Abstract
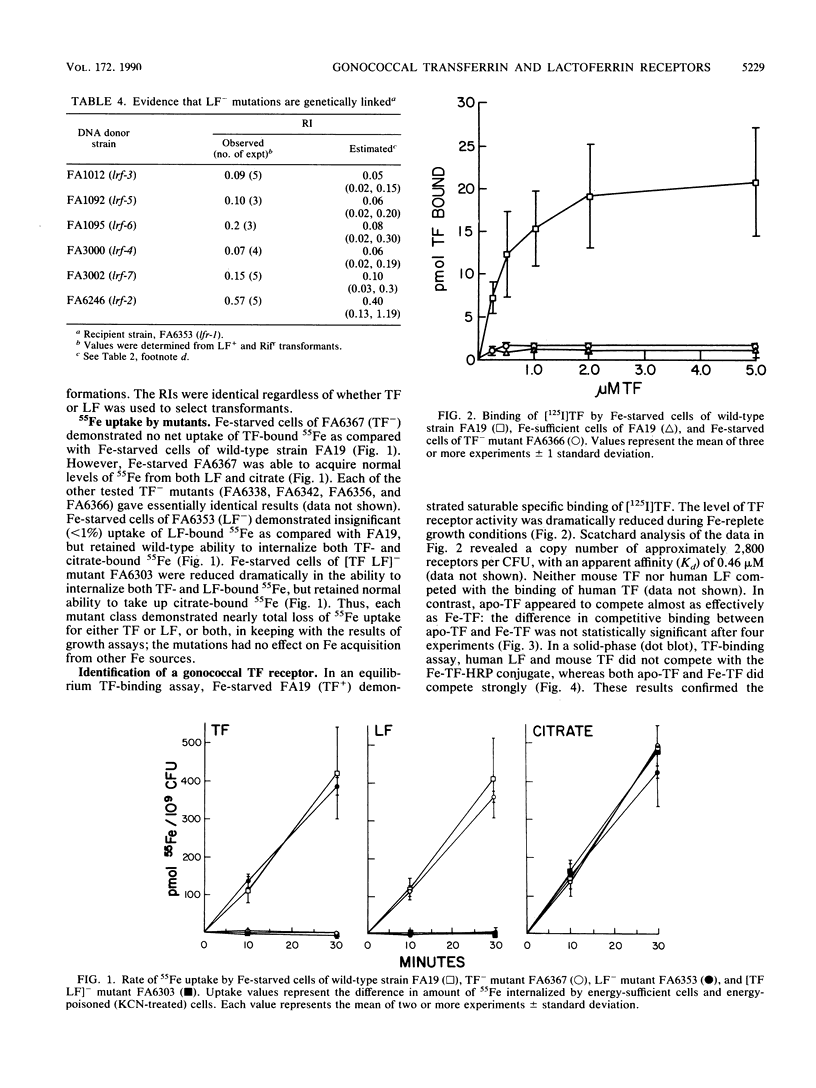
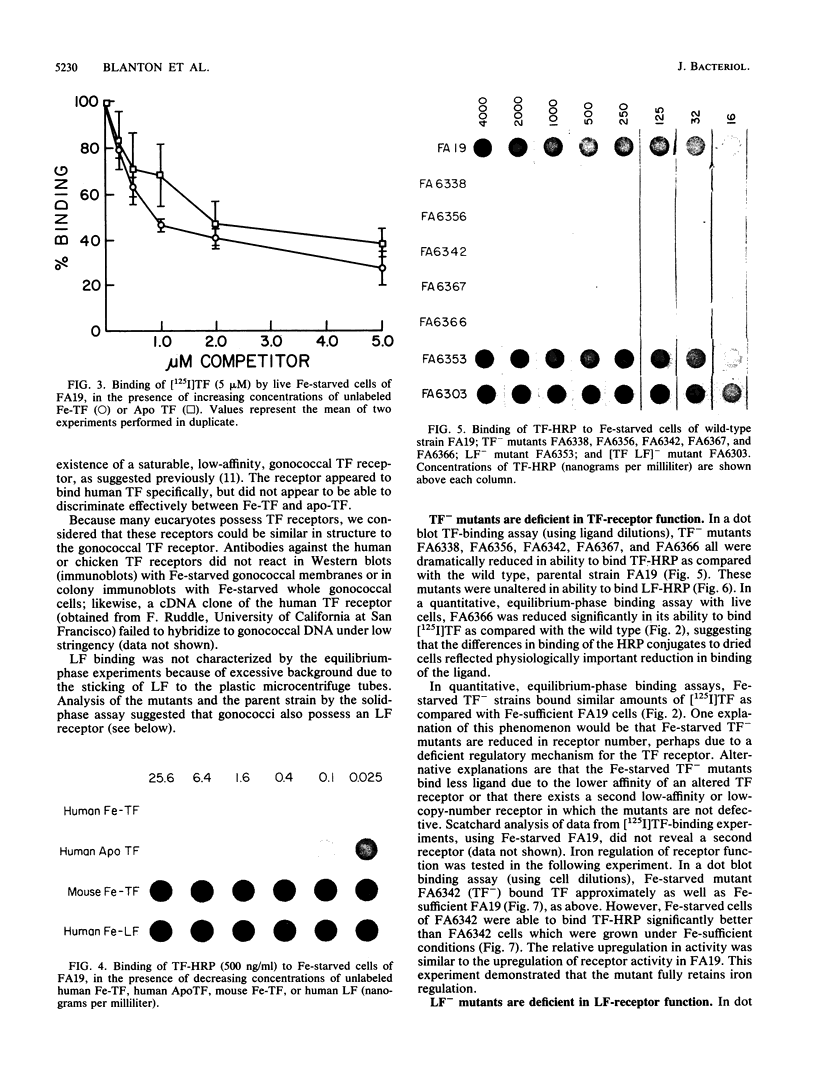
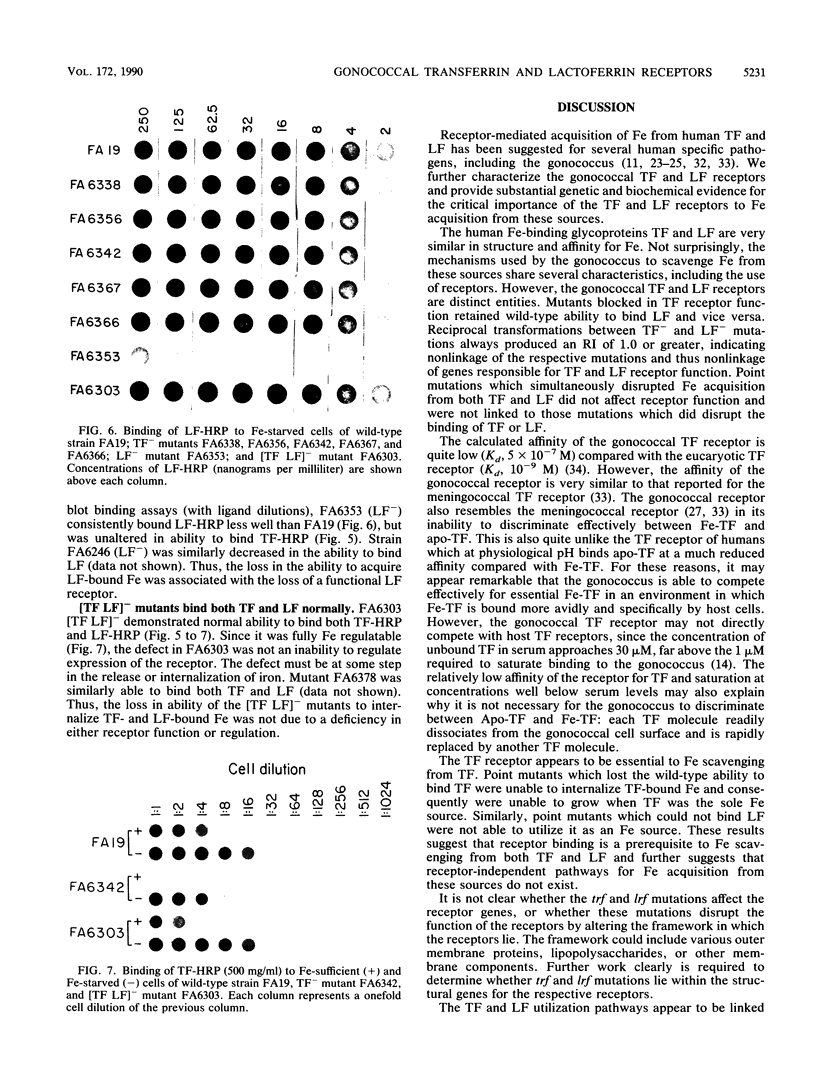
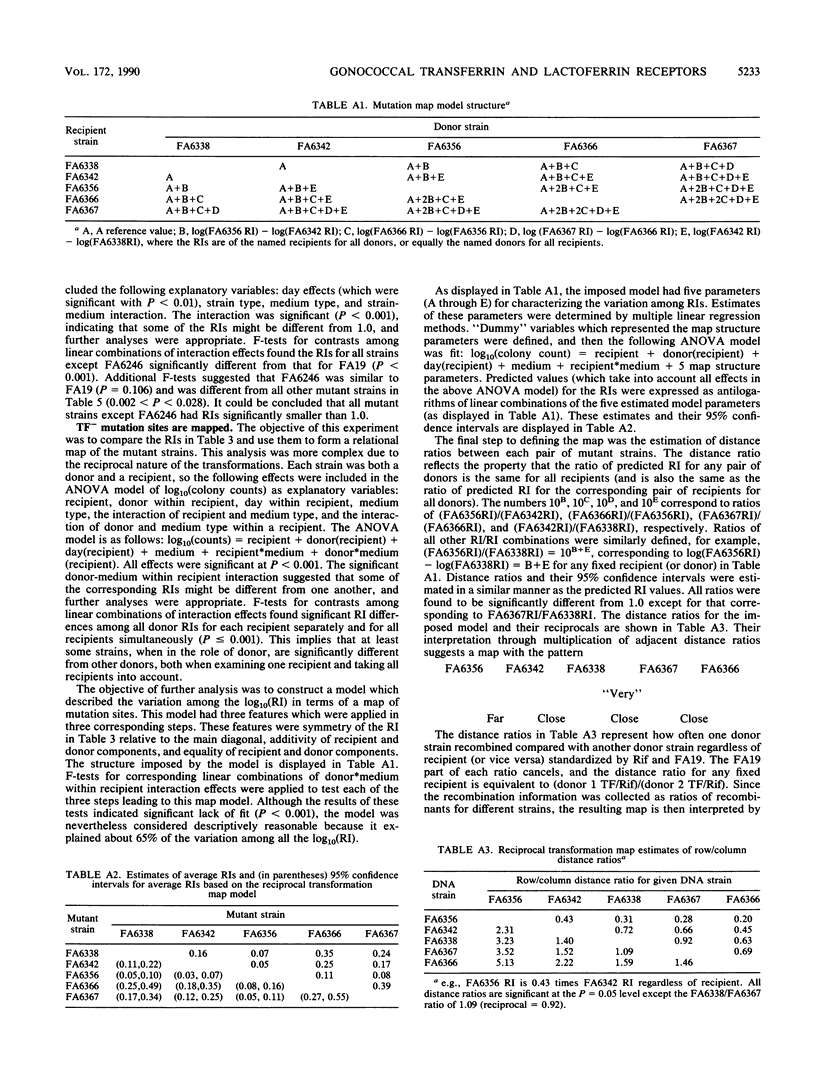
Transferrin (TF) and lactoferrin (LF) are probably the major sources of iron (Fe) for Neisseria gonorrhoeae in vivo. We isolated mutants of N. gonorrhoeae FA19 that were unable to grow with Fe bound to either TF (TF-) or LF (LF-) or to both TF and LF ([TF LF]-). The amount of Fe internalized by each of the mutants was reduced to background levels from the relevant iron source(s). The wild-type parent strain exhibited saturable specific binding of TF and LF; receptor activity was induced by Fe starvation. The TF(-)-specific or LF(-)-specific mutants were almost completely lacking in receptor activity for TF or LF, respectively, whereas the [TF LF]- mutants bound both TF and LF as well as the wild-type strain. All mutants utilized citrate and heme normally as Fe sources. These results demonstrate that ability to bind TF or LF is essential for gonococci to scavenge appreciable amounts of Fe from these sources in vitro. In addition, the TF and LF Fe acquisition pathways are linked by the mutual use of a nonreceptor gene product that is essential to Fe scavenging from both of these sources; this gene product is not required for Fe acquisition from other sources.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Graves J. F., Sox T. E., Tenover F. C., Sparling P. F. Marker rescue by a homologous recipient plasmid during transformation of gonococci by a hybrid Pcr plasmid. J Bacteriol. 1982 Jul;151(1):77–82. doi: 10.1128/jb.151.1.77-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., McKenna W., Woods J. P., Sparling P. F. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb Pathog. 1987 Nov;3(5):351–363. doi: 10.1016/0882-4010(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., West E. P., McKenna W., Thompson S. A., Sparling P. F. A pleiotropic iron-uptake mutant of Neisseria meningitidis lacks a 70-kilodalton iron-regulated protein. Infect Immun. 1988 Apr;56(4):977–983. doi: 10.1128/iai.56.4.977-983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRATI-ELIZUR E., SRINIVASAN P. R., ZAMENHOF S. Genetic analysis, by means of transformation, of histidine linkage groups in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Jan 15;47:56–63. doi: 10.1073/pnas.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. W., Brandt P., Mahoney J. R., Lee J. T., Jr Haptoglobin: a natural bacteriostat. Science. 1982 Feb 5;215(4533):691–693. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- McKenna W. R., Mickelsen P. A., Sparling P. F., Dyer D. W. Iron uptake from lactoferrin and transferrin by Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):785–791. doi: 10.1128/iai.56.4.785-791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Eberhard U. Hemopexin. N Engl J Med. 1970 Nov 12;283(20):1090–1094. doi: 10.1056/NEJM197011122832007. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984 Aug 1;160(2):398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead K., Hill T., Chart H. Interaction of lactoferrin and transferrins with the outer membrane of Bordetella pertussis. J Gen Microbiol. 1987 Apr;133(4):891–898. doi: 10.1099/00221287-133-4-891. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol Microbiol. 1988 Jul;2(4):467–472. doi: 10.1111/j.1365-2958.1988.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon V. V., Baseman J. B. The acquisition of human lactoferrin by Mycoplasma pneumoniae. Microb Pathog. 1987 Dec;3(6):437–443. doi: 10.1016/0882-4010(87)90013-1. [DOI] [PubMed] [Google Scholar]
- Tsai J., Dyer D. W., Sparling P. F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988 Dec;56(12):3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H. The structure, function, and regulation of transferrin receptors. Invest Radiol. 1987 Jan;22(1):74–83. doi: 10.1097/00004424-198701000-00017. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987 Aug;169(8):3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]