PDH) was expressed as the quotient of active and total PDC multiplied by 100.
2.4.2. PDK activity
PDK activity was determined by measuring the rate at which activated, dephosphorylated PDC was inactivated by the endogenous PDK upon incubation with ATP. Fresh heart supernatant fractions were prepared using homogenization buffer as described above, omitting sodium fluoride and DCA. Following PDC dephosphorylation, phosphorylation was then initiated by adding ATP (5 mmol/l) and sodium fluoride (50 mmol/l). Aliquots were removed just prior to and at 0.5, 1, 2, 5, 10, and 20 min after the addition of ATP to determine PDC activity in buffer containing pyruvate and DCA, as described above. Controls for the ATP-dependent inhibition included parallel incubation of the PDC samples under identical conditions except for omission of ATP. PDC activity changed by less than 10% over 20-min incubation in the absence of ATP, confirming that ATP-independent changes in PDC activity were insignificant.
2.5. PDK mRNA expression
Northern blot analysis was performed essentially as described [37]. Hybridization was performed with [32P]-labelled cDNA probes of rat PDK1 [38], PDK2 [37], and PDK4 [21]. RNA lane loading was monitored by repeat hybridization of the membrane with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using a GAPDH cDNA probe [39]. Band signal intensity was quantified by densitometry and expressed in relation to GAPDH signal intensity.
2.6. Immunoblot analysis of PDC and PDP protein expression
Protein samples from mitochondria isolated from un-perfused rat hearts [40] were subjected to 10% SDS–PAGE [41] and transferred to nitrocellulose membranes. Membranes were blocked and incubated with rabbit polyclonal antibodies against PDC or PDP [22,34,42]. Chemiluminescence-based detection (NEN, Boston, MA) and quantitation by densitometry followed incubation of the membranes with anti-rabbit secondary antibody.
2.7. Statistical analysis
Weight, enzyme activity, and expression data were analyzed using a two-way analysis of variance (ANOVA). Left ventricular function, glucose oxidation, and glycolysis were examined using the repeated measures two-way ANOVA. A corrected P-value >0.05 was considered non-significant (NS). Values are expressed as the mean±standard error of the mean (S.E.M.).
3. Results
3.1. Animal data
The weight of hearts from aortic-banded rats (2.30±0.05 g, n=30) was significantly increased compared to that of sham-operated control rats (1.90±0.04 g, n=30, P<0.05). Body weights of aortic-banded rats (473±6 g) were not significantly different from sham-operated control rats (458±7 g, P=NS).
3.2. Mechanical function of control and hypertrophied hearts
All functional parameters were lower in hypertrophied hearts than control hearts under basal conditions (Table 1). Exposure to 1 mM DCA significantly improved cardiac output and coronary flow in hypertrophied hearts. Perfusion with 3 mM DCA had minor effects on function, the only significant effect being a modest increase in heart rate in hypertrophied hearts. Most functional parameters remained lower in DCA-treated hypertrophied hearts than in DCA-treated control hearts. The absence of palmitate also had minor effects on function with an increase in heart rate and a decrease in peak systolic pressure in both groups. Exposure to both 3 mM DCA and 3 mM lactate had no significant effects on heart performance (data not shown).
Table 1.
Mechanical function of control and hypertrophied hearts perfused with or without dichloroacetate (DCA) or in the absence of palmitate
| Control
|
Hypertrophy
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Basal (8) | 1 mM DCA(8) | 3 mM DCA (5) | No palmitate (4) | Basal (8) | 1 mM DCA (8) | 3 mM DCA (5) | No palmitate (4) | |
| Heart rate | 269±6 | 268±9 | 276±5 | 282±11 | 239±7* | 252±9 | 261±5† | 269±4† |
| Peak systolic pressure | 119±3 | 120±3 | 116±2 | 110±3* | 114±2* | 118±5 | 110±2*‡ | 105±2*†‡ |
| Cardiac output | 69.6±1.3 | 73.0±1.1 | 70.4±2.4 | 70.7±0.4 | 57.0±1.0* | 62.8±1.2*†‡ | 58.6±1.8*‡ | 57.8±1.3*‡ |
| Rate pressure product | 32.0±0.6 | 32.0±0.4 | 32.0±0.9 | 31.1±0.6 | 27.2±0.6* | 29.4±0.6 | 28.6±0.3* | 28.1±0.4* |
| Hydraulic work | 83.0±2.5 | 87.7±2.7 | 81.8±3.9 | 77.5±8.0 | 65.1±1.8* | 73.9±3.8*‡ | 64.2±2.1*‡ | 59.3±2.4*†‡ |
| Coronary flow | 19.9±1.0 | 20.3±0.9 | 18.5±1.0 | 17.3±0.5 | 10.5±0.8* | 14.2±0.5*†‡ | 12.4±1.1*‡ | 12.8±0.1*‡ |
Values are those at end of the perfusion period. Numbers per group are in parentheses. Heart rate, bpm; peak systolic pressure, mmHg; cardiac output, ml/min; rate pressure product, mmHg bpm/1000; hydraulic work, mmHg ml/min per 100; coronary flow, ml/min per g wet wt.
Different from Basal Control, P<0.05;
different from corresponding Basal group, P<0.05;
different from corresponding DCA-treated or No palmitate Control, P<0.05.
3.3. Glucose oxidation and glycolysis
In the absence of DCA, glucose oxidation was slightly but not significantly lower in hypertrophied hearts than in control hearts (Fig. 1A). Glycolysis, on the other hand, was significantly accelerated in hypertrophied hearts compared to control hearts (Fig. 1B).
Fig. 1.
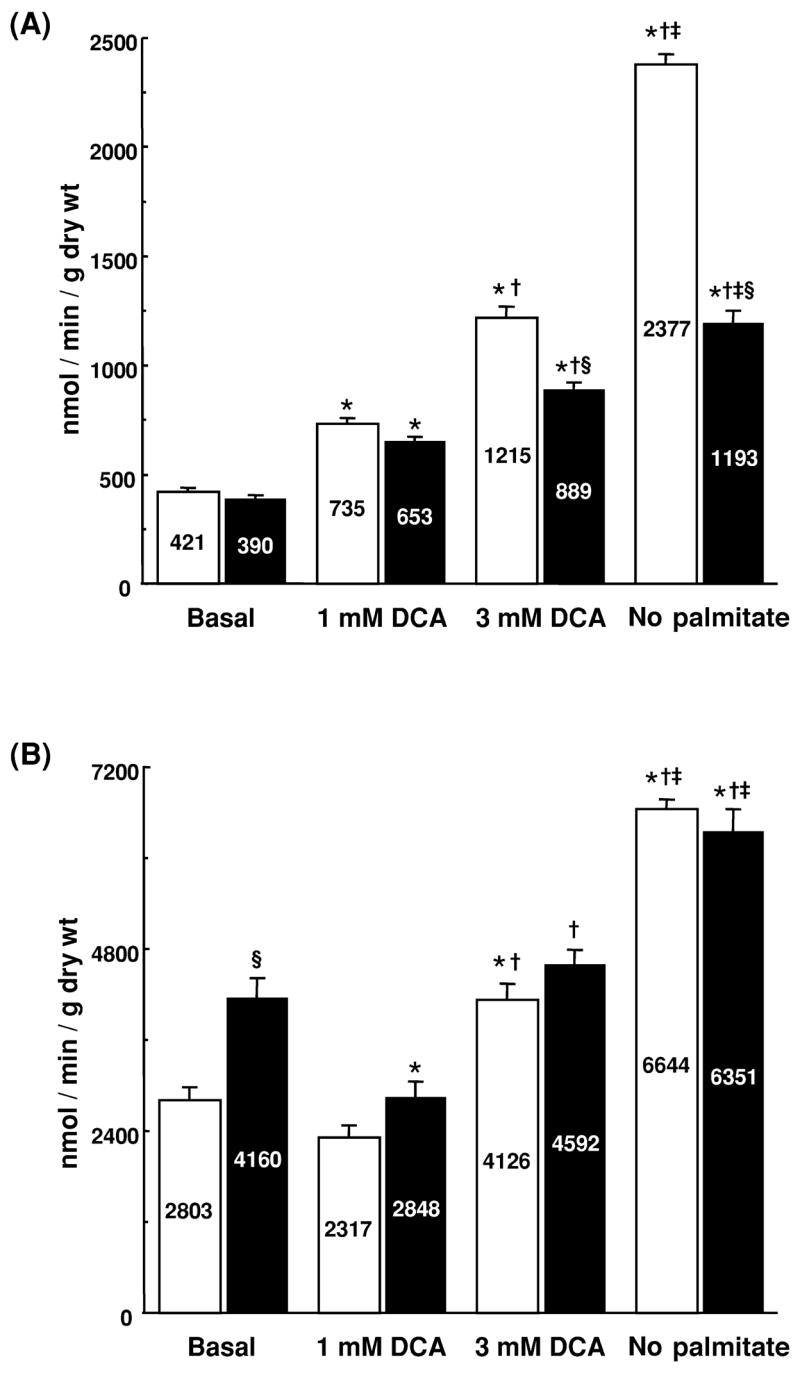
Glucose oxidation (A) and glycolysis (B) in isolated working control (open bars) and hypertrophied (solid bars) rat hearts perfused with or without dichloroacetate (DCA) or in the absence of palmitate (No palmitate). *Different from corresponding Basal group, P<0.05. †Different from corresponding 1 mM DCA group, P>0.05. ‡Different from corresponding 3 mM DCA group, P>0.05. §Different from corresponding DCA-treated or No palmitate Control, P<0.05. n=5–8 hearts per group.
Glucose oxidation was stimulated 2–3-fold by DCA in both groups. However, absolute rates of glucose oxidation were lower in hypertrophied hearts than in control hearts (Fig. 1A), particularly at the highest concentration of DCA (3 mM). The effects of DCA on glycolysis were concentration dependent and complex (Fig. 1B). In the presence of 1 mM DCA, glycolysis was significantly inhibited in hypertrophied hearts but was not significantly inhibited in control hearts. In contrast, exposure of hearts to 3 mM DCA led to higher rates of glycolysis than those in hearts exposed to 1 mM DCA. As a result, rates of glycolysis in control hearts exposed to 3 mM DCA were significantly greater than the rates seen in the absence of DCA. Rates of glycolysis in hypertrophied hearts exposed to 3 mM DCA did not differ from those perfused without DCA. Interestingly, glycolysis did not differ between control and hypertrophied hearts at either concentration of DCA.
Rates of glycolysis and glucose oxidation were stimulated more dramatically in the absence of palmitate than in the presence of DCA (Fig. 1A,B). Under these conditions, glycolysis did not differ between the two groups. Glucose oxidation, on the other hand, was dramatically lower in hypertrophied hearts than in control hearts. Normalization of glycolysis and glucose oxidation to work performed by the heart did not significantly change the findings (data not shown).
The proportion of glucose passing through glycolysis that is oxidized, which is a measure of coupling of glucose oxidation to glycolysis, is summarized in Table 2. Under basal conditions, 16% of the glucose was oxidized in control hearts, while only 10% was oxidized in hypertrophied hearts. As expected, activation of PDC by DCA and the omission of palmitate from the perfusate increased the proportion of glucose oxidized in both groups. However, the proportion oxidized in hypertrophied hearts (a range of 19±1 to 25±2%) remained significantly lower than in control hearts (a range of 30±2 to 36±1%).
Table 2.
Percentage of glucose oxidized in isolated working control and hypertrophied rat hearts perfused with and without dichloroacetate (DCA) and in the absence of palmitate
| Condition of study | Control | Hypertrophy |
|---|---|---|
| Basal | 15.7±0.9 (8) | 9.7±0.5* (8) |
| 1.0 mM DCA | 33.3±2.0*† (8) | 24.5±1.6*†‡ (8) |
| 3.0 mM DCA | 30.2±2.0*† (5) | 19.5±0.8*†‡ (5) |
| No palmitate | 35.9±.2*† (4) | 19.0±1.2*†‡ (4) |
Values are expressed as % glucose oxidized. Numbers per group are in parentheses. % Glucose oxidized was calculated as the quotient of glucose oxidation and glycolysis multiplied by 100.
Different from Basal Control, P<0.05;
different from corresponding Basal group, P<0.05;
different from corresponding DCA-treated or No palmitate Control, P<0.05.
Compared to values in hearts perfused with 0.5 mM lactate and 3 mM DCA (Fig. 1), 3 mM lactate and 3 mM DCA did not significantly alter rates of glycolysis (Control, 3942±245; Hypertrophy, 4104±198 nmol/min per g dry wt) or glucose oxidation (Control, 1075±81; Hypertrophy, 737±60 nmol/min per g dry wt). Importantly, the extent of coupling between glycolysis and glucose oxidation remained significantly lower in hypertrophied hearts (18.0±1.1%) than in control hearts (27.9±2.6%, n=3 per group, P<0.05). Alterations in perfusate lactate concentrations cannot, therefore, account for the changes in glucose catabolism observed in hypertrophied hearts.
3.4. PDC activity
Contrary to our initial hypothesis, the amount of PDC recovered in the active form was actually greater in extracts from hypertrophied hearts than from control hearts (Table 3). Total PDC activity did not differ significantly between groups (Table 3). Similarly, the activation state of PDC was generally higher in hypertrophied hearts than control hearts, but again, this difference was not significant. Similar findings (data not shown) were obtained when PDC activities were expressed in relation to dry heart weight or in relation to mitochondrial glutamate dehydrogenase (GDH) activity. The total PDC values using these alternative calculations were 9.6±1.8 U/g dry wt (Control) and 9.1±0.9 U/g dry wt (Hypertrophy) and 0.57±0.11 U PDC/U GDH (Control) and 0.69±0.05 U PDC/U GDH (Hypertrophy).
Table 3.
PDC activity in isolated working control and hypertrophied rat hearts
| PDC activity | Control | Hypertrophy |
|---|---|---|
| Active | 3.3±0.6 | 7.7±0.9* |
| Total | 29.2±5.2 | 39.4±3.3 |
| % Active | 12.6±2.7 | 20.0±3.0 |
Values are expressed as mU/mg protein; n=4 per group.
Different from Control, P<0.05.
Maximal and comparable activation of PDC was achieved in both control and hypertrophied hearts following perfusion with 3 mM DCA (Control, 26.0±5.8 vs. Hypertrophy, 28.9±4.2 mU/mg protein, n=3, P=NS). No further activation of PDC was obtained with higher concentrations of DCA (up to 5 mM) and no significant differences in PDC activity between control and hypertrophied hearts were observed with 1 mM DCA (data not shown). PDC activity did not differ significantly following perfusion without palmitate (Control, 17.1±2.0, Hypertrophy 25.1±1.1 mU/mg protein, n=3, P=NS).
3.5. Expression of PDC, PDK and PDP
Based on Western blot analysis (Fig. 2), no significant difference in the expression of E1α, E1β, or E2 subunits of PDC was observed between control and hypertrophied hearts. E3 subunits were not detected with this preparation of antiserum. Interestingly, expression of E3-binding protein was higher in hypertrophied hearts than in control hearts. The expression of mRNA encoding PDK isoforms 1, 2 and 4, as assessed by densitometry, was not significantly different between control and hypertrophied hearts (Fig. 3). In keeping with the mRNA expression data, no significant differences in PDK activity were detected between control and hypertrophied hearts; endogenous PDK activity in tissue homogenates was assessed by fully dephosphorylating PDC and following the time-dependency for inactivation of dephospho-PDC in the presence of ATP. These studies showed that total PDC activities following PDP treatment and also the subsequent rates of in vitro PDC inactivation during incubation with ATP were very similar in extracts of control and hypertrophied hearts. For example, after 1 min in the presence of ATP, PDC had declined to 52.1±4.5% (Control) and 47.7±4.5% (Hypertrophy) of maximum. In both cases, PDC activities declined to less than 25% maximum within 20 min. Expression of PDP isoforms (Fig. 4) was not significantly different between the two groups.
Fig. 2.
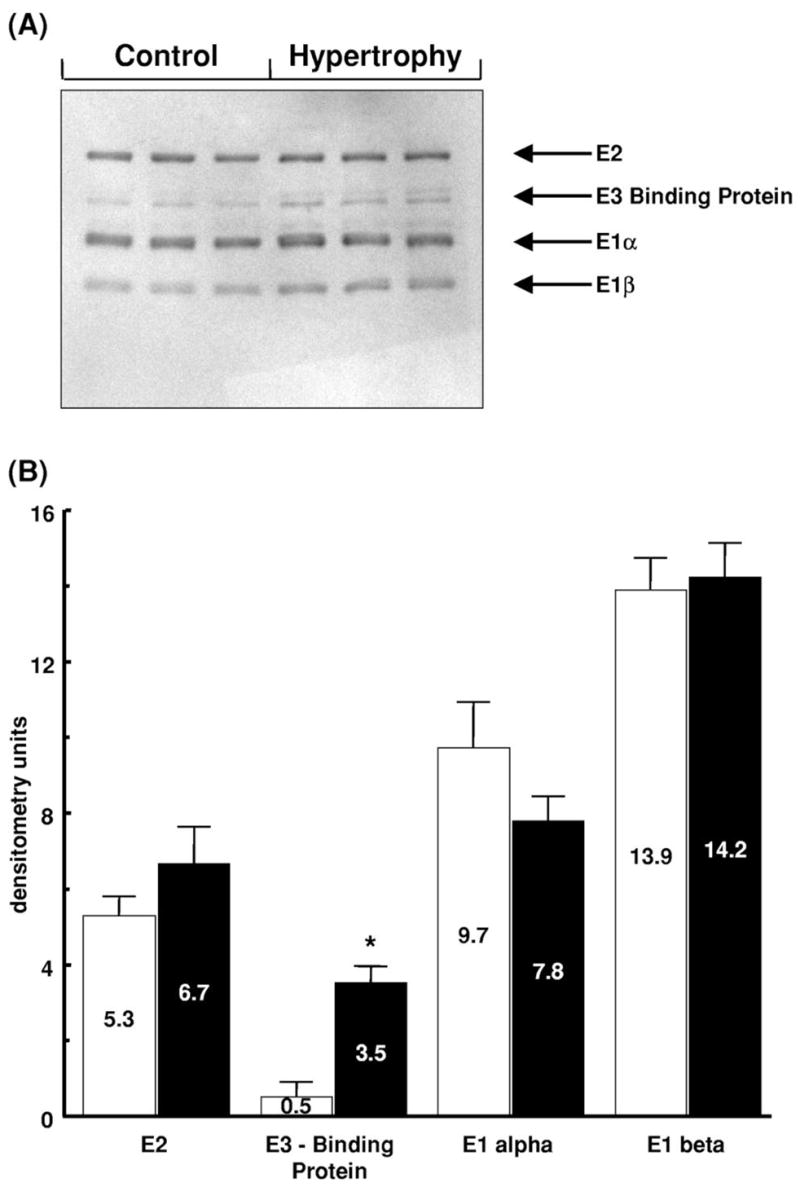
Expression of PDC protein subunits in control (Control) and hypertrophied (Hypertrophy) rat hearts. (A) Representative immunoblot, each lane represents a different heart; n=3 per group. Subunit sizes are as follows: E2, 68,000; E3-binding protein (Protein X), 56,000; E1α, 41,000; E1β, 35,000. (B) Densitometric analysis of protein subunit levels in control (open bars) and hypertrophied (solid bars) hearts expressed in arbitrary units with values as mean±S.E.M.; n=3 hearts per group. *Significantly different from non-hypertrophied control.
Fig. 3.
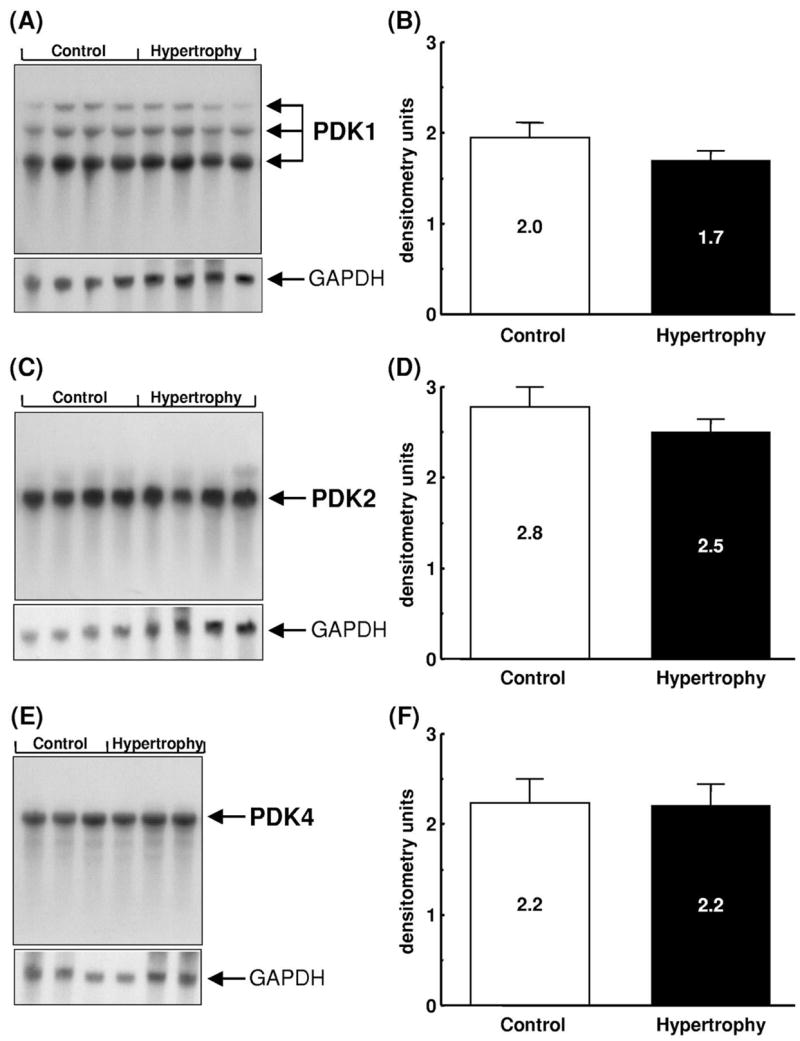
Expression of PDK mRNA in control (Control) and hypertrophied (Hypertrophy) rat hearts. (A,C,E) Representative Northern blots of PDK isoforms -1, -2 and -4, respectively. Messenger RNA sizes are as follows: PDK1, 5.5, 3.5 and 2.0 kb; PDK2, 2.4 kb; PDK4, 3.9 kb; GAPDH, 1.4 kb. Densitometric analysis of mRNA levels expressed in arbitrary units with values as mean±S.E.M.; n=3 hearts per group. (B) PDK1; (D) PDK2; (F) PDK4.
Fig. 4.
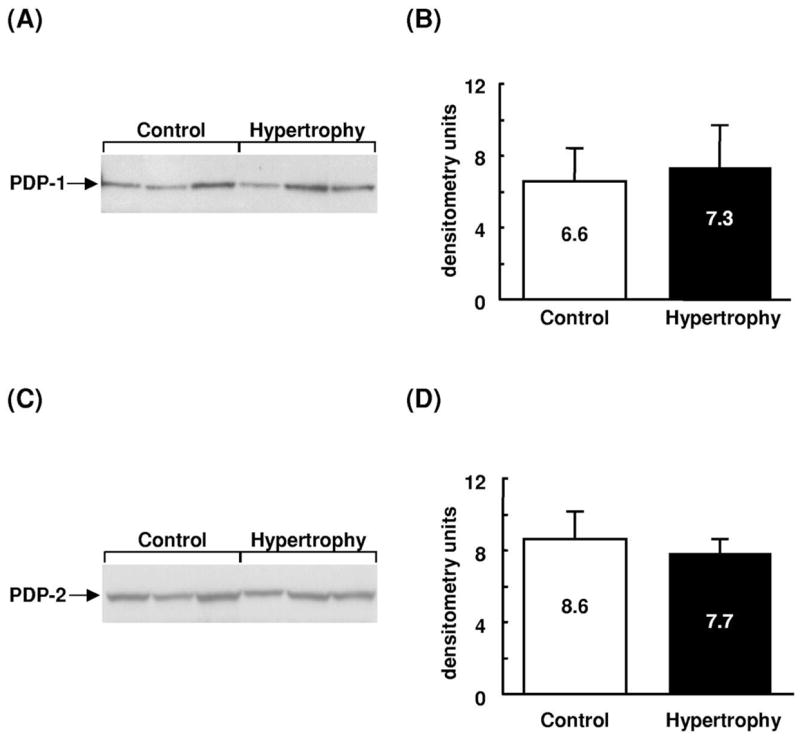
Expression of PDP protein in control (Control) and hypertrophied (Hypertrophy) rat hearts. (A,C) Representative immunoblots of PDP isoforms. Each lane represents a different heart. Sizes are as follows: PDP1, 53,000; PDP2, 53,000. Densitometric analysis shows protein subunit expression in arbitrary units with values as mean±S.E.M.; n=3 hearts per group. (B) PDP1; (D) PDP2.
4. Discussion
In this study, we confirm that coupling of glucose oxidation to glycolysis is low in the hypertrophied heart [9,13]. We now demonstrate that this reduction cannot be explained by alterations in measured PDC activity. Notably, glucose oxidation is significantly repressed in hypertrophied hearts despite comparable activation of PDC in hypertrophied and non-hypertrophied hearts by DCA or in the absence of perfusate palmitate. This observation stands in marked contrast to those made on hearts from diabetic or hyperthyroid animals [17,22–24], in which PDC activation state is dramatically reduced, in concert with enhanced expression of PDK. Taken together, our data indicate that changes in factors other than PDC must be responsible for the low coupling of glucose oxidation to glycolysis observed in hypertrophied hearts.
In agreement with our previous studies [4,9–12], we found, in the presence of palmitate, that glucose oxidation is not increased in hypertrophied hearts compared to control hearts, even though glycolysis is accelerated in hypertrophied hearts. This observation is surprising because the enhanced pyruvate production would be expected to induce PDC activation through non-competitive inhibition of PDK [15–19,43] with consequent stimulation of glucose oxidation. Furthermore, the finding that glucose oxidation and coupling of glucose oxidation to glycolysis are substantially lower in hypertrophied hearts than in control hearts when PDC is comparably activated by DCA or the absence of palmitate (Fig. 1A) provides unequivocal evidence that glucose oxidation is limited in the hypertrophied heart.
In contrast to our initial hypothesis, and to the situation in diabetic or hyperthyroid hearts, impaired coupling of glucose oxidation to glycolysis in the hypertrophied heart is not due to a reduction in the proportion of PDC in the active form (Table 3). Two further key observations also underline the fact that the limitation of glucose oxidation in hypertrophied hearts cannot be explained by a reduction in total PDC capacity. First, the expression of E1α, E1β, and E2 subunits of PDC does not differ significantly between control and hypertrophied hearts (Fig. 2). Second, total PDC activity measured in myocardial extracts is not lower in hypertrophied hearts than that in non-hypertrophied hearts, whether expressed in relation to total protein (Table 3) or mitochondrial GDH activity. With respect to PDC subunit expression, one caveat remains, in that the expression of E3-binding protein was significantly increased in hypertrophied hearts. The E3-binding protein (Protein X) links E3 to the E2 core and is required for full PDC activity [42]. Differential expression of PDC subunits has been documented in rat white adipose tissue during the suckling to weaning transition period [44], but the significance of the increased expression of E3-binding protein in hypertrophied hearts is unclear.
One potential explanation for low rates of glucose oxidation in hypertrophied hearts is a limitation of mitochondrial oxidative metabolism. However, hypertrophied hearts are known to oxidize octanoate at rates comparable to those in non-hypertrophied hearts [45]. Moreover, rates of oxygen consumption per gram of tissue in hearts with compensated hypertrophy are not significantly different from non-hypertrophied hearts [45–47]. Taken together, these data suggest that terminal oxidation of substrates distal to the PDC is not significantly impaired in hearts with compensated cardiac hypertrophy.
As a source of unlabelled acetyl-CoA, endogenous glycogen and triglyceride could potentially contribute to the decreased glucose oxidation measured in hypertrophied hearts, especially in the absence of exogenous palmitate. In this regard, myocardial glycogen content did not significantly differ between hypertrophied and control hearts under the conditions studied here (data not shown) and the extent of net glycogen degradation was small in both groups, accounting for no more than 10% of total glycolytic flux. Degradation of glycogen therefore does not account for the low glucose oxidation rates observed in the hypertrophied hearts. Degradation of endogenous tri-glycerides, likely substantial in the absence of exogenous palmitate, was not assessed in the current experiments and might contribute to decreased glucose oxidation and reduced coupling of glucose oxidation to glycolysis observed in hypertrophied hearts perfused in the absence of exogenous palmitate. However, net mobilization of endogenous triglyceride is unlikely to account for these findings in the presence of exogenous palmitate with and without DCA. Nevertheless, it will be important to specifically examine the possible role of endogenous triglycerides in the metabolism of hypertrophied hearts in future studies.
Because changes in PDK or PDP might be responsible for alterations in PDC activity, we also determined the expression of PDK and PDP isoforms in hypertrophied hearts and control hearts. Four isoforms of PDK have been described [21,37,38] that differ in kinetic and regulatory properties and in tissue-specific expression [21]. In the heart, PDK isoforms 1, 2 and 4 predominate [21]. In the current study, no differences in expression of any of the PDK isoforms were detected at the mRNA level between control and hypertrophied hearts (Fig. 3), unlike the situation in hearts from diabetic and hyperthyroid animals, in which PDK-4 expression is increased.
The two recognized mammalian isoforms of PDP differ with respect to tissue distribution, kinetics, and regulatory properties [34]. Here, we show that, in contrast to skeletal muscle, both PDP1 and PDP2 isoforms are expressed in heart muscle (Fig. 4) and that expression of both isoforms was unchanged as a result of cardiac hypertrophy.
We are aware of two previous reports in which PDC activity has been determined in hypertrophied hearts. In one study, using pulmonary artery banding in adult ferrets, total PDC activity was unchanged over 4–6 weeks of pressure overload [48], in keeping with the findings in the current investigation. In another study, abdominal aortic banding in rats was employed for 5 weeks prior to determination of PDC activity [49]. The investigators found that PDC activation state was reduced in hypertrophied hearts. The explanation for the discrepant findings between the latter study and ours is not immediately apparent. The studies differ in the model of cardiac hypertrophy used, in the fact that hearts were not perfused in the earlier study, and in methods of calculating and expressing PDC activity.
Accelerated rates of glycolysis are consistent with reported changes in glycolytic enzymes in the myocardium of hearts exposed to a pressure overload [1,50,51]. Inhibitory effects on myocardial glycolysis (observed at 1 mM DCA) might be expected according to the Pasteur effect, assuming a constant or lowered energy demand. Moreover, DCA may also directly inhibit glycolysis by reducing the activities of hexokinase and pyruvate kinase [52]. The effects of DCA observed here, however, were more complex in that glycolysis was stimulated by 3 mM DCA, relative to values seen at 1 mM DCA. Conceivably, the effects of 3 mM DCA could be mediated by inhibition of fatty acid oxidation with reduction in myocardial citrate and activation of phosphofructokinase [53,54], but definition of the exact mechanism will require further study.
4.1. Potential limitations
The findings of this present study cannot necessarily be extrapolated to the in vivo setting, because in vitro models fail to adequately reproduce in vivo conditions including workload, heart rate, neural and hormonal stimulation and oxygen delivery. The workload achieved in the current study and how it relates to the in vivo situation and to control hearts may be a particularly important consideration. When the rate pressure product, a functional parameter highly correlated with myocardial oxygen consumption [55], and mass of hypertrophied hearts are considered, it is likely that energy requirements in the current experiments were lower in hypertrophied hearts than the situation in vivo and lower than in control hearts. In light of the importance of workload to oxidative catabolism of glucose [32], it is possible that the lower energy requirements may have affected coupling between glucose oxidation and glycolysis in hypertrophied hearts. Of potential relevance, Massie et al. found that glucose oxidation and coupling to glycolysis were not suppressed in hypertrophied pig hearts studied in vivo under conditions in which estimated energy requirements were comparable [56]. Given these considerations, it is clearly important to determine the extent of coupling between glucose oxidation and glycolysis in hypertrophied rat hearts studied in vivo as well as under more extreme workload conditions in vitro in future experiments.
Notwithstanding this important caveat, the results of this current investigation may have important clinical ramifications. Stimulation of pyruvate oxidation by activation of PDC, which improves coupling between glucose oxidation and glycolysis, is a metabolic approach that improves function of non-hypertrophied hearts during ischemia and reperfusion [8]. The data in this investigation indicate that this approach may not be as beneficial to the hypertrophied heart as it is to the non-hypertrophied heart [9], because glucose oxidation is evidently limited in hypertrophied hearts by other factors. Determination of the factors responsible for impaired coupling of glucose oxidation to glycolysis in hypertrophied hearts is, therefore, of great importance because it will identify novel therapeutic targets.
Acknowledgments
Supported by grants from the Medical Research Council of Canada (now the Canadian Institutes for Health Research) and the Heart and Stroke Foundation of BC and Yukon. N.S. is a Research Fellow of the Alberta Heritage Foundation for Medical Research and the Heart and Stroke Foundation of Canada. M.F.A. is a Career Investigator of the Heart and Stroke Foundation of BC and Yukon. We are grateful to Yulia D’yachkova for her assistance with statistical analysis.
References
- 1.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11(5):416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 2.Sack MN, Harrington LS, Jonassen AK, Mjos OD, Yellon DM. Coordinate regulation of metabolic enzyme encoding genes during cardiac development and following carvedilol therapy in spontaneously hypertensive rats. Cardiovasc Drugs Ther. 2000;14(1):31–39. doi: 10.1023/a:1007887020332. [DOI] [PubMed] [Google Scholar]
- 3.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970;218(1):153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 4.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267(2 Pt 2):H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 5.El Alaoui-Talibi Z, Guendouz A, Moravec M, Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine. Am J Physiol. 1997;272(4 Pt 2):H1615–H1624. doi: 10.1152/ajpheart.1997.272.4.H1615. [DOI] [PubMed] [Google Scholar]
- 6.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–457. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 7.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99(4):578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 8.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33(2):243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 9.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves post-ischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36(4):1378–1385. doi: 10.1016/s0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 10.Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. J Mol Cell Cardiol. 1999;31(3):493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- 11.Schonekess BO, Allard MF, Lopaschuk GD. Recovery of glycolysis and oxidative metabolism during postischemic reperfusion of hypertrophied rat hearts. Am J Physiol. 1996;271(2 Pt 2):H798–H805. doi: 10.1152/ajpheart.1996.271.2.H798. [DOI] [PubMed] [Google Scholar]
- 12.Allard MF, Lopaschuk GD. Ischemia and reperfusion injury in the hypertrophied heart. In: Karmazyn M, editor. Myocardial ischemia: mechanisms, reperfusion, protection. Basel: Birkhauser; 1996. pp. 423–452. [DOI] [PubMed] [Google Scholar]
- 13.Allard MF, Wambolt RB, Longnus SL, et al. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. Am J Physiol. 2000;273(3):E487–E493. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- 14.Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. 1993;264(1):135–144. [PubMed] [Google Scholar]
- 15.Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4(14):3224– 3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- 16.Randle PJ. Fuel selection in animals. Biochem Soc Trans. 1986;14(5):799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- 17.Randle PJ, Priestman DA, Mistry S, Halsall A. Mechanisms modifying glucose oxidation in diabetes mellitus. Diabetologia. 1994;37(Suppl 2):S155–S161. doi: 10.1007/BF00400839. [DOI] [PubMed] [Google Scholar]
- 18.Priestman DA, Orfali KA, Sugden MC. Inhibition of pyruvate dehydrogenase kinase by pyruvate in cultured cardiac myocytes. Biochem Soc Trans. 1997;25(1):101S. doi: 10.1042/bst025101s. [DOI] [PubMed] [Google Scholar]
- 19.Priestman DA, Orfali KA, Sugden MC. Pyruvate inhibition of pyruvate dehydrogenase kinase. Effects of progressive starvation and hyperthyroidism in vivo, and of dibutyryl cyclic AMP and fatty acids in cultured cardiac myocytes. FEBS Lett. 1996;393(2–3):174– 178. doi: 10.1016/0014-5793(96)00877-0. [DOI] [PubMed] [Google Scholar]
- 20.Sugden MC, Holness MJ. Interactive regulation of the pyruvate dehydrogenase complex and the carnitine palmitoyltransferase system. FASEB J. 1994;8(1):54–61. doi: 10.1096/fasebj.8.1.8299890. [DOI] [PubMed] [Google Scholar]
- 21.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998;329(Pt 1):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: role of thyroid hormone status and lipid supply. Biochem J. 2000;352(Pt 3):731–738. [PMC free article] [PubMed] [Google Scholar]
- 24.Priestman DA, Donald E, Holness MJ, Sugden MC. Different mechanisms underlie the long-term regulation of pyruvate dehydrogenase kinase (PDHK) by tri-iodothyronine in heart and liver. FEBS Lett. 1997;419(1):55–57. doi: 10.1016/s0014-5793(97)01430-0. [DOI] [PubMed] [Google Scholar]
- 25.Gamble J, Lopaschuk GD. Glycolysis and glucose oxidation during reperfusion of ischemic hearts from diabetic rats. Biochim Biophys Acta. 1994;1225(2):191–199. doi: 10.1016/0925-4439(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 26.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39(1):89–105. doi: 10.1016/s0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 27.Kolar F, Papousek F, Pelouch V, Ostadal B, Rakusan K. Pressure overload induced in newborn rats: effects on left ventricular growth, morphology, and function. Pediatr Res. 1998;43(4 Pt 1):521–526. doi: 10.1203/00006450-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Linehan KA, Seymour AM, Williams PE. Semiquantitative analysis of collagen types in the hypertrophied left ventricle. J Anat. 2001;198(Pt 1):83–92. doi: 10.1046/j.1469-7580.2001.19810083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopaschuk GD, Barr RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem. 1997;172(1–2):137–147. [PubMed] [Google Scholar]
- 30.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation. 1996;93(8):1549–1555. doi: 10.1161/01.cir.93.8.1549. [DOI] [PubMed] [Google Scholar]
- 31.Cederblad G, Carlin JI, Constantin-Teodosiu D, Harper P, Hultman E. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal Biochem. 1990;185(2):274–278. doi: 10.1016/0003-2697(90)90292-h. [DOI] [PubMed] [Google Scholar]
- 32.Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am J Physiol. 1994;267(5 Pt 2):H1862–H1871. doi: 10.1152/ajpheart.1994.267.5.H1862. [DOI] [PubMed] [Google Scholar]
- 33.Constantin-Teodosiu D, Cederblad G, Hultman E. A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem. 1991;198(2):347–351. doi: 10.1016/0003-2697(91)90437-x. [DOI] [PubMed] [Google Scholar]
- 34.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem. 1998;273(28):17680–17688. doi: 10.1074/jbc.273.28.17680. [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.McDaniel HG, Yeh M, Jenkins R, Razzaque A. Glutamic dehydrogenase from rat heart mitochondria. I. Purification and physical properties including molecular weight determination. J Mol Cell Cardiol. 1984;16(4):295–301. doi: 10.1016/s0022-2828(84)80600-8. [DOI] [PubMed] [Google Scholar]
- 37.Popov KM, Kedishvili NY, Zhao Y, Gudi R, Harris RA. Molecular cloning of the p45 subunit of pyruvate dehydrogenase kinase. J Biol Chem. 1994;269(47):29720–29724. [PubMed] [Google Scholar]
- 38.Popov KM, Kedishvili NY, Zhao Y, Shimomura Y, Crabb DW, Harris RA. Primary structure of pyruvate dehydrogenase kinase establishes a new family of eukaryotic protein kinases. J Biol Chem. 1993;268(35):26602–26606. [PubMed] [Google Scholar]
- 39.Yu JZ, Bondy GP, Allard MF, Thompson CR, Levin A, McManus BM. Serum from patients with chronic renal insufficiency alters growth characteristics and ANP mRNA expression of adult rat cardiac myocytes. J Mol Cell Cardiol. 1996;28(12):2429–2441. doi: 10.1006/jmcc.1996.0236. [DOI] [PubMed] [Google Scholar]
- 40.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272(32):19746–19751. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- 43.Cooper RH, Randle PJ, Denton RM. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maury J, Kerbey AL, Priestman DA, Patel MS, Girard J, Ferre P. Pretranslational regulation of pyruvate dehydrogenase complex subunits in white adipose tissue during the suckling-weaning transition in the rat. Biochem J. 1995;311(Pt 2):531–535. doi: 10.1042/bj3110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.el Alaoui-Talibi Z, Landormy S, Loireau A, Moravec J. Fatty acid oxidation and mechanical performance of volume-overloaded rat hearts. Am J Physiol. 1992;262(4 Pt 2):H1068–H1074. doi: 10.1152/ajpheart.1992.262.4.H1068. [DOI] [PubMed] [Google Scholar]
- 46.Bache RJ, Zhang J, Murakami Y, et al. Myocardial oxygenation at high workstates in hearts with left ventricular hypertrophy. Cardiovasc Res. 1999;42(3):616–626. doi: 10.1016/s0008-6363(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 47.Izzi G, Zile MR, Gaasch WH. Myocardial oxygen consumption and the left ventricular pressure-volume area in normal and hypertrophic canine hearts. Circulation. 1991;84(3):1384–1392. doi: 10.1161/01.cir.84.3.1384. [DOI] [PubMed] [Google Scholar]
- 48.Do E, Baudet S, Verdys M, et al. Energy metabolism in normal and hypertrophied right ventricle of the ferret heart. J Mol Cell Cardiol. 1997;29(7):1903–1913. doi: 10.1006/jmcc.1997.0429. [DOI] [PubMed] [Google Scholar]
- 49.Seymour AM, Chatham JC. The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. J Mol Cell Cardiol. 1997;29(10):2771–2778. doi: 10.1006/jmcc.1997.0512. [DOI] [PubMed] [Google Scholar]
- 50.Keller A, Rouzeau JD, Farhadian F, et al. Differential expression of alpha- and beta-enolase genes during rat heart development and hypertrophy. Am J Physiol. 1995;269(6 Pt 2):H1843–H1851. doi: 10.1152/ajpheart.1995.269.6.H1843. [DOI] [PubMed] [Google Scholar]
- 51.Bishop SP, Altschuld RA. Evidence for increased glycolytic metabolism in cardiac hypertrophy and congestive heart failure. In: Alpert N, editor. Cardiac hypertrophy. New York: Academic Press; 1971. pp. 567–585. [Google Scholar]
- 52.Anderson JW, Karounos D, Yoneyama T, Hollingsworth JW. Dichloroacetate-induced changes in liver of normal and diabetic rats. Proc Soc Exp Biol Med. 1975;149(3):814–821. doi: 10.3181/00379727-149-38905. [DOI] [PubMed] [Google Scholar]
- 53.McAllister A, Allison SP, Randle PJ. Effects of dichloroacetate on the metabolism of glucose, pyruvate, acetate, 3-hydroxybutyrate and palmitate in rat diaphragm and heart muscle in vitro and on extraction of glucose, lactate, pyruvate and free fatty acids by dog heart in vivo. Biochem J. 1973;134(4):1067–1081. doi: 10.1042/bj1341067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268(34):25836–25845. [PubMed] [Google Scholar]
- 55.Neely JR, Whitmer M, Mochizuki S. Effects of mechanical activity and hormones on myocardial glucose and fatty acid utilization. Circ Res. 1976;38(5 Suppl 1):I22–30. [PubMed] [Google Scholar]
- 56.Massie BM, Schaefer S, Garcia J, et al. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91(6):1814–1823. doi: 10.1161/01.cir.91.6.1814. [DOI] [PubMed] [Google Scholar]


