Abstract
Background
The growing field of proteomics and systems biology is resulting in an ever increasing demand for purified recombinant proteins for structural and functional studies. Here, we show a systematic approach to successfully express a full-length protein of interest by using cell-free and cell-based expression systems.
Results
In a pre-screen, we evaluated the expression of 960 human full-length open reading frames in Escherichia coli (in vivo and in vitro). After analysing the protein expression rate and solubility, we chose a subset of 87 plasmids yielding no protein product in E. coli in vivo. These targets were subjected to a more detailed analysis comparing a prokaryotic cell-free E. coli system with an eukaryotic wheat germ system. In addition, we determined the expression rate, yield and solubility of those proteins. After sequence optimisation for the E. coli in vitro system and generating linear templates for wheat germ expression, the success rate of cell-free protein expression reached 93%.
Conclusion
We have demonstrated that protein expression in cell-free systems is an appropriate technology for the successful expression of soluble full-length proteins. In our study, wheat germ expression using a two compartment system is the method of choice as it shows high solubility and high protein yield.
Background
With the sequencing of the human genome completed and with mRNA/cDNA identification rapidly progressing, many potential novel genes have been discovered and attention has turned to the function and structure of the predicted proteins [1-4]. In order to study these novel gene products, sufficient amounts of protein generally obtained through recombinant protein expression are required. The (high-throughput) expression and characterisation of unknown and poorly characterised human proteins is a main objective of recombinant proteomic studies today.
Escherichia coli is the most commonly used prokaryotic expression system for the high-level production of recombinant proteins in vivo [5] and has already been used successfully in high-throughput protein expression and purification studies [4,6]. The use of E. coli has many advantages, including the ease of growth and manipulation of the organism and the availability of many different vectors and host strains that have been developed over the years. However, the use of E. coli also has limitations, such as the aggregation of protein in insoluble inclusion bodies, problems with the expression of gene products toxic to the physiology of the host cell or proteolytic degradation of proteins in the cytoplasm [7]. In light of these difficulties, cell-free expression systems are becoming increasingly popular [8-14]. The in vitro systems have several advantages, including rapid protein synthesis [15], the possibility to express toxic gene products [16] and constructs that otherwise would be proteolytically degraded. Furthermore, it is possible to express proteins with up to 10 putative transmembrane domains as reported recently [17]. The compatibility with PCR-generated templates as well as plasmids allows the in vitro expression reaction with E. coli extract to be optimised using silent mutations within PCR products [18]. These sequence optimisations reduce unfavourable secondary structures in mRNA and thus improve the success rate of translation and protein expression. In contrast, for cell-free protein expression with wheat germ lysate sequence optimisation is not necessary because of the eukaryotic nature of this source.
For protein expression analyses, a comprehensive cDNA collection is available at the German Ressource Center (RZPD). The full-length open reading frames (ORFs) are cloned into an entry vector by utilizing the Gateway® cloning technology (Invitrogen). Untranslated regions are excluded and only the open reading frame is cloned into the selected vector, either with or without a stop codon. For protein expression, the open reading frame can be moved into any desired expression vector by homologous recombination. Thus, a protein can be expressed with or without a tag and the tag itself can easily be selected and altered by choosing the appropriate destination vector.
The aim of this study was to evaluate alternatives to protein expression in E.coli in vivo especially for those ORFs yielding no protein in this system. Therefore we investigated protein expression in two different in vitro systems: E. coli and wheat germ extract. The performance of these systems was analysed and optimised in respect to expression rate, protein yield and solubility. Altogether, we tested the expression of 960 human full-length proteins in vivo and in vitro using standardised conditions.
Results
Comparison of in vivo and in vitro Escherichia coli expressions
We used 960 randomly selected fully sequence-verified human open reading frames with a broad range of expected molecular weights (from less than 8 kDa up to 134 kDa, average of 35 kDa), different predicted subcellular localisations and biochemical functions including membrane proteins. The ORFs were cloned into an expression vector (pDEST17-D18), for production of proteins with an N-terminal 6xHis-tag. Identical constructs were used for protein expression in vitro and also for transformation of bacteria and expression in vivo. Protein expression was analysed by western blotting using an anti-His antibody.
In E. coli in vivo 629 out of 960 proteins, and in vitro 456 out of 960 proteins were successfully expressed. Protein expression in bacteria was unsuccessful either because clones were generated, which did not show protein expression (233 samples) or the transformation failed completely (98 samples). Considering the overlap of both expression systems, 206 full open reading frames yielded no protein product in vivo andin vitro. In contrast, 331 targets were expressed in vivo andin vitro with an average molecular weight of 33 kDa. Among these, 57 clones showed an expression rate of 4, 86 clones of 3 and 7 clones of 2. Furthermore, 424 targets expressed in either system with an average moleclar weight of 32 kDa.
Optimisation of E. coli expression in vitro
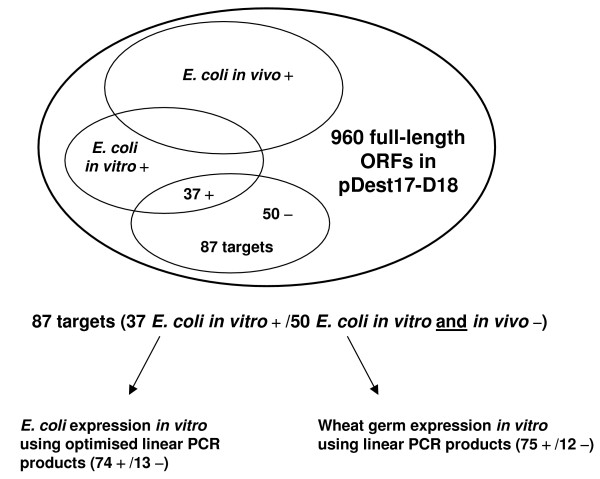
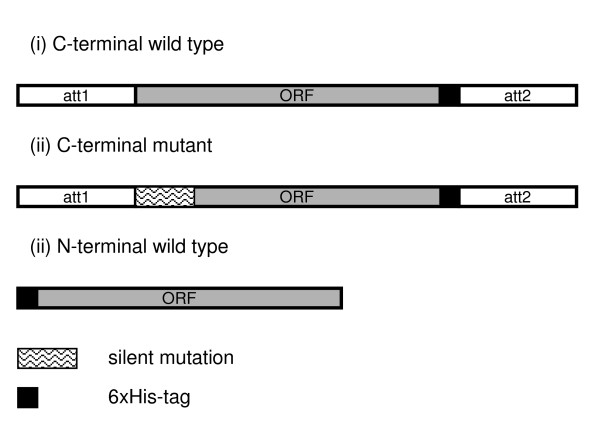
We next examined the effect of sequence optimisation on protein expression rate and protein yield by selecting randomly 87 out of the 960 ORFs (Figure 1, Table 1) where protein expression had been unsuccessful in vivo or where transformation had failed in BL21(DE3)pLysS. Three kinds of linear PCR templates were generated (Figure 2): (i) a C-terminal wild type with C-terminal 6xHis-tag (ii) a C-terminal mutant with C-terminal 6xHis-tag and inserted silent mutation at the N-terminus (iii) a N-terminal wild type with N-terminal 6xHis-tag and no attachment sites (att-sites); For the C- and N-terminal wild type template the ORF is identical to the original ORF in the plasmid DNA.
Figure 1.
Scheme of the experimental strategy. Successful protein expression is indicated by +, unsuccessful protein expression by -.
Table 1.
Subset of 87 clones tested in E. coli in vitro and in wheat germ expression. ORF Nr.: Clone identifier; RZPD Clone ID: Available clones at RZPD GmbH; Hit Acc. Nr.: Best BLAST hit of DNA sequence. Molecular weight was calculated by translation of the DNA sequence. Expression/solubility were assigned values from 0 (no expression/no protein detectable in the supernatant) to 4 (very strong band/protein band in the supernatant is stronger than in the pellet). The column 'yielded by' is indicated as follows: WG C: wheat germ C-terminal, WG: wheat germ, C- and N-terminal, WG N: wheat germ, N-terminal, RTS CW: E. coli in vitro C-terminal wildtype, RTS CM: E. coli in vitro C-terminal mutant, RTS NW: E. coli in vitro N-terminal wildtype, RTS Pl: E. coli in vitro original plasmid.
| ORF Nr | RZPDCloneID | Hit Acc No | Gene symbol | Mw in kDa | Best expr. rate | Yielded by | Best solubility |
| 264 | RZPDo834B052 | NM_001677 | ATP1B1 | 36 | 4 | RTS NW | 3 |
| 400 | RZPDo834D022 | NM_002573 | PAFAH1B3 | 27 | 4 | WG, RTS CM NW | 4 |
| 433 | NM_000318 | PXMP3 | 32 | 4 | WG, RTS CW CM NW | 4 | |
| 464 | RZPDo834F012 | NM_006793 | PRDX3 | 29 | 4 | WG C, RTS CM | 4 |
| 505 | NM_003187 | TAF9 | 30 | 4 | WG | 3 | |
| 531 | NM_012222 | MUTYH | 23 | 4 | WG | 3 | |
| 562 | RZPDo834B043 | NM_000075 | CDK4 | 35 | 4 | RTS CM NW Pl | 3 |
| 571 | RZPDo834B083 | NM_003182 | TAC1 | 13 | 4 | WG C, RTS CW CM | 4 |
| 616 | RZPDo834D033 | NM_000550 | TYRP1 | 61 | 3 | RTS Pl | 2 |
| 636 | NM_000612 | IGF2 | 22 | 4 | WG C, RTS CW CM | 4 | |
| 637 | RZPDo834E013 | NM_015646 | RAP1B | 22 | 4 | WG, RTS CW CM NW | 4 |
| 639 | NM_002512 | NME2 | 14 | 4 | WG N, RTS NW | 3 | |
| 690 | RZPDo834F123 | NM_006923 | SDF2 | 23 | 4 | WG C, RTS CW CM | 4 |
| 694 | RZPDo834G023 | NM_020470 | YIF1 | 32 | 4 | WG N | 3 |
| 728 | RZPDo834E0511 | NM_000434 | NEU1 | 46 | 4 | RTS NW | 2 |
| 741 | RZPDo834H073 | NM_002799 | PSMB7 | 30 | 4 | WG C | 4 |
| 772 | RZPDo834C0311 | NM_002804 | PSMC3 | 46 | 4 | WG N, RTS CM | 4 |
| 777 | RZPDo834C0411 | NM_003908 | EIF2S2 | 39 | 4 | WG | 4 |
| 831 | NM_004394 | DAP | 12 | 3 | WG | 4 | |
| 832 | NM_002966 | S100A10 | 12 | 4 | WG, RTS CM NW | 4 | |
| 833 | RZPDo834A124 | NM_017503 | SURF2 | 30 | 4 | WG C | 4 |
| 840 | RZPDo834B024 | NM_005499 | UBA2 | 72 | 3 | WG C | 4 |
| 842 | NM_005942 | MOCS1 | 24 | 4 | WG N, RTS CM | 3 | |
| 855 | NM_002134 | HMOX2 | 36 | 4 | RTS NW | 4 | |
| 861 | RZPDo834C034 | NM_006370 | VTI1B | 27 | 4 | WG N, RTS NW Pl | 3 |
| 868 | RZPDo834C084 | NM_005892 | FMNL1 | 53 | 4 | WG, RTS CM NW | 3 |
| 873 | RZPDo834C104 | NM_007363 | NONO | 55 | 4 | WG C | 3 |
| 881 | NM_002622 | PFDN1 | 15 | 4 | WG C | 4 | |
| 898 | RZPDo834D114 | NM_006117 | PECI | 21 | 4 | WG N, RTS CW | 4 |
| 901 | RZPDo834E024 | NM_031263 | HNRPK | 51 | 4 | RTS CW NW | 4 |
| 904 | NM_004401 | DFFA | 13 | 4 | WG N | 4 | |
| 906 | NM_006693 | CPSF4 | 31 | 4 | WG C, RTS CM | 4 | |
| 915 | RZPDo834F024 | M55654 | TBP | 38 | 4 | WG N, RTS NW Pl | 3 |
| 918 | NM_004184 | WARS | 54 | 3 | WG N | 4 | |
| 921 | RZPDo834F064 | NM_004309 | ARHGDIA | 23 | 4 | RTS CM NW | 3 |
| 924 | RZPDo834F094 | NM_002861 | PCYT2 | 44 | 4 | WG C | 4 |
| 930 | RZPDo834G014 | NM_001551 | IGBP1 | 40 | 4 | WG, RTS Pl | 4 |
| 932 | RZPDo834G034 | NM_002070 | GNAI2 | 41 | 4 | WG C, RTS NW Pl | 4 |
| 935 | NM_001014835 | PAK4 | 64 | 2 | WG C | 3 | |
| 936 | RZPDo834G064 | NM_002074 | GNB1 | 38 | 3 | RTS NW | 3 |
| 939 | RZPDo834G074 | NM_006321 | ARIH2 | 58 | 3 | WG C, RTS Pl | 4 |
| 940 | RZPDo834G084 | NM_013296 | GPSM2 | 55 | 2 | WG C | 4 |
| 943 | RZPDo834G114 | NM_001863 | COX6B1 | 11 | 4 | WG, RTS CW CM Pl | 4 |
| 944 | NM_004537 | NAP1L1 | 46 | 4 | WG | 4 | |
| 945 | RZPDo834H014 | NM_152925 | RBM12 | 59 | 4 | WG C | 4 |
| 947 | RZPDo834H034 | NM_001017957 | OS-9 | 70 | 4 | WG C | 4 |
| 1033 | NM_007317 | KIF22 | 74 | 0 | 0 | ||
| 1068 | NM_206900 | RTN2 | 52 | 4 | WG C | 4 | |
| 1082 | NM_018074 | FLJ10374 | 37 | 4 | WG; RTS CW CM | 4 | |
| 1091 | NM_001512 | GSTA4 | 26 | 4 | WG | 4 | |
| 1093 | RZPDo834A015 | NM_001647 | APOD | 22 | 4 | WG C, RTS CM | 4 |
| 1101 | NM_001643 | APOA2 | 12 | 4 | WG N | 3 | |
| 1115 | NM_007261 | CMRF-35H | 25 | 4 | WG, RTS CM NW | 4 | |
| 1189 | RZPDo834H106 | NM_001425 | EMP3 | 19 | 4 | WG | 4 |
| 1294 | RZPDo834A035 | NM_014876 | KIAA0063 | 24 | 4 | WG | 4 |
| 1330 | NM_002816 | PSMD12 | 53 | 4 | WG C | 4 | |
| 1453 | RZPDo834B115 | NM_198216 | SNRPB | 25 | 4 | WG C, RTS CM | 3 |
| 1454 | RZPDo834H0711 | NM_006841 | SLC38A3 | 56 | 4 | WG, RTS NW | 3 |
| 1461 | RZPDo834C025 | NM_004047 | ATP6V0B | 22 | 3 | WG C | 1 |
| 1462 | RZPDo834C035 | NM_003145 | SSR2 | 21 | 4 | WG C, RTS CM | 3 |
| 1480 | NM_000984 | RPL23A | 18 | 3 | WG | 4 | |
| 1485 | RZPDo834D025 | NM_014860 | SUPT7L | 47 | 4 | WG | 4 |
| 1487 | RZPDo834D035 | NM_198120 | EBAG9 | 25 | 4 | WG C | 3 |
| 1533 | RZPDo834F075 | NM_013300 | HSU79274 | 31 | 4 | WG C | 3 |
| 1554 | RZPDo834G095 | NM_001778 | CD48 | 28 | 4 | RTS NW | 3 |
| 1555 | RZPDo834G105 | NM_019111 | HLA-DRA | 29 | 4 | RTS CM | 3 |
| 1575 | RZPDo834H085 | NM_004233 | CD83 | 23 | 4 | WG, RTS CM | 4 |
| 1576 | RZPDo834H095 | NM_007024 | PL6 | 39 | 2 | WG N | 1 |
| 1642 | NM_003490 | SYN3 | 64 | 2 | WG | 4 | |
| 1670 | NM_006841 | SLC38A3 | 56 | 4 | WG N, RTS NW | 3 | |
| 1734 | RZPDo834F0511 | NM_001436 | FBL | 34 | 4 | WG C | 4 |
| 1736 | RZPDo834G0511 | NM_004343 | CALR | 49 | 4 | WG C | 4 |
| 2066 | NM_015723 | PNPLA8 | 89 | 0 | 0 | ||
| 2225 | RZPDo834H0221 | NM_018127 | ELAC2 | 93 | 0 | 0 | |
| 2229 | RZPDo834A046 | NM_00513 | REC8L1 | 63 | 3 | WG C, RTS CM | 4 |
| 2504 | NM_199053 | FLJ12716 | 65 | 4 | RTS CW CM NW | 3 | |
| 2724 | NM_173157 | NR4A1 | 65 | 4 | WG N | 2 | |
| 2871 | NM_018099 | MLSTD1 | 60 | 4 | RTS CW CM NW | 3 | |
| 2938 | RZPDo834H0421 | NM_015072 | TTLL5 | 92 | 0 | 0 | |
| 2949 | RZPDo834E1121 | NM_020748 | KIAA1287 | 135 | 0 | 0 | |
| 2959 | NM_021932 | RIC8 | 17 | 4 | WG, RTS Pl | 4 | |
| 2962 | RZPDo834H0621 | NM_014149 | HSPC049 | 78 | 2 | RTS CM NW | 0 |
| 2964 | RZPDo834F1121 | NM_003263 | TLR1 | 91 | 4 | RTS CM NW | 4 |
| 2968 | RZPDo834G0821 | NM_001040428 | SPATA7 | 65 | 4 | RTS CW CM NW | 3 |
| 2973 | NM_032292 | FLJ20203 | 91 | 4 | RTS CW CM NW | 3 | |
| 2978 | RZPDo834H0721 | NM_014585 | SLC40A1 | 63 | 0 | 0 | |
| 2979 | RZPDo834E0821 | NM_013277 | RACGAP1 | 71 | 4 | RTS CM | 0 |
Figure 2.
Optimised linear templates for E. coli in vitro expression. Three kinds of linear PCR-products were generated to investigate the effect on protein expression rate and yield. (i) C-terminal wild type with C-terminal 6xHis-tag (ii) C-terminal mutant with C-terminal 6xHis-tag and inserted silent mutation at the N-terminus (iii) N-terminal wild type with N-terminal 6xHis-tag and no attachment sites (att-sites); For the C- and N-terminal wild type template the ORF is identical to the original ORF in the plasmid DNA.
Influence of sequence optimisation on protein expression rate
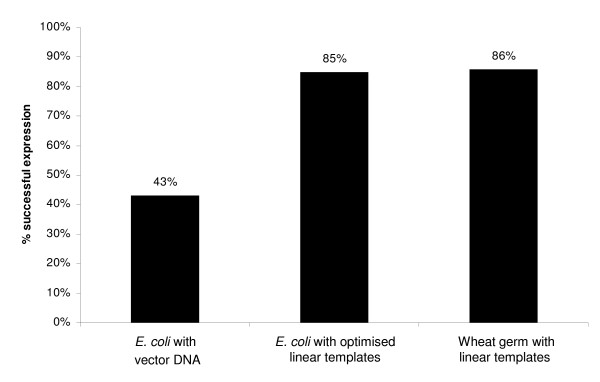
Of these 87 samples, 37 samples (43%) were successfully expressed in vitro using the original plasmid DNA. After sequence optimisation, we increased the success rate of protein expression up to 74 samples (85%) in the cell-free E. coli system (Figure 3, 4).
Figure 3.
Comparison of in vitro expression of 87 targets in E. coli and wheat germ.
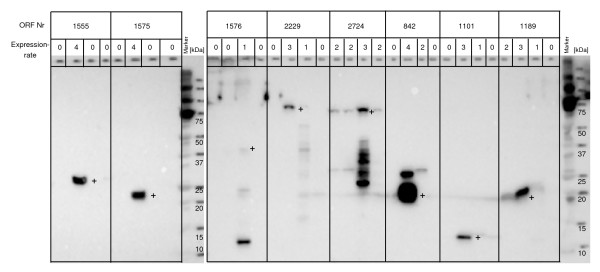
Figure 4.
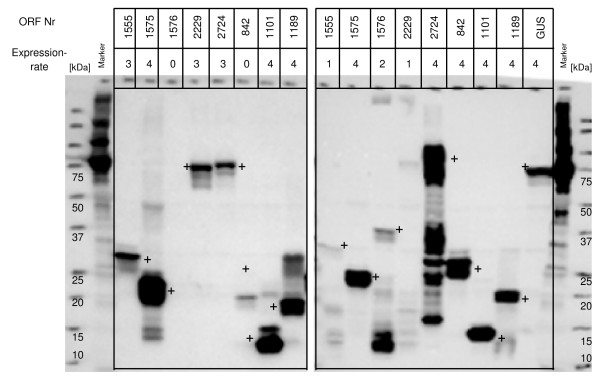
E.coli expression in vitro. Presented are western blots of 8 targets expressed with C-terminal wild type, C-terminal mutant, N-terminal wild type and original plasmid DNA templates (from left to right). Successful protein expression was defined for values 2 – 4 and unsuccessful protein expression for values of 0 and 1. Bands of the expected size are marked with a +.
When analysing the results of those samples which had previously not shown expression in vitro (50 samples), we found that following sequence optimisation 37 (74%) proteins were expressed.
Influence of sequence optimisation on protein yield
To assess the protein expression yield of PCR products after sequence optimisation, we evaluated 37 samples that had previously expressed protein in vitro from original plasmid DNA. Protein yield was determined by analysis of protein bands on western blots. Bands were given marks from 0 (no expression) to 4 (very strong). Here, we discovered that 65% of expressions (24 samples) showed an improvement in the protein yield compared to expressions using original plasmid DNA and another 19% showed similar protein yields. A smaller amount of protein was expressed in only 6 cases (16%) using the optimised PCR products. Among these were 3 samples which did not express protein at all. In summary, after analysis of 87 expressions in vitro with optimised PCR-products, 16 samples (18%) revealed no protein product in vivo or in vitro in the E. coli systems (Table 2).
Table 2.
Proteins not expressing in E. coli in vitro or in wheat germ or in both systems. Molecular weight was calculated by translation of the DNA sequence. Localization information was taken from the Uniprot database. Domain information was retrieved from the Pfam database: cc: coiled coil, tms: transmembrane segment, sp signal peptide. Empty fields correspond to no assignment in the database.
| Hit Acc. No. | Gene symbol | Mw in kDa | Localization | Domains | Expression in E. coli in vitro | Expression in wheat germ |
| NM_007317 | KIF22 | 74 | nuclear | 1cc,, | no | no |
| NM_015723 | PNPLA8 | 89 | membrane | no | no | |
| NM_020748 | KIAA1287 | 135 | n/a | no | no | |
| NM_018127 | ELAC2 | 93 | nuclear | no | no | |
| NM_015072 | TTLL5 | 92 | n/a | 3cc,, | no | no |
| NM_014585 | SLC40A1 | 63 | membrane | ,,10tms | no | no |
| NM_020470 | YIF1A | 32 | membrane | ,,5tms | no | yes |
| NM_004394 | DAP | 12 | secreted | no | yes | |
| NM_001014835 | PAK4 | 64 | n/a | no | yes | |
| NM_013296 | GPSM2 | 55 | n/a | no | yes | |
| NM_006812 | OS-9 | 70 | n/a | 1cc1sp1tms | no | yes |
| NM_014860 | SUPT7L | 47 | n/a | 1cc,, | no | yes |
| NM_003908 | EIF2S2 | 39 | nuclear | no | yes | |
| NM_006321 | ARIH2 | 58 | nucleus | 2cc,, | no | yes |
| NM_002816 | PSMD12 | 53 | cytosol | 1cc,, | no | yes |
| NM_000984 | RPL23A | 18 | cytosol | no | yes | |
| NM_002134 | HMOX2 | 36 | microsomal | 1cc,1tms | yes | no |
| NM_018099 | MLSTD1 | 60 | intracellular | ,,2tms | yes | no |
| NM_013277 | RACGAP1 | 71 | intracellular | 1cc,, | yes | no |
| NM_003263 | TLR1 | 91 | membrane | 1sp,1tms | yes | no |
| NM_032292 | FLJ20203 | 91 | n/a | 1cc,, | yes | no |
| NM_014149 | HSPC049 | 78 | n/a | 1cc,, | yes | no |
Wheat germ expression in vitro
The aim of this experiment was to elucidate whether the wheat germ system would show an increase in the success rate and protein yield of the 87 selected open reading frames compared to the optimised in vitro expressions in E. coli. Two wild type PCR constructs were made for each open reading frame, one for production of a protein with a C-terminal 6xHis-tag and another for a protein with a N-terminal 6xHis-tag (Figure 5). A total of 75 proteins could be expressed in wheat germ lysate with either a C- or a N-terminal 6xHis-tag (86%, Figure 3). Out of the 16 open reading frames which were not expressed in the E. coli systems, 10 were now successfully expressed using wheat germ lysate (Table 2). However, 6 open reading frames did not express in the wheat germ system, but were previously successfully expressed in vitro in E. coli (Table 2). On average, based on western blotting analyses, the protein yield was higher in the wheat germ compared to expressions in the E. coli in vitro system, for identical human ORFs.
Figure 5.
Wheat germ expression in vitro. Presented are western blots of 8 targets expressed with C-terminal (left) and N-terminal (right) 6xHis-tag. ORF Nr.: clone identifier. GUS: glucuronidase is the positive control. Successful protein expression was defined for values 2 – 4 and unsuccessful protein expression for values of 0 and 1. Bands of theexpected size are marked with a +.
Influence of tag position on protein expression in vitro
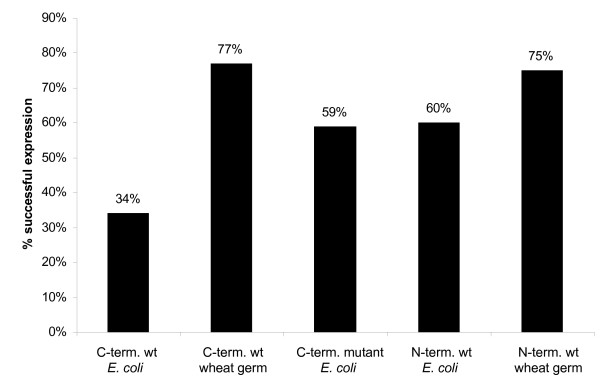
To assess the influence of either N- or C-terminal tag positions on expression rate, the 87 open reading frames were evaluated in both in vitro expression systems, E. coli and wheat germ (Figure 6). In the E. coli in vitro system, protein expressions using optimised PCR products were evaluated. Here, 52 (60%) N-terminal tagged wild type PCR products expressed protein compared to only 30 (34%) with C-terminal wild type PCR products (Figure 4). With the C-terminal mutant product 51 (59%) proteins were expressed. In the wheat germ system, 65 were expressed using the N-terminal wild type construct (75%) and 67 with the C-terminal tag (78%) (Figure 5).
Figure 6.
Influence of tag on in vitro expression. We compared 87 targets expressed in E.coli in vitro and in wheat germ. C-term. wt: C-terminal wild type; C-term. mutant: C-terminal mutant; N-term. wt: N-terminal wild type.
In summary, 81 out of 87 open reading frames were expressed in both in vitro systems, corresponding to a success rate of 93%. Only 6 ORFs yielded no protein in any of the systems tested (Table 2).
Comparison of solubility of proteins expressed in E. coli in vivo, in vitro and in wheat germ system
For solubility studies, the lysis supernatant of those targets revealing expression was analysed by western blot. 483 proteins, expressed in E. coli in vivo, were tested and 193 proteins were soluble (40%). For E. coli expressions in vitro (with original plasmid DNA), 388 were analysed and 185 proteins were soluble (48%). In the wheat germ system with a C-terminal 6xHis-tag 66 of the 68 (97%) expressing PCR products showed soluble protein and 95% with an N-terminal 6xHis-tag.
Discussion and conclusion
With this approach we evaluated the performance of three different protein expression systems in vivo and in vitro with a set of 960 full-length open reading frames. For our investigations of protein synthesis we chose Escherichia coli bacteria as it is one of the most common and easy to use systems. For cell-free in vitro expression, we compared the E. coli with the wheat germ protocol.
First, we analysed the protein expression rate in the two E. coli systems (in vitro and in vivo) and found that expression is higher in the in vivo system (66% compared to 48%). Regarding the success rate of both protocols, only 22% of plasmids yielded no protein.
We then focused on a subset of 87 targets which had yielded no protein in E. coli in vivo. These targets expressed with the cell-free wheat germ and E. coli protocol yielded very different protein expression rates. In wheat germ 86% of the targets were expressed and in cell-free E. coli only 43%. One of the reasons for unsuccessful in vitro protein expression in E. coli may be the presence of secondary structures in mRNA, which may inhibit translation [19]. To solve this problem, we made use of the ProteoExpert software, which predicts possible sequence-related problems and proposes optimised sequences with potentially reduced unfavourable secondary structures [18,20]. Out of the 87 proteins that were not expressed in E. coli in vivo, 37 were expressed in vitro using the wild type sequence. Another set of 37 human proteins could be rescued by sequence optimisation and using linear templates for in vitro expression. Therefore, the overall success rate of in vivo negative clones was 85%. This result clearly demonstrates that sequence optimisation is necessary to improve protein synthesis in the E. coli in vitro system.
Furthermore, we analysed the influence of tag position on protein expression rate. We found no difference between C- or N-terminal tag in the wheat germ system. However, considering the cell-free E. coli system, 60% successful expression was obtained with the N-terminal wild type PCR product in contrast to only 34% with the C-terminal one. In this context it is important to realize that this 60% expression with the N-terminal tag matches with the 59% obtained with the C-terminal mutant. Obviously, modifying the sequence by adding a sequence optimised peptide tag also avoids expression problems associated with the inition of translation.
After analysis of the 87 optimised expressions in vitro, 6 samples remained that were not expressed in vivo or in vitro (Table 2). This corresponds to a protein expression success rate of 93%. Regarding those proteins, which could not be expressed in either system, it is striking that the molecular mass of all of these targets is higher than 63 kDa with an average molecular weight of 91 kDa. Two membrane associated proteins belong to the unsuccessful targets: PNPLA8 (89 kDa) and SLC40A1 (63 kDa), the latter with more than 10 transmembrane domains. Furthermore, a DNA binding protein KIF22 (74 kDa) of the Kinesin family, involved in spindle formation, ELAC2 (93 kDa) an endonuclease, TTLL5 (92 kDa), a tubulin tyrosin ligase-like protein and KIAA1287 (135 kDa), a hypothetical protein with one transmembrane domain, are among the non-expressing targets. At this point it is unclear whether these human proteins are functionally expressed in any of the systems. Therefore, we can not speculate about the interference between the protein function and the different expression systems.
However, 6 proteins with an average molecular weight of 72 kDa were expressed in E. coli in vitro but not in wheat germ. Among these proteins is HMOX2 which belongs to the heme oxygenase family, an iron-containing protein with one transmembrane domain. As reported recently, iron-containing proteins require supplemented iron sources which were not added in this case [21]. Further proteins are two with transmembrane domains (MLSTD1 with two transmembrane domains and TLR1 with one). The three proteins RACGAP1, FLJ20203 and HSPC049 each contain one coiled coil domain and have molecular weights higher than 70 kDa. Obviously, the expression of proteins with molecular weights higher than 70 kDa are critical for the wheat germ system [22].
Ten proteins with an average molecular weight of 45 kDa also remain which were expressed in wheat germ but not in E. coli in vitro. An explanation for this can not be found in the structural domains, because a coiled coil and one transmembrane domain were not a hindrance for expression of the proteins mentioned before. Also the molecular weight is not the problem. Regarding the function of these proteins, SUPT7L (transcription regulation factor), EIF2S2 (translation initiation factor) and RPL23A (rRNA binding protein) are proteins which interfere with DNA or RNA. It seems that those proteins are likely to have negative effects on their recombinant expression, when functional active in E.coli cells. Also proteins influencing the cell cycle like DAP (involved in cell death), KIAA1142 (has a kinase motif), PAK4 (kinase, involved in the JNK pathway), GPSM2 (a signalling modulator) and OS-9 (influences cell growth viability) seem to hamper recombinant protein expression.
Based on western blotting analyses, the protein yield in wheat germ was higher compared to expressions in the E. coli in vitro. This may be due to the fact that the in vitro E. coli expression system is a batch method for protein expression, whereas the wheat germ system is based on a two-compartment system. The two chambers are separated by a semi-permeable membrane which concentrates the expressed protein in the 50 μl reaction chamber, but lets compounds required for protein synthesis such as substrates and energy components pass through into the larger feeding chamber. At the same time, potentially inhibitory by-products are diluted via diffusion across the membrane. The wheat germ system showed the highest rate of success compared to expression in E. coli in vitro or in vivo. Thus, for in vitro protein expression, specifically for toxic proteins which can not be expressed in bacteria, the wheat germ system is the method of choice.
Comparing protein solubility in E. coli bacteria and the cell-free E. coli and wheat germ systems, we found that the wheat germ system produces the highest solubility rate (97%). This was also reported previously [22]. It should be mentioned that our experimental procedure does not exclude the formation of protein aggregates. Moreover, the data show that the proteins expressed in vitro are more likely to be soluble than those expressed in vivo. However, even though the E. coli in vivo expressions showed, in a first approach, a higher success rate than in vitro, the in vitro system does have advantages. Protein expression is very fast and can be accomplished within a few hours. The expression of toxic gene products allows proteins to be expressed, which are impossible to express in bacteria. Also the use of PCR products is possible, and no clones are necessary for protein expression. However, linear DNA needs to be protected during the in vitro reactions to suppress nuclease activity. In addition, proteins are also more likely to be soluble when expressed in any of the in vitro systems used compared to expression in bacteria.
In summary, we have demonstrated that cell-free protein expression leads to the desired full-length protein with an overall success rate of up to 93%. In our study, wheat germ expression using a two compartment system is the method of choice as it shows high solubility and high protein yield.
Methods
Expression-vector construction
The genes used in this study are available from the RZPD full-ORF clone collection. Entry clones containing the genes of interest were generated by utilising the Gateway® Cloning technology (Invitrogen). All entry clones were fully sequenced in order to verify the insert within pDONR201. From the entry clone, the ORF was sub-cloned to a Gateway® destination vector (pDEST17-D18, a modification of pDEST17, Invitrogen) creating an expression clone (LR reaction), which was then transformed into DH10B bacteria. Plasmid DNA of individual clones was used for transformation of BL21 (DE3) pLysS bacteria and for protein expression in vivo as well as for protein expression in the cell-free E. coli system. The pDEST17-D18 http://www.rzpd.de destination vector was used to express selected recombinant proteins controlled by the T7 promoter with an N-terminal 6xHis-tag. Identical constructs were used for protein expression in E. coli as well as for expressions in the cell-free E. coli system. All DNA preparations were carried out by a Qiagen Biorobot 9600 using Qiawell 96 Ultra Plasmid Kits (Qiagen).
In vivo protein expression using E. coli bacteria
Competent BL21 (DE3) pLysS (Novagen) bacteria were transformed with plasmid DNA (pDEST17-D18 containing the gene of interest). The generated expression clones were cultured overnight, diluted 1:50 to a final volume of 3 ml, and incubated in 24-well plates at 30°C or 3.5 h (until the OD600 was 0,4–0,6). Expression was induced with 1 mM IPTG and bacteria cultured for a further 3,5 h at 30°C. Cells were harvested by centrifugation. A 5 μl aliquot of cell-pellet was removed and added to 45 μl of SDS sample buffer. 10 μl of the sample were then loaded onto a gel for western blotting analysis. An aliquot of the original sample was also saved for analysis of protein solubility.
In vitro protein expression (E. coli) using vector DNA
In vitro protein expression was carried out using pDEST17-D18 plasmid DNA containing the ORF of interest. A cell-free batch expression system (RTS 100 E. coli HY kit, Roche Diagnostics) was utilised and 50 μl reactions were prepared according to the manufacturer's instructions. In brief, the samples were incubated at 30°C for 4 hours in a thermal cycler. Green fluorescent protein was expressed as control protein. Following incubation, a 5 μl aliquot was removed and added to 45 μl of SDS sample buffer. 10 μl of sample were then loaded onto a gel for Western blotting analysis. An aliquot of the original sample was also saved for analysis of protein solubility.
In vitro protein expression (E. coli) using optimised linear PCR products
Three PCR products were created for each ORF, a C-terminal wild type, a C-terminal mutant and a N-terminal wild type product (Figure 2). Sequence-verified templates were applied for the amplification of PCR products with the Linear Template Generation Set (LTGS, Roche Diagnostics). For the C-terminal mutant template, silent mutations as proposed by ProteoExpert http://www.proteoexpert.com were introduced at the N-terminus of the sequence. PCR was performed using partially matching primers along the first 15 to 20 nucleotides of each ORF. One gene-specific sense primer containing silent mutations, one gene-specific anti-sense and one wild type primer were used to produce the first PCR product. Different primers were applied depending on whether a C- or a N-terminal 6xHis-tag was desired. The PCR products were checked on agarose gels, and the second amplification step was carried out according to the supplier's instructions. As positive control protein, green fluorescent protein was expressed. Prior to in vitro expression, all products were verified for correct size and purity. In vitro expression was carried out according to instructions and SDS samples prepared.
In vitro protein expression (wheat germ) using linear PCR products
Specific PCR products were generated to achieve translation in wheat germ lysate. The first wild type PCR product generated for optimisation in the E. coli in vitro system was utilised to produce a second PCR product for the wheat germ system. Linear templates with a T7 promoter and a Kozak sequence were generated for protein expression in wheat germ lysate. In contrast to PCR products created for the E. coli in vitro system, these products did not contain silent mutations. The first PCR products were made using gene-specific primer pairs and the second amplification step was carried out by the RTS Wheat Germ LTGS kit (Roche Diagnostics) according to instructions. The PCR products were again checked for correct size and purity. Proteins were expressed using the RTS 100 Wheat Germ CECF kit (Roche Diagnostics, positive control: glucuronidase) and contained either a C- or an N-terminal 6xHis-tag. Samples (50 μl) were incubated at 24°C, 900 rpm for 24 h (ProteoMaster Instrument, Roche Diagnostics), SDS samples prepared for western blotting and an aliquot saved for analysis of protein solubility.
Analysis of protein solubility
An aliquot of the induced bacterial culture was mixed with a lysis reagent (Pop Culture Reagent, Novagen) and 0.1% Tween 20 and incubated for 10 min at room temperature. The sample was centrifuged at 10000 g for 20 min, the supernatant and the pellet were separated and SDS samples prepared for western blotting analysis. For the in vitro systems, samples were centrifuged directly and the pellet and supernatant separated. Results were expressed as values ranging from 0 (no protein detectable in the supernatant) to 4 (the protein band in the supernatant is stronger than in the pellet). Values of 0 to 1 were defined as insoluble and values of 2 to 4 as soluble protein. Values correspond to: 4 > 70%; 3 > 40%; 2 > 10%; 1 < 10% solubility; 0 = unsoluble.
Western blotting
Western blotting was performed with the Criterion System (BioRad) and 10–20% gradient pre-cast gels. Samples (10 μl) were heated at 95°C for 5 min and loaded onto the gel, which was run at 200 V, 400 mA for 1 h. Following electrophoresis, gels were blotted onto PVDF membranes (Hybond P, Amersham Pharmacia) at 100 V, 1000 mA for 1 h and protein transfer checked by briefly immersing the membrane in Ponceau S solution (Sigma). Membranes were thoroughly washed in TBST (2 mM Tris/HCl, pH 7.6; 13.7 mM NaCl and 0.1% (v/v) Tween 20) and then blocked for 1 h in 5% (w/v) non-fat milk/TBST. Following another 3 × 15 min washes in TBST, membranes were incubated with the anti-His mouse antibody (Qiagen, 1:2000 in 3% (w/v) bovine serum albumin/TBST) overnight at 4°C. Following incubation with the secondary antibody (Anti-mouse IgG HRP, Southern Biotech) for 1 h, membranes were washed three times in TBST and developed with ChemiGlow® (Alpha Innotech) chemiluminescent substrate for 5 min. Images were obtained using a CCD camera system (ChemiImager 5500, Alpha Innotech). Protein bands on western blots were assigned values from 0 (no expression) to 4 (very strong band). Successful protein expression was defined for values of 2 to 4 and unsuccessful expression for values of 0 and 1. The ratings reflect the relative amount of human fusion protein compared to the reference protein (positive control). 4 ≥ reference protein; 3 ≥ 50% of r. p.; 2 ≥ 10% of r. p.; no expression <1< 10% of r.p.; 0 = no expression.
Authors' contributions
CL coordinated the experiments and helped to draft the manuscript. BG drafted the manuscript. NW and TS performed the experiments. LE built the database. JL provided plasmids. BK organised funding and helped to draft the manuscript and coordinated the study. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by Roche Diagnostics GmbH. We thank Andrea Graentzdoerffer, Cordula Nemetz, Kairat Madin, Manfred Watzele and Bernd Buchberger for advice and helpful discussions.
Contributor Information
Claudia Langlais, Email: cl40@leicester.ac.uk.
Birgit Guilleaume, Email: bguilleaume@web.de.
Nadja Wermke, Email: n.wermke@dkfz.de.
Tina Scheuermann, Email: t.scheuermann@dkfz.de.
Lars Ebert, Email: l.ebert@dkfz.de.
Joshua LaBaer, Email: josh@hms.harvard.edu.
Bernhard Korn, Email: b.korn@dkfz.de.
References
- Brenner SE. A tour of structural genomics. Nature Reviews Genetics. 2001;2:801–809. doi: 10.1038/35093574. [DOI] [PubMed] [Google Scholar]
- Wiemann S, Bechtel S, Bannasch D, Pepperkok R, Poustka A. The German cDNA network: cDNAs, functional genomics and proteomics. J Struct Funct Genomics. 2003;4:87–96. doi: 10.1023/A:1026148428520. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Itoh T, Suzuki Y, O'Donovan C, Fukuchi S, Koyanagi KO, Barrero RA, Tamura T, Yamaguchi-Kabata Y, Tanino M, et al. Integrative annotation of 21,037 human genes validated by full-length cDNA clones. Plos Biology. 2004;2:856–875. doi: 10.1371/journal.pbio.0020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussow K, Scheich C, Sievert V, Harttig U, Schultz J, Simon B, Bork P, Lehrach H, Heinemann U. Structural genomics of human proteins – target selection and generation of a public catalogue of expression clones. Microbial Cell Factories. 2005;4 doi: 10.1186/1475-2859-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning G, Makrides S. Strategies for optimising heterologous protein expression in Escherchia coli. TIBTECH. 1998;16:54–60. doi: 10.1016/s0167-7799(97)01155-4. [DOI] [PubMed] [Google Scholar]
- Braun P, Hu YH, Shen BH, Halleck A, Koundinya M, Harlow E, LaBaer J. Proteome-scale purification of human proteins from bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2654–2659. doi: 10.1073/pnas.042684199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx F. Recombinant protein expression in Escherichia coli. Current Opinion in Biotechnology. 1999;10:411–421. doi: 10.1016/S0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Madin K, Sawasaki T, Ogasawara T, Endo Y. A highly efficient and robust cell-free protein synthesis system prepared from wheat embryos: plants apparently contain a suicide system directed at ribosomes. Proc Natl Acad Sci USA. 2000;97:559–564. doi: 10.1073/pnas.97.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki T, Ogasawara T, Morishita R, Endo Y. A cell-free protein synthesis system for high-throughput proteomics. Proc Natl Acad Sci USA. 2002;99:14652–14657. doi: 10.1073/pnas.232580399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki T, Seki M, Sinozaki K, Endo Y. High-throughput expression of proteins from cDNAs catalogue from Arabidopsis in wheat germ cell-free protein synthesis system. Tanpakushitsu Kakusan Koso. 2002;47:1003–1008. [PubMed] [Google Scholar]
- Endo Y, Sawasaki T. High-throughput, genome-scale protein production method based on the wheat germ cell-free expression system. Biotechnology Advances. 2003;21:695–713. doi: 10.1016/S0734-9750(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Endo Y, Sawasaki T. Cell-free expression systems for eukaryotic protein production. Curr Opin Biotechnol. 2006;17:373–380. doi: 10.1016/j.copbio.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Vinarov DA, Lytle BL, Peterson FC, Tyler EM, Volkman BF, Markley JL. Cell-free protein production and labeling protocol for NMR-based structural proteomics. Nature Methods. 2004;1:149–153. doi: 10.1038/nmeth716. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Hirota H, Kigawa T, Yabuki T, Shlrouzu M, Terada T, Ito Y, Matsuo Y, Kuroda Y, Nishimura Y, et al. Structural genomics projects in Japan. Nature Structural Biology. 2000;7:943–945. doi: 10.1038/80712. [DOI] [PubMed] [Google Scholar]
- Busso D, Kim R, Kim SH. Expression of soluble recombinant proteins in a cell-free system using a 96-well format. Journal of Biochemical and Biophysical Methods. 2003;55:233–240. doi: 10.1016/S0165-022X(03)00049-6. [DOI] [PubMed] [Google Scholar]
- Renesto P, Raoult D. From genes to proteins – In vitro expression of rickettsial proteins. Rickettsiology: Present and Future Directions. 2003;990:642–652. doi: 10.1111/j.1749-6632.2003.tb07439.x. [DOI] [PubMed] [Google Scholar]
- Klammt C, Lohr F, Schafer B, Haase W, Dotsch V, Ruterjans H, Glaubitz C, Bernhard F. High level cell-free expression and specific labeling of integral membrane proteins. European Journal of Biochemistry. 2004;271:568–580. doi: 10.1111/j.1432-1033.2003.03959.x. [DOI] [PubMed] [Google Scholar]
- Langlais C, Gernold N, Scheuermann T, Korn B. The Linear Template Generation set: Optimization of Protein expression in the RTS 100 HY. Biochemica. 2003;3:22–25. [Google Scholar]
- de Smit M, J vD. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci USA. 1990;87:7668–7672. doi: 10.1073/pnas.87.19.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges D, Watzele M, Nemetz C, Wizemann S, Buchberger B. Analyzing and enhancing mRNA translational efficiency in an Escherichia coli in vitro expression system. Biochemical and Biophysical Research Communications. 2004;318:601–614. doi: 10.1016/j.bbrc.2004.04.064. [DOI] [PubMed] [Google Scholar]
- Boyer ME, Wang CW, Swartz JR. Simultaneous expression and maturation of the iron-sulfur protein ferredoxin in a cell-free system. Biotechnology and Bioengineering. 2006;94:128–138. doi: 10.1002/bit.20830. [DOI] [PubMed] [Google Scholar]
- Tyler RC, Aceti DJ, Bingman CA, Cornilescu CC, Fox BG, Frederick RO, Jeon WB, Lee MS, Newman CS, Peterson FC, et al. Comparison of cell-based and cell-free protocols for producing target proteins from the Arabidopsis thaliana genome for structural studies. Proteins-Structure Function and Bioinformatics. 2005;59:633–643. doi: 10.1002/prot.20436. [DOI] [PubMed] [Google Scholar]