Abstract
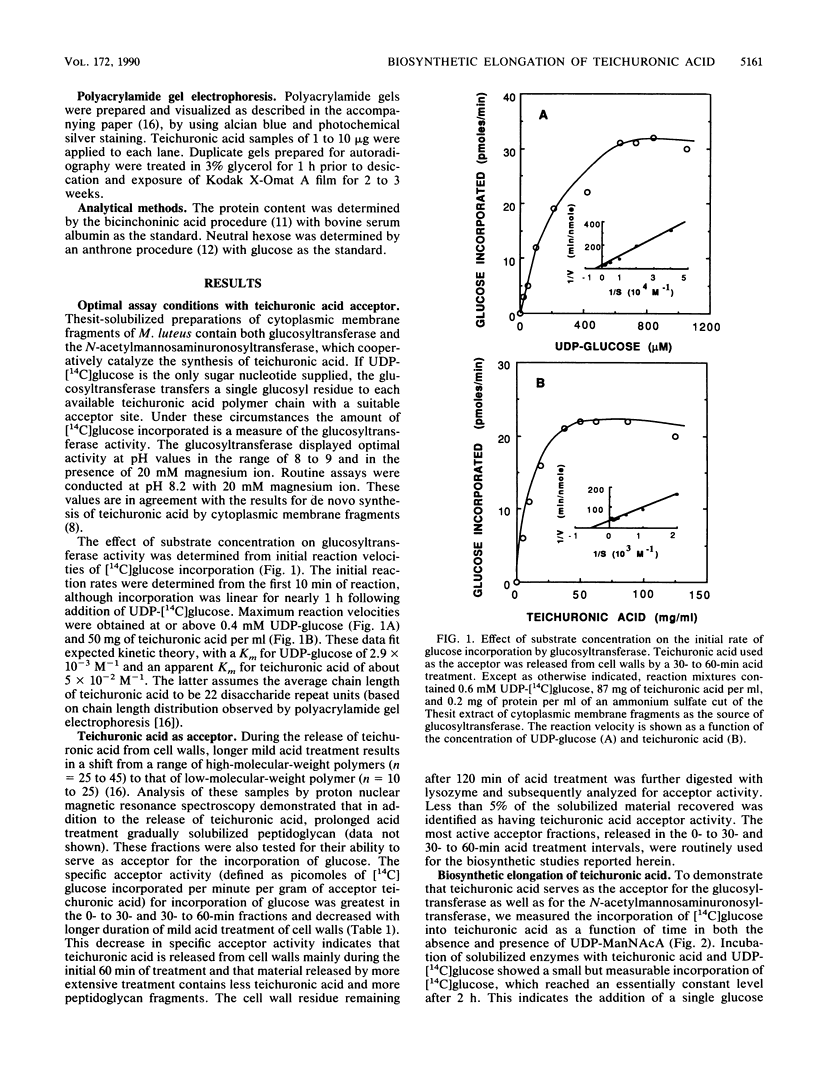
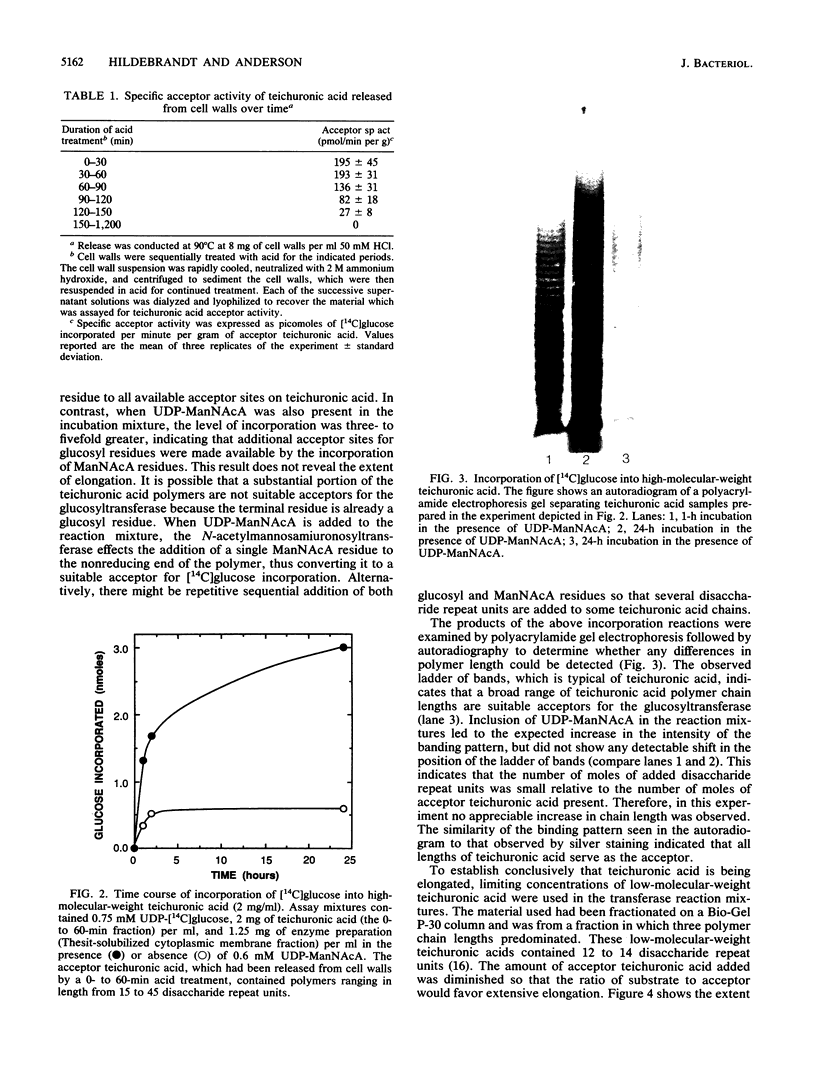
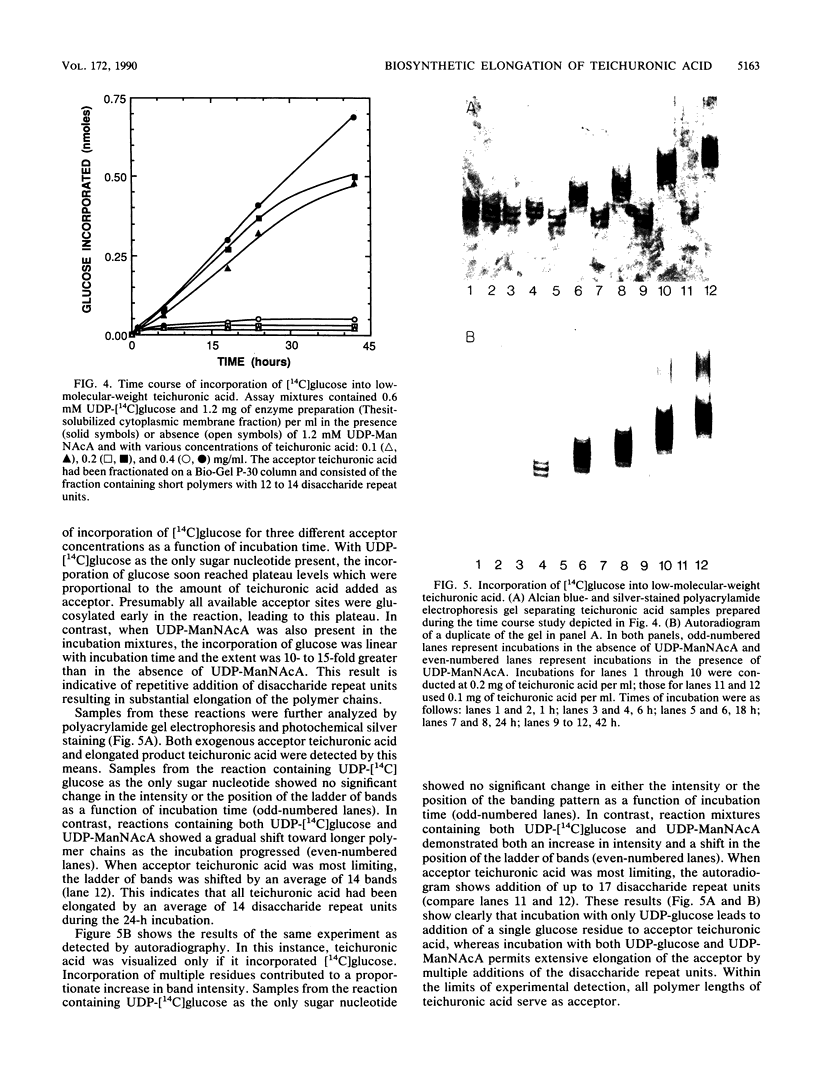
Cytoplasmic membrane fragments of Micrococcus luteus catalyze in vitro biosynthesis of teichuronic acid from uridine diphosphate D-glucose (UDP-glucose), uridine diphosphate N-acetyl-D-mannosaminuronic acid (UDP-ManNAcA), and uridine diphosphate N-acetyl-D-glucosamine. Membrane fragments solubilized with Thesit (dodecyl alcohol polyoxyethylene ether) can utilize UDP-glucose and UDP-ManNAcA to effect elongation of teichuronic acid isolated from native cell walls. When UDP-glucose is the only substrate supplied, the detergent-solubilized glucosyltransferase incorporates a single glucosyl residue onto each teichuronic acid acceptor. When both UDP-glucose and UDP-ManNAcA are supplied, the glucosyltransferase and the N-acetylmannosaminuronosyltransferase act cooperatively to elongate the teichuronic acid acceptor by multiple additions of the disaccharide repeat unit. As shown by polyacrylamide gel electrophoresis, low-molecular-weight fractions of teichuronic acid are converted to higher-molecular-weight polymers by the addition of as many as 17 disaccharide repeat units.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gassner G. T., Dickie J. P., Hamerski D. A., Magnuson J. K., Anderson J. S. Teichuronic acid reducing terminal N-acetylglucosamine residue linked by phosphodiester to peptidoglycan of Micrococcus luteus. J Bacteriol. 1990 May;172(5):2273–2279. doi: 10.1128/jb.172.5.2273-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. The structure of the branching point between acidic polysaccharide and peptidoglycan in Micrococcus lysodeikticus cell wall. J Biochem. 1977 May;81(5):1181–1186. [PubMed] [Google Scholar]
- Ichihara N., Ishimoto N., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosaminuronic acid. FEBS Lett. 1974 Feb 1;39(1):46–48. doi: 10.1016/0014-5793(74)80013-x. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Hoger J. H., Ratnayake J. H., Anderson J. S. Characterization of three intermediates in the biosynthesis of teichuronic acid of Micrococcus luteus. Arch Biochem Biophys. 1984 Dec;235(2):679–691. doi: 10.1016/0003-9861(84)90244-3. [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Lacher K. P., Anderson J. S. Carbon-13 nuclear magnetic resonance spectroscopic study of teichuronic acid from Micrococcus luteus cell walls. Comparison of the polysaccharide isolated from cells with that synthesized in vitro. Biochemistry. 1981 Aug 4;20(16):4781–4785. doi: 10.1021/bi00519a039. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. L., Anderson J. S. Biosynthesis of the polysaccharide of micrococcus lysodekticus cells walls. I. Characterization of an in vitro system for polysaccharide biosynthesis. J Biol Chem. 1972 Apr 25;247(8):2471–2479. [PubMed] [Google Scholar]
- Rohr T. E., Levy G. N., Stark N. J., Anderson J. S. Initial reactions in biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3460–3465. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stark N. J., Levy G. N., Rohr T. E., Anderson J. S. Reactions of second stage of biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3466–3472. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Traxler C. I., Goustin A. S., Anderson J. S. Elongation of teichuronic acid chains by a wall-membrane preparation from Micrococcus luteus. J Bacteriol. 1982 May;150(2):649–656. doi: 10.1128/jb.150.2.649-656.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters P. J., Hildebrandt K. M., Dickie J. P., Anderson J. S. Polymer length of teichuronic acid released from cell walls of Micrococcus luteus. J Bacteriol. 1990 Sep;172(9):5154–5159. doi: 10.1128/jb.172.9.5154-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]