Abstract
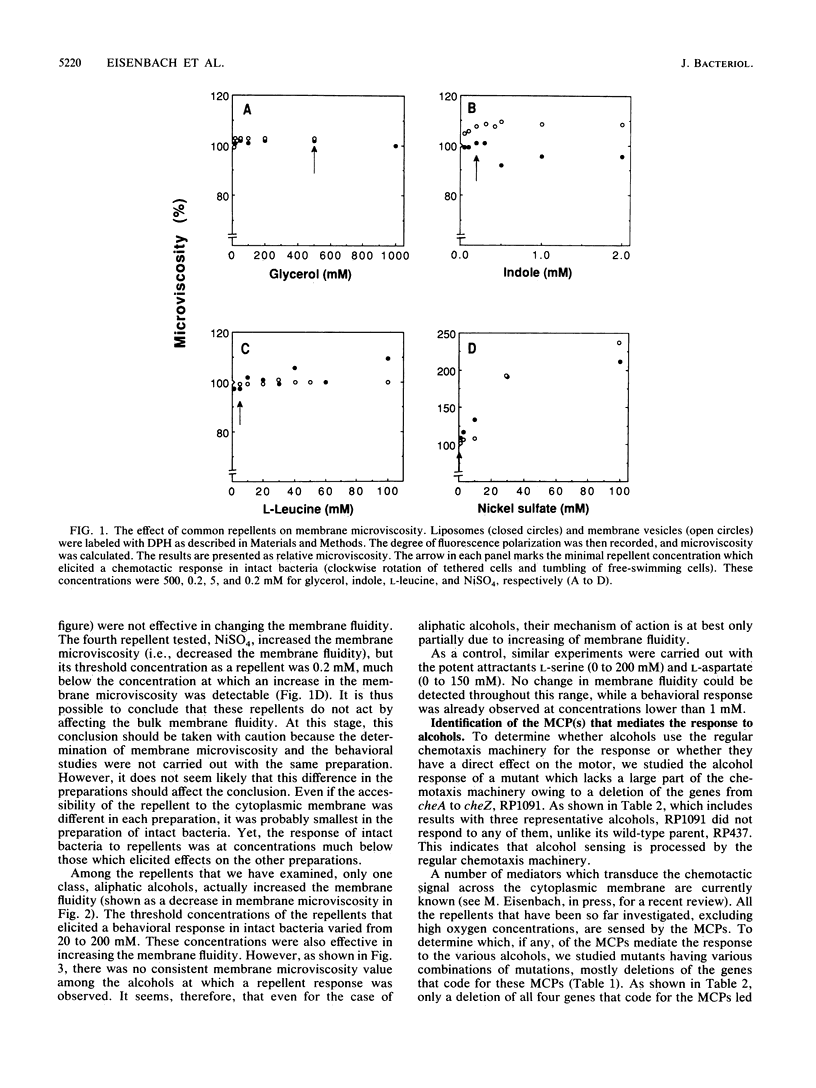
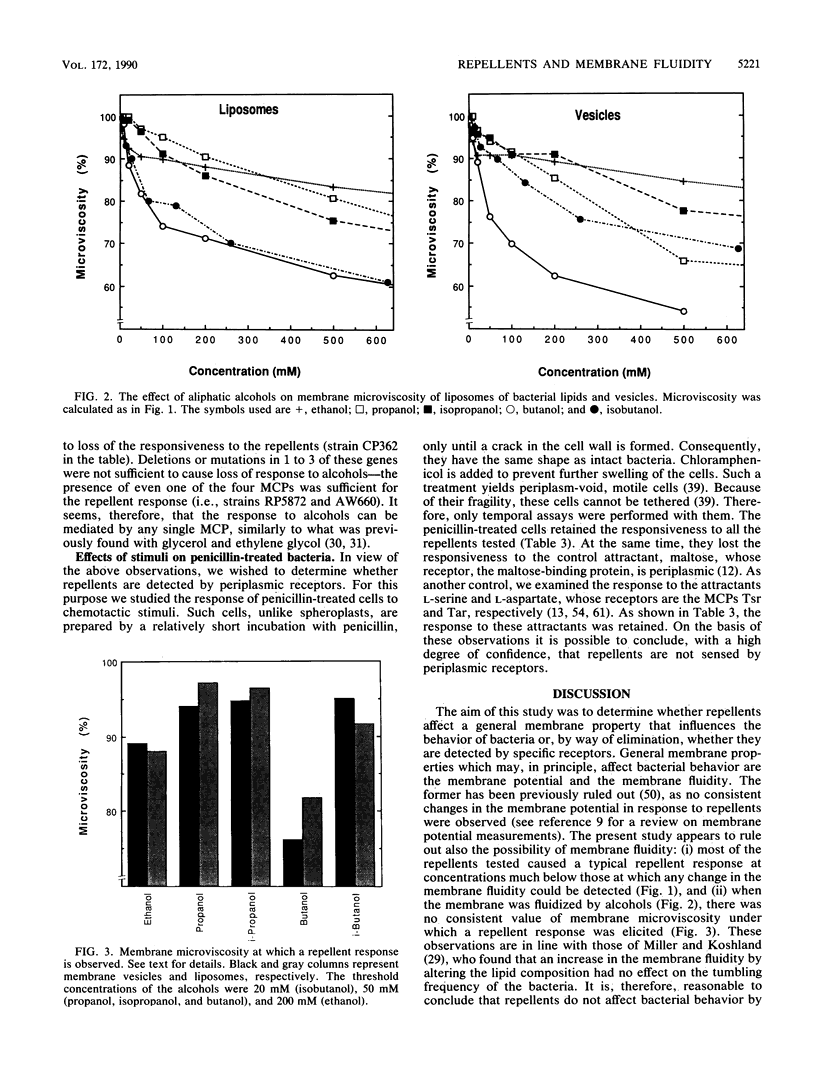
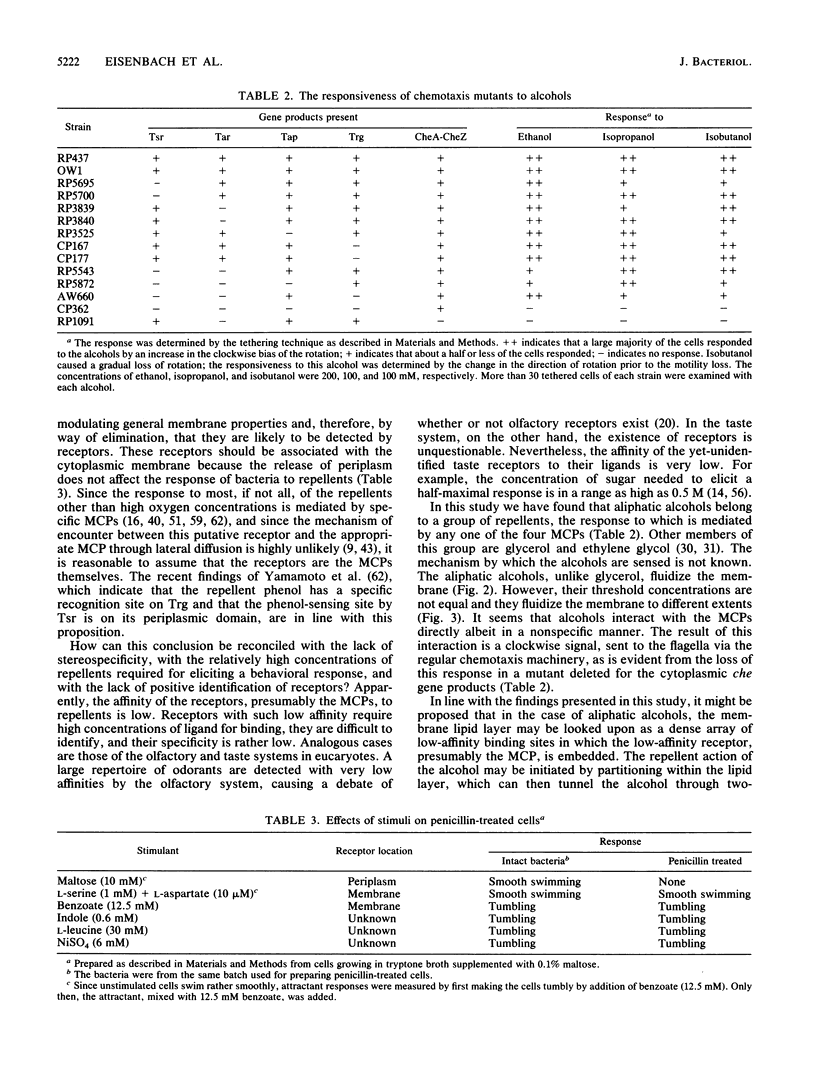
A long-standing question in bacterial chemotaxis is whether repellents are sensed by receptors or whether they change a general membrane property such as the membrane fluidity and this change, in turn, is sensed by the chemotaxis system. This study addressed this question. The effects of common repellents on the membrane fluidity of Escherichia coli were measured by the fluorescence polarization of the probe 1,6-diphenyl-1,3,5-hexatriene in liposomes made of lipids extracted from the bacteria and in membrane vesicles. Glycerol, indole, and L-leucine had no significant effect on the membrane fluidity. NiSO4 decreased the membrane fluidity but only at concentrations much higher than those which elicit a repellent response in intact bacteria. This indicated that these repellents are not sensed by modulating the membrane fluidity. Aliphatic alcohols, on the other hand, fluidized the membrane, but the concentrations that elicited a repellent response were not equally effective in fluidizing the membrane. The response of intact bacteria to alcohols was monitored in various chemotaxis mutants and found to be missing in mutants lacking all the four methyl-accepting chemotaxis proteins (MCPs) or the cytoplasmic che gene products. The presence of any single MCP was sufficient for the expression of a repellent response. It is concluded (i) that the repellent response to aliphatic alcohols can be mediated by any MCP and (ii) that although an increase in membrane fluidity may take part in a repellent response, it is not the only mechanism by which aliphatic alcohols, or at least some of them, are effective as repellents. To determine whether any of the E. coli repellents are sensed by periplasmic receptors, the effects of repellents from various classes on periplasm-void cells were examined. The responses to all the repellents tested (sodium benzoate, indole, L-leucine, and NiSO4) were retained in these cells. In a control experiment, the response of the attractant maltose, whose receptor is periplasmic, was lost. This indicates that these repellents are not sensed by periplasmic receptors. In view of this finding and the involvement of the MCPs in repellent sensing, it is proposed that the MCPs themselves are low-affinity receptors for the repellents.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., Parkinson J. S. Transmembrane signaling by bacterial chemoreceptors: E. coli transducers with locked signal output. Cell. 1988 Dec 2;55(5):817–826. doi: 10.1016/0092-8674(88)90137-7. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Callahan A. M., Frazier B. L., Parkinson J. S. Chemotaxis in Escherichia coli: construction and properties of lambda tsr transducing phage. J Bacteriol. 1987 Mar;169(3):1246–1253. doi: 10.1128/jb.169.3.1246-1253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M., Adler J. Bacterial cell envelopes with functional flagella. J Biol Chem. 1981 Aug 25;256(16):8807–8814. [PubMed] [Google Scholar]
- Eisenbach M. Changes in membrane potential of Escherichia coli in response to temporal gradients of chemicals. Biochemistry. 1982 Dec 21;21(26):6818–6825. doi: 10.1021/bi00269a030. [DOI] [PubMed] [Google Scholar]
- Eisenbach M. Functions of the flagellar modes of rotation in bacterial motility and chemotaxis. Mol Microbiol. 1990 Feb;4(2):161–167. doi: 10.1111/j.1365-2958.1990.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Wolf A., Welch M., Caplan S. R., Lapidus I. R., Macnab R. M., Aloni H., Asher O. Pausing, switching and speed fluctuation of the bacterial flagellar motor and their relation to motility and chemotaxis. J Mol Biol. 1990 Feb 5;211(3):551–563. doi: 10.1016/0022-2836(90)90265-N. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Failure of sensory adaptation in bacterial mutants that are defective in a protein methylation reaction. Cell. 1978 Dec;15(4):1231–1240. doi: 10.1016/0092-8674(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. Maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Apr;122(1):206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblom M. L., Adler J. Genetic and biochemical properties of Escherichia coli mutants with defects in serine chemotaxis. J Bacteriol. 1980 Dec;144(3):1048–1060. doi: 10.1128/jb.144.3.1048-1060.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S. J., Hobson A. C., Adler J. Attractants and repellents influence methylation and demethylation of methyl-accepting chemotaxis proteins in an extract of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6309–6313. doi: 10.1073/pnas.76.12.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Ball C. B., Adler J. Identification of a methyl-accepting chemotaxis protein for the ribose and galactose chemoreceptors of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):260–264. doi: 10.1073/pnas.76.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikos A., Conley M. P., Boyd A., Berg H. C., Simon M. I. Chimeric chemosensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus I. R., Welch M., Eisenbach M. Pausing of flagellar rotation is a component of bacterial motility and chemotaxis. J Bacteriol. 1988 Aug;170(8):3627–3632. doi: 10.1128/jb.170.8.3627-3632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Lofgren K. W., Fox C. F. Attractant-directed motility in Escherichia coli: requirement for a fluid lipid phase. J Bacteriol. 1974 Jun;118(3):1181–1182. doi: 10.1128/jb.118.3.1181-1182.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Membrane fluidity and chemotaxis: effects of temperature and membrane lipid composition on the swimming behavior of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1977 Apr;111(2):183–201. doi: 10.1016/s0022-2836(77)80122-8. [DOI] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Demethylation of methyl-accepting chemotaxis proteins in Escherichia coli induced by the repellents glycerol and ethylene glycol. J Bacteriol. 1984 Feb;157(2):576–581. doi: 10.1128/jb.157.2.576-581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J Bacteriol. 1983 Apr;154(1):104–112. doi: 10.1128/jb.154.1.104-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotactic repellents of Bacillus subtilis. J Mol Biol. 1976 Jan 5;100(1):103–108. doi: 10.1016/s0022-2836(76)80037-x. [DOI] [PubMed] [Google Scholar]
- Park C., Hazelbauer G. L. Mutations specifically affecting ligand interaction of the Trg chemosensory transducer. J Bacteriol. 1986 Jul;167(1):101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Hazelbauer G. L. Transfer of chromosomal mutations to plasmids via Hfr-mediated conduction. J Bacteriol. 1986 Jan;165(1):312–314. doi: 10.1128/jb.165.1.312-314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978 Jul;135(1):45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Houts S. E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982 Jul;151(1):106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Correlation between bacteriophage chi adsorption and mode of flagellar rotation of Escherichia coli chemotaxis mutants. J Bacteriol. 1983 May;154(2):604–611. doi: 10.1128/jb.154.2.604-611.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Direction of flagellar rotation in bacterial cell envelopes. J Bacteriol. 1984 Apr;158(1):222–230. doi: 10.1128/jb.158.1.222-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P. H., Eigen M. Diffusion controlled reaction rates in spheroidal geometry. Application to repressor--operator association and membrane bound enzymes. Biophys Chem. 1974 Oct;2(3):255–263. doi: 10.1016/0301-4622(74)80050-5. [DOI] [PubMed] [Google Scholar]
- Segall J. E., Ishihara A., Berg H. C. Chemotactic signaling in filamentous cells of Escherichia coli. J Bacteriol. 1985 Jan;161(1):51–59. doi: 10.1128/jb.161.1.51-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour F. W., Doetsch R. N. Chemotactic responses by motile bacteria. J Gen Microbiol. 1973 Oct;78(2):287–296. doi: 10.1099/00221287-78-2-287. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Slocum M. K., Parkinson J. S. Genetics of methyl-accepting chemotaxis proteins in Escherichia coli: null phenotypes of the tar and tap genes. J Bacteriol. 1985 Aug;163(2):586–594. doi: 10.1128/jb.163.2.586-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Macnab R. M., Alger J. R., Castle A. M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982 Oct;152(1):384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Stock J. B., Koshland D. E., Jr Role of membrane potential and calcium in chemotactic sensing by bacteria. J Mol Biol. 1981 Jun 25;149(2):241–257. doi: 10.1016/0022-2836(81)90300-4. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Kort E. N., Larsen S. H., Ordal G. W., Reader R. W., Adler J. Role of methionine in bacterial chemotaxis: requirement for tumbling and involvement in information processing. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4640–4644. doi: 10.1073/pnas.72.11.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Koshland D. E., Jr Changing reactivity of receptor carboxyl groups during bacterial sensing. J Biol Chem. 1981 Nov 10;256(21):10826–10833. [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Plachy W. Z., Nikaido H. Partitioning of hydrophobic probes into lipopolysaccharide bilayers. Biochim Biophys Acta. 1990 May 9;1024(1):152–158. doi: 10.1016/0005-2736(90)90218-d. [DOI] [PubMed] [Google Scholar]
- Wang E. A., Koshland D. E., Jr Receptor structure in the bacterial sensing system. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7157–7161. doi: 10.1073/pnas.77.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Macnab R. M., Imae Y. Repellent response functions of the Trg and Tap chemoreceptors of Escherichia coli. J Bacteriol. 1990 Jan;172(1):383–388. doi: 10.1128/jb.172.1.383-388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Parola A. H., Abdah M., Masalha H. Homeoviscous adaptation, growth rate, and morphogenesis in bacteria. Biophys J. 1985 Aug;48(2):337–339. doi: 10.1016/S0006-3495(85)83788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]