Abstract
The mechanism of superoxide generation by endothelial nitric oxide synthase (eNOS) was investigated by the electron spin resonance spin-trapping technique using 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide. In the absence of calcium/calmodulin, eNOS produces low amounts of superoxide. Upon activating eNOS electron transfer reactions by calcium/calmodulin binding, superoxide formation is increased. Heme-iron ligands, cyanide, imidazole, and the phenyl(diazene)-derived radical inhibit superoxide generation. No inhibition is observed after addition of l-arginine, NG-hydroxy-l-arginine, l-thiocitrulline, and l-NG-monomethyl arginine to activated eNOS. These results demonstrate that superoxide is generated from the oxygenase domain by dissociation of the ferrous–dioxygen complex and that occupation of the l-arginine binding site does not inhibit this process. However, the concomitant addition of l-arginine and tetrahydrobiopterin (BH4) abolishes superoxide generation by eNOS. Under these conditions, l-citrulline production is close to maximal. Our data indicate that BH4 fully couples l-arginine oxidation to NADPH consumption and prevents dissociation of the ferrous–dioxygen complex. Under these conditions, eNOS does not generate superoxide. The presence of flavins, at concentrations commonly employed in NOS assay systems, enhances superoxide generation from the reductase domain. Our data indicate that modulation of BH4 concentration may regulate the ratio of superoxide to nitric oxide generated by eNOS.
Keywords: electron spin-resonance spin trapping/tetrahydrobiopterin/flavins/l-arginine/nitric oxide synthase inhibitors
The endothelial nitric oxide synthase (eNOS) contributes to the regulation of systemic blood pressure by the production of nitric oxide (⋅NO) (1–3). It has been demonstrated in blood vessels that ⋅NO activates intracellular signaling pathways controlling intracellular calcium levels in vascular smooth muscle cells, resulting in vasorelaxation (3, 4). The role of ⋅NO in pathophysiological conditions, such as hypercholesterolemia and atherosclerosis, is under active investigation. Under these conditions, an impaired endothelial-dependent vasorelaxation is observed, which is associated with enhanced oxidative vascular damage (5–7). It has been proposed that an alteration in the levels of ⋅NO and superoxide may be responsible for endothelial dysfunction (7–9). The reaction between ⋅NO and superoxide generates peroxynitrite (ONOO−), a potent oxidizing agent (10–12). Although in vitro data are consistent with this proposal, it is as yet unclear how the endothelium regulates the production of these radicals. It has been shown that chronic exposure of endothelial cells to hypercholesterolemic levels of low density lipoprotein (240 mg of cholesterol/dl) increases the release of superoxide by an eNOS-dependent mechanism (13). This indicates that eNOS may play an important role in the production of both superoxide and ⋅NO. Superoxide generation by a purified preparation of the neuronal isoform of NOS (nNOS) has been demonstrated (14). By analogy, the direct generation of superoxide by eNOS is a possibility. We have recently presented preliminary evidence for purified eNOS-dependent superoxide generation (15).
Constitutive NOS monomers consist of a flavin-containing reductase domain, a heme containing oxygenase domain, and a regulatory calmodulin-binding linker sequence (16, 17). On calcium/calmodulin binding, a flow of NADPH-derived electrons is established from the reductase to the oxygenase domain of NOS, which thereby activates enzyme catalysis (18). It has been demonstrated that calcium/calmodulin binding to nNOS increases the rate of reduction of both flavins and the heme iron (19). Low temperature spectroscopy and magnetic circular dichroism have demonstrated that reduction of iron(III) to iron(II) facilitates oxygen binding to the heme group to form a transient ferrous–dioxygen complex (J. H. Dawson, A. P. Ledbetter, M. Sono, K. McMillan, and B.S.S.M., unpublished results). This complex is reduced to form water and a hydroxylating heme iron(IV)-oxo species, which oxidizes l-arginine to ⋅NO and l-citrulline (16, 20). However, the ferrous–dioxygen complex may also dissociate to form superoxide and to regenerate heme iron(III) (14, 21). In addition to calcium/calmodulin, NOS also requires (6R)-5,6,7,8-tetrahydrobiopterin (BH4) for enzyme activity. Evidence indicates that this cofactor may be a key molecule in the control of both ⋅NO and superoxide generation, and consequently the formation of hydrogen peroxide and peroxynitrite, by nNOS (21, 22).
There are few reliable methods for the detection and quantification of NOS-dependent superoxide formation. The superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c has long been used as an indirect but specific assay for the detection of superoxide (23). However, the direct calcium/calmodulin-enhanced reduction of ferricytochrome c by the flavin-containing reductase domain of eNOS precludes the use of ferricytochrome c in this system (24, 25). Acetylation of ferricytochrome c has been reported to minimize direct reduction by nNOS (26). The direct reduction of lucigenin and nitroblue tetrazolium by NOS invalidates the use of these chemicals as superoxide detectors (27). Electron spin resonance (ESR) is the only methodology available to directly detect and quantify radical production. However, ESR cannot directly detect superoxide at room temperature because of its rapid decay. The reaction of superoxide with spin-trapping agents produces a radical adduct that is stable at ambient conditions. To detect superoxide from nNOS both 5,5-dimethyl pyrroline N-oxide (DMPO) (14) and its phosphorylated analog 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO) (27) have been used. The DEPMPO–superoxide radical adduct (DEPMPO-OOH) is more stable than DMPO-OOH. It has been reported that DEPMPO is 40-fold more sensitive than the cytochrome c assay for the detection of superoxide (28). In addition, DEPMPO traps superoxide with an efficiency of 60–70% (28). Thus, DEPMPO is an ideal spin trap for detecting and quantifying superoxide production from eNOS by ESR.
eNOS-dependent superoxide formation may play an important role in the pathology of conditions like diabetes, hypertension, aging, and atherosclerosis (29, 30). Alterations of NOS activity in favor of superoxide formation will be proinflammatory and proatherogenic. We have previously demonstrated that redox-cycling compounds like lucigenin (31) and adriamycin (32) shift eNOS activity from a ⋅NO synthase to a NADPH oxidase. In this work we present direct evidence for the generation of superoxide by eNOS by a calcium/calmodulin-dependent mechanism. In particular, the effect of BH4, in the presence and absence of l-arginine, reveals that changes in BH4 concentration can regulate the formation of superoxide relative to ⋅NO. The physiological implications of this regulation are discussed.
MATERIALS AND METHODS
Materials.
Bovine brain calmodulin and diphenyleneiodonium chloride were obtained from Calbiochem. l-[14C]arginine was obtained from DuPont/NEN. NADPH, l-arginine, calcium chloride, EGTA, flavin mononucleotide (FMN), flavin-adenine dinucleotide (FAD), glutathione, imidazole, and BSA were obtained from Sigma. l-Thiocitrulline, NG-nitro-l-arginine methyl ester (l-NAME), NG-nitro-l-arginine, NG-hydroxy-l-arginine monoacetate, and BH4 were obtained from Alexis (San Diego, CA). Diethylenetriaminepentaacetic acid (DTPA) was obtained from Fluka and bovine Cu/Zn-SOD (5,000 units/mg) was obtained from Boehringer Mannheim. DEPMPO was synthesized as described (33). Recombinant eNOS was purified in the absence of BH4 as described (34). eNOS was purified by HPLC by using a Superose G HR 10/30 column (Pharmacia) and the top fractions of the peak corresponding to dimeric eNOS were collected. eNOS containing BH4 was prepared by incubating the enzyme with BH4 (18-fold molar excess) for 15 min at 4°C before concentration and HPLC purification. Enzyme concentration is expressed on the basis of heme content.
ESR Measurements.
ESR spectra were recorded at room temperature on a Varian E-109 spectrometer operating at 9.03 GHz and 100 KHz field modulation equipped with a loop–gap resonator (27). Reactions were initiated by the addition of eNOS to the incubation mixtures containing DEPMPO. After 1 min incubation the sample was scanned 10 times and the resultant ESR spectrum quantified. Concentration of the radical adducts was calculated by double integration of the computer-based simulation spectra from the experimental data. 3-Carbamoyl-2,2,5,5-tetramethylpyrrolidine N-oxyl was used as standard (N.H., J.V.-V., and B.K., unpublished results). Computer simulation was performed by using software written by D. Duling (35).
Biochemical Assays.
eNOS Activity. eNOS activity was determined by quantifying the conversion of l-[14C]arginine to l-[14C]citrulline as previously described (32). Briefly, 11.1 pmol eNOS was added to reaction mixtures (final volume, 0.22 ml) containing 50 mM Hepes (pH 7.4), 0.1 mM DTPA, 0.1 mM (0.625 μCi) l-[14C]arginine, 0.3–0.5 mM NADPH, 0.2 mM calcium chloride, 20 μg/ml calmodulin, 1 μM FMN, 1 μM FAD, 10 μM BH4, 100 μM glutathione, and 200 μg/ml BSA. To stop the reaction, an aliquot of the reaction mixture (50 μl) was diluted in 50 mM Hepes (pH 5.5) containing 0.5 mM EGTA, and chilled on ice. l-[14C]Citrulline was isolated from the excess of l-[14C]arginine by using a Dowex 50W cation-exchange column, and its concentration was determined by liquid scintillation counting.
NADPH Consumption by eNOS.
The initial rates of NADPH oxidation were determined spectrophotometrically at 340 nm. NADPH concentration was calculated by using a molar extinction coefficient of 6.22 mM−1⋅cm−1. Reactions were initiated by addition of 0.3 mM NADPH to reaction mixtures (final volume, 0.25 ml) containing 18.5 pmol of eNOS in 50 mM Hepes buffer (pH 7.4), 0.1 mM DTPA in the presence of cofactors, inhibitors, and l-arginine when indicated. To determine the ratio of NADPH consumption to l-citrulline formed by eNOS, aliquots of the reaction mixtures containing l-[14C]arginine, were analyzed for l-[14C]citrulline concentration at different times.
RESULTS AND DISCUSSION
Generation of Superoxide by eNOS: Dependence on Calcium/Calmodulin.
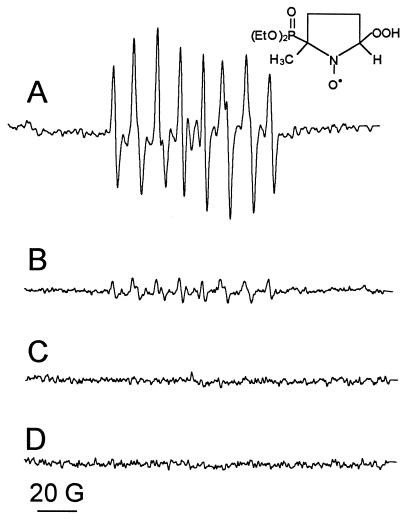
Fig. 1A shows the ESR spectrum, characteristic of DEPMPO-OOH, generated upon activating eNOS electron transfer reactions by calcium/calmodulin binding in the presence of NADPH and DEPMPO. The spectral intensity of DEPMPO-OOH detected in incubations of “resting” enzyme, i.e., in the absence of calcium/calmodulin, was only 15% of that detected with activated eNOS (Fig. 1B). Furthermore, DEPMPO-OOH formation was SOD-inhibitable and entirely dependent on the presence of the enzyme (Fig. 1 C and D). The identity of the spin adduct was confirmed by spectral simulation (Fig. 1A). Incubation of eNOS (7.0 pmol) with 0.2 mM calcium, 20 μg/ml calmodulin, 0.1 mM NADPH, and 50 mM DEPMPO in 50 mM Hepes buffer (pH 7.4) containing 0.1 mM DTPA generated 6.7 ± 0.3 μM DEPMPO-OOH and consumed 147 ± 6 nmol of NADPH per min per mg of protein (Table 1, experiment A), at room temperature. eNOS incubated at 37°C in the presence of calcium/calmodulin consumed 380 nmol NADPH per min per mg of protein. Unfortunately, spin-trapping experiments using a loop–gap resonator are technically difficult to perform at 37°C. Therefore, all our studies were performed at room temperature. In the absence of calcium/calmodulin, the yield of DEPMPO-OOH was reduced to 1.5 ± 0.5 μM (Fig. 1B) and 50 ± 5.3 nmol of NADPH per min per mg of protein were consumed (Table 1, experiment A). This indicates that calcium/calmodulin enhanced both superoxide formation and NADPH oxidation by a factor of 3–5.
Figure 1.
Calcium/calmodulin-dependent superoxide generation from eNOS. eNOS (7 pmol) was incubated with (A), 0.2 mM calcium, 20 μg/ml calmodulin, 0.1 mM NADPH, 50 mM DEPMPO, and 0.1 mM DTPA in 50 mM Hepes (pH 7.4). (B) As A, in the absence of Ca2+/calmodulin. (C) As A, in the presence of 10 μg/ml SOD. (D) As A, but without enzyme. Incubations were made at room temperature and the spectra were acquired by using a loop–gap resonator. Instrumental conditions: microwave power 2 mW, modulation amplitude 1 G, time constant 0.128 s, scan rate 1.6 G/s, gain 1.25 × 105, number of scans, 10. ESR data can be fitted by considering two isomers of DEPMPO-OOH (hyperfine coupling constants are given in Gauss). Isomer 1 (63% contribution), aN = 13.0, aP = 50.6, aH = 11.7; isomer 2 (37% contribution), aN = 13.1, aP = 48.4, aH = 9.9; and DEPMPO-OH (13% contribution), aN = 13.9, aP = 46.7, aH = 13.5.
Table 1.
Effect of cofactors, l-arginine, and analogs of l-arginine on NADPH oxidation, l-citrulline, and DEPMPO-OOH formation by eNOS
| Experiment | Enzyme | Addition | NADPH oxidation, nmol⋅min−1 per mg protein | [14C]Citrulline, nmol⋅min−1 per mg protein | DEPMPO-OOH, μM | |
|---|---|---|---|---|---|---|
| A | eNOS | —* | 50.0 ± 5.3 | —† | 1.5 ± 1.5 | |
| +Ca2+/CaM | + | None | 147.0 ± 6.0 | —† | 6.7 ± 0.3 | |
| +Ca2+/CaM | + | 1 mM l-Arg | ND | —† | 6.5 | |
| +Ca2+/CaM | + | 10 μM BH4 | 139.5 | —† | 4.2 | |
| +Ca2+/CaM | + | l-Arg + BH4¶ | 288.7 | 133.3 | 0.0 | |
| +Ca2+/CaM | + | 0.1 mM l-NAME | 107.1 ± 3.4 | —† | 5.2 | |
| +Ca2+/CaM | + | 1 mM CN− | 49.0 | —† | <1.0 | |
| B | eNOS/+ BH4‡ | —* | 47.0 | —† | <1.0 | |
| +Ca2+/CaM | + | None | 122.2 | —† | 2.5 | |
| C | eNOS | —* | 50.0 ± 5.3 | —† | 1.5 ± 1.5 | |
| +Flavins§ | + | None | ND | —† | 7.2 | |
| +Flavins§ | + | Ca2+/CaM | 334.5 ± 34 | —† | 13.6 | |
| +Flavins§ | + | Ca2+/CaM l-Arg + BH4¶ | 564.3 ± 44.3 | 138.2 ± 9.1 | 1.9 | |
| +Flavins§ | + | 0.1 mM DPI | 1.0 | —† | 0.0 |
Values represent mean ± SD (n = 3), where appropriate. ND, not determined; DPI, diphenyleneiodonium chloride.
Enzyme was incubated with NADPH in 50 mM Hepes buffer (pH 7.4) containing 0.1 mM DTPA.
Assay does not contain either substrate or cofactor necessary for activity.
Enzyme isolated in the presence of BH4.
FAD (1 μM) and FMN (1 μM).
l-Arg (0.1 mM) and BH4 (10 μM).
The Role of the Oxygenase Domain.
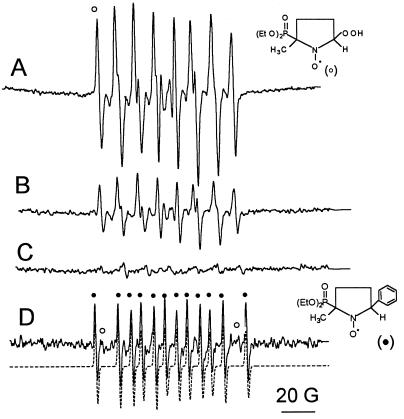
The enhancement of both NADPH consumption and superoxide formation upon binding of calcium/calmodulin to NOS is likely to be due to increased reduction of the heme iron and, hence, enhanced dissociation of the ferrous–dioxygen complex (14, 19, 20). To corroborate a role for the heme group in superoxide generation, the effect of cytochrome P450 inhibitors and eNOS substrates on the activated enzyme was analyzed. Imidazole (0.5 mM), a well-known type II heme-iron ligand that binds to the heme of NOS, inhibited superoxide generation by about 60% (Fig. 2B). Cyanide (1 mM) inhibited superoxide generation by 87% (Fig. 2C, Table 1, experiment A). In addition, cyanide inhibited l-citrulline formation by 95% (i.e., from 138.2 to 5.9 nmol of citrulline per min per mg of protein) and NADPH consumption by 68% (Table 1, experiment A). Higher concentrations of cyanide (10 mM) decreased superoxide generation to levels below those observed in the absence of calcium/calmodulin (data not shown). Therefore, it is likely that cyanide interferes with both heme-dependent and flavin-dependent superoxide formation. Phenyldiazene, an inhibitor of NOS (36), also inhibited superoxide generation by eNOS (Fig. 2D). Concomitantly, a DEPMPO-phenyl radical adduct, formed by trapping of phenyl radical by DEPMPO, was detected (Fig. 2D). This is consistent with the observation that phenyldiazene inhibits ⋅NO synthesis by the formation of an aryl–heme iron complex (36). It is likely that the formation of the phenyl–heme complex inhibits the generation of superoxide.
Figure 2.
Inhibition of eNOS-dependent superoxide generation by cytochrome P450 type inhibitors. eNOS (7 pmol) was incubated with 0.1 mM NADPH/50 mM DEPMPO in 50 mM Hepes (pH 7.4) containing 0.1 mM DTPA and (A) 0.2 mM calcium and 20 μg/ml calmodulin. (B) As A, in the presence of 0.5 mM imidazole. (C) As A, in the presence of 1 mM cyanide. (D) As A, after 15 min incubation with 0.1 mM phenyldiazene. ○, DEPMPO-OOH; •, DEPMPO-phenyl radical and was simulated (broken line in D) by using the hyperfine coupling constants (in Gauss). aN = 14.8, aP = 44.9, aH = 23.3. Instrumental conditions: microwave power 2 mW, modulation amplitude 1 G, time constant 0.128 s, scan rate 1.6 G/s, gain 1.25 × 105, number of scans, 10.
These results indicate that superoxide generation is inhibited by heme iron ligands such as phenyl radical generators, imidazole, and cyanide, supporting further the hypothesis that superoxide is generated from the dissociation of the heme ferrous–dioxygen complex in the oxygenase domain of eNOS.
l-Arginine (1 mM, Table 1, experiment A) or 0.1 mM l-NG-hydroxy-l-arginine monoacetate (data not shown) did not inhibit the generation of superoxide by eNOS. This indicates that l-arginine alone does not control superoxide generation by eNOS. This is in contrast to the inhibitory effect of the addition of substrates to cytochrome P450-dependent superoxide production (37).
The effect of l-arginine analogs was also investigated. Superoxide generation was not inhibited by the addition of l-NMA or l-thiocitrulline (data not shown). The effect of l-NAME on superoxide generation was variable. Maximal inhibition of approximately 30%, in both superoxide formation and NADPH consumption, was observed by the addition of 1 mM l-NAME to activated eNOS (Table 1, experiment A). Competition binding experiments of l-arginine and analogs to eNOS have demonstrated that the guanidino group of l-arginine is oriented toward the sixth heme-ligand site (38). Q-Band pulsed electron nuclear double resonance spectroscopy of eNOS (39) demonstrated that the distance between the guanidino nitrogen of l-arginine and the heme group is 4 Å. These results indicate that the occupation of the l-arginine binding site, and any consequent conformational changes of the heme pocket, does not prevent the binding and reduction of oxygen by the heme group. Our data also suggest that l-arginine does not interfere with the release of superoxide from the heme iron.
The Role of BH4.
The role of BH4 in superoxide generation was assessed by using eNOS isolated in the presence of BH4 (eNOS/+BH4). On activation with calcium/calmodulin, one-third the amount of superoxide, but only a 17% decrease in NADPH consumption, was observed by using eNOS/+BH4 compared with eNOS isolated in the absence of BH4 (Table 1, experiment B). Addition of BH4 (10 μM) to pterin-free eNOS results in a 6% decrease in NADPH consumption and a 40% decrease in DEPMPO-OOH formation (Table 1, experiment B). These results show that BH4 decreases the detectable levels of superoxide without a corresponding decrease in the rate of NADPH oxidation.
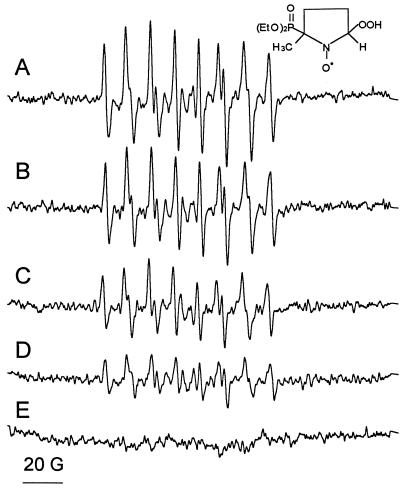
To understand how BH4 controls superoxide generation, eNOS was incubated with increasing amounts of BH4. Superoxide yields in the presence of 1, 10, and 100 μM BH4 were 5.2, 4.2, and 2.6 μM, respectively (Fig. 3). This concentration-dependence is intriguing as only 1 μM of BH4 was sufficient to activate eNOS to generate 113.4 nmol of citrulline per min per mg of protein, which corresponds to 80% of its maximal activity (see Table 1, experiment C). The concentration-dependent inhibition of superoxide generation, without a corresponding decrease in NADPH consumption (Table 1, experiment A), indicates that BH4 directly scavenges superoxide. The direct reaction between BH4 and superoxide was investigated further by adding BH4 to 0.4 mM xanthine/0.01 unit/ml xanthine oxidase. BH4 inhibited the yields of DEPMPO-OOH in a concentration dependent manner and had no effect on xanthine oxidase activity as monitored by the rate of urate formation (J.V.-V., N.H., K.A.P., Jr., and B.K., unpublished data).
Figure 3.
Effect of BH4 on the generation of superoxide by eNOS. eNOS (7 pmol) was incubated with (A) 0.2 mM calcium, 20 μg/ml calmodulin, 0.1 mM NADPH, 50 mM DEPMPO, and 0.1 mM DTPA in 50 mM Hepes (pH 7.4). (B) As A, in the presence of 1 μm BH4. (C) As A, in the presence of 10 μM BH4. (D) As A, in the presence of 100 μM BH4. (E) As C, in the presence of 0.1 mM l-arginine. Instrumental conditions: microwave power 2 mW, modulation amplitude 1 G, time constant 0.128 s, scan rate 1.6 G/s, gain 1.25 × 105, number of scans, 10.
In contrast to the control of l-arginine oxidation, BH4 does not appear to regulate NADPH oxidation by eNOS. This supports the view that BH4 acts to stabilize and increase l-arginine binding, thereby acting as an allosteric effector of NOS activity (40). It has been proposed that BH4 promotes eNOS dimerization (41). Although this is controversial (42), the modest effect of BH4 on NADPH consumption suggests that neither the binding nor the oxidation of NADPH by eNOS depend on NOS dimerization. The data also indicate that BH4 does not control NADPH binding.
The above data demonstrate that neither l-arginine nor BH4 alone are able to control eNOS NADPH oxidase activity. This result is not in agreement with a previous report that indicates l-arginine controls superoxide generation from purified nNOS (14). Moreover, it is likely that this preparation (14) contains significant amounts of bound BH4. Therefore, the role of l-arginine and BH4 in combination was investigated further. As shown in Fig. 3E, DEPMPO-OOH was not detected in incubations of eNOS with 10 μM BH4 and 100 μM l-arginine. However, under these conditions, enzyme turnover occurs and any superoxide that is formed will be rapidly scavenged by ⋅NO. Consequently, increased NADPH oxidation may be attributable to increases in both superoxide and l-citrulline formation. We have used the ratio of NADPH oxidation/l-citrulline formation to quantify the formation of superoxide under this condition.
In the presence of BH4, the ratio of NADPH oxidation (corrected for NADPH oxidation in the absence of calcium/calmodulin) to l-citrulline formation was 1.79. This compares favorably to a reported value of 1.73 (43) and is close to the value of 1.5 (44) that would be expected if superoxide is not being formed. Under these conditions no superoxide was detected by ESR spin trapping. The modest difference between the theoretical and observed values for the ratio of NADPH/l-citrulline suggest that, simultaneous with ⋅NO and l-citrulline generation, a low level of superoxide may be formed. The inability of DEPMPO to detect superoxide under these conditions is likely to be a result of the rapid scavenging of superoxide by ⋅NO.
The Role of the Reductase Domain.
According to the proposed model for electron transfer between the reductase and oxygenase domains of NOS, NADPH-derived electrons are transferred to flavins (FAD/FMN) that, upon calcium/calmodulin binding, transfer electrons to the heme iron enabling it to catalyze the oxygen-dependent oxidation of l-arginine (16, 18, 32). It has been demonstrated that this electron transfer sequence can be disrupted by electron acceptors such as cytochrome c, ferricyanide, lucigenin, and adriamycin (18, 26, 27, 31, 32). These compounds are reduced at the reductase domain of eNOS, increasing the rate of NADPH consumption and inhibiting l-citrulline formation (18, 32). In the case of redox-cycling compounds like lucigenin and adriamycin, an increase in superoxide generation is also observed (31, 32).
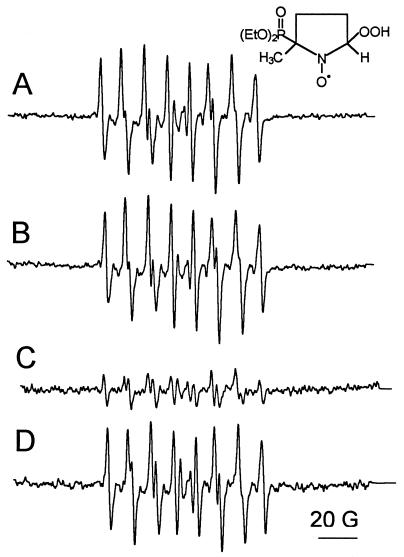
Although it has been demonstrated that exogenous flavins are not required for NOS activity (34, 45), most published assays include the addition of flavin cofactors (FAD and FMN) (21, 22, 40). We investigated whether these cofactors can act as electron acceptors for the reductase domain of eNOS and subsequently catalyze the reduction of oxygen by NADPH. Addition of 1 μM FAD and 1 μM FMN to incubations of eNOS in the presence of calcium/calmodulin generated two times more superoxide (Table 1, experiment C) than in the presence of either calcium/calmodulin (Table 1, experiment A) or flavins (Table 1, experiment C) alone. Consequently, the effects of flavins and calcium/calmodulin in eNOS-dependent superoxide production are independent and additive. Superoxide formation was abolished by the addition of diphenyleneiodonium chloride, a flavoprotein inhibitor (Table 1, experiment C). l-Arginine did not affect DEPMPO-OOH formation (Fig. 4B), but a dramatic decrease was observed upon coaddition of l-arginine and BH4. Interestingly, addition of l-NAME, which inhibits ⋅NO formation, reversed the inhibitory effect of BH4 (Fig. 4D). This suggests that the decrease in DEPMPO-OOH yield as a result of BH4 addition is due to the rapid scavenging of superoxide by ⋅NO to form peroxynitrite (10).
Figure 4.
Effects of l-arginine, BH4, and l-NAME on the generation of superoxide by eNOS. eNOS (7 pmol) was incubated with 0.2 mM calcium, 20 μg calmodulin, 0.1 mM NADPH, 50 mM DEPMPO, and 0.1 mM DTPA in 50 mM Hepes (pH 7.4) with (A) 1 μM FAD and 1 μM FMN. (B) As A, in the presence of 0.1 mM l-arginine. (C) As B, in the presence of 10 μM BH4. (D) As C, in the presence of 0.1 mM l-NAME. Instrumental conditions: microwave power 2 mW, modulation amplitude 1 G, time constant 0.128 s, scan rate 1.6 G/s, gain 1.25 × 105, number of scans, 10.
Flavins increased NADPH consumption by calcium/calmodulin-activated eNOS by 2.3-fold (Table 1). These data indicate that eNOS has a flavin reductase activity. After reduction, unbound flavins are reoxidized, by oxygen, producing superoxide (Fig. 4A) and regenerating oxidized flavins. This mechanism implies that flavins will inhibit eNOS activity. In agreement, the addition of 10 μM FAD and FMN, a concentration 10 times higher than that commonly used for enzyme activity assays, caused a reduction in enzyme activity from 138.2 to 106.0 nmol of l-citrulline per min per mg of protein. This inhibitory effect, however, was mostly because of FMN, which alone inhibited eNOS activity by approximately 18% to 113.6 nmol of l-citrulline per min per mg of protein. Interestingly, it has been known for some time that eNOS activity, quantified by monitoring ⋅NO generation, is increased by the addition of the superoxide-scavenging enzyme, SOD (46, 47). This stimulatory effect has been attributed to the scavenging of superoxide generated from the autoxidation of BH4 (47), although it is likely that this effect is also because of the scavenging of superoxide generated during the redox cycling of unbound flavins.
Activation of eNOS in the presence of flavins (1 μM FAD and 1 μM FMN), 0.1 mM l-arginine, and 10 μM BH4 produced 138.2 ± 9.1 nmol of l-citrulline per min per mg of protein (Table 1, experiment C) and consumed 564.3 ± 44.3 nmol of NADPH per min per mg of protein (Table 1, experiment C), giving a ratio of 3.7 NADPH/l-citrulline (after correction for background). Under these conditions 1.9 μM DEPMPO-OOH was detected (Fig. 4C). Therefore, in the presence of flavins, superoxide formation occurs at a faster rate than ⋅NO formation and hence all the ⋅NO generated by eNOS is likely to be converted to peroxynitrite.
Considerations for NOS Activity.
Addition of FAD/FMN to eNOS activity assays has been employed to maximize the rate of l-citrulline formation (21, 22, 31, 43). However, at higher concentrations, flavins can inhibit l-citrulline formation by competing with l-arginine for NADPH-derived electrons. The reduction of flavins by eNOS results in the formation of superoxide, which scavenges ⋅NO to generate peroxynitrite. Our data indicate that the concentrations of flavins commonly used in eNOS assays may be high enough to generate significant amounts of superoxide. The flavin reductase activity of eNOS emphasizes the need to consider the ability of eNOS to generate superoxide by reducing redox-cycling compounds at the reductase domain of the enzyme. As a result of this activity, these compounds may switch eNOS activity from a ⋅NO synthase to a peroxynitrite synthase.
Implications in Pathophysiological Conditions.
This study indicates that eNOS generates superoxide on activation with calcium/calmodulin in the presence of l-arginine. However, upon addition of BH4 to the above incubation mixture, superoxide formation is inhibited. This suggests that optimal BH4 is critical for calcium-dependent production of ⋅NO and l-citrulline in vivo. It is conceivable that BH4 may control eNOS activity at two levels. Decreases in BH4 binding affinity, or decreases in cellular BH4 concentration, may alter enzymatic activity in favor of superoxide generation. BH4 has been used to reverse vascular dysfunction induced by hypercholesterolemia (49) and diabetes (50), suggesting that BH4 levels are compromised in these situations. It has been demonstrated that blood vessels depleted of BH4 produce hydrogen peroxide (49). It therefore appears likely that inhibition of BH4 synthesis and/or BH4 binding will result in alterations in NOS activity and consequently in endothelial dysfunction. The regulation of BH4 synthesis and its ability to modulate ⋅NO/superoxide balance is undoubtedly an exciting area of future research.
Acknowledgments
This research was supported by National Institutes of Health Grants RR01008, GM27665, HL45058, HL47250, and HL 48251 from the National Heart, Lung and Blood Institute; by Grant GM55792 from the National Institute of General Medical Sciences to N.H.; by Grants GM52419 from the National Institute of General Medical Sciences and AQ-1192 from the Robert A. Welch Foundation Grant to B.S.S.M.; and by an American Heart Association Postdoctoral Fellowship to J.V.-V.
ABBREVIATIONS
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- DEPMPO
5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide
- DEPMPO-OOH
DEPMPO-superoxide radical adduct
- SOD
superoxide dismutase
- DTPA
diethylenetriaminepentaacetic acid
- eNOS
endothelial NOS
- l-NMA
NG-methyl-l-arginine
- l-NAME
NG-nitro-l-arginine methyl ester
References
- 1.Rees D D, Palmer R M J, Moncada S. Proc Natl Acad Sci USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moncada S, Holman A E, Vanhoutte P M. Trends Pharmacol Sci. 1987;8:365–368. [Google Scholar]
- 3.Furchgott R F. In: Vasodilation: Vascular Smooth Muscle, Peptides, Autonomic Nerves and Endothelium. Vanhoutte P M, editor. New York: Raven; 1988. pp. 401–404. [Google Scholar]
- 4.Ignarro L J, Buga G M, Wood K S, Byrns R E, Chaudhuri G. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cayatte A J, Palacino J J, Horten K, Cohen R A. Arterioscler Thromb. 1994;14:753–759. doi: 10.1161/01.atv.14.5.753. [DOI] [PubMed] [Google Scholar]
- 6.Creager M A, Gallagher J, Girerd X J, Coleman S M, Dzau V J, Cooke J P. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girerd X J, Hirsch A T, Cooke J P, Dzau V J, Creager M A. Circ Res. 1990;67:1301–1308. doi: 10.1161/01.res.67.6.1301. [DOI] [PubMed] [Google Scholar]
- 8.Bode-Boger S M, Boger R H, Kienke S, Junker W, Frolich J C. Biochem Biophys Res Commun. 1996;219:598–603. doi: 10.1006/bbrc.1996.0279. [DOI] [PubMed] [Google Scholar]
- 9.Cooke J P, Tsao P S. Circulation. 1997;95:311–312. doi: 10.1161/01.cir.95.2.311. [DOI] [PubMed] [Google Scholar]
- 10.Huie R E, Padmaja S. Free Radical Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 11.Vásquez-Vivar J M, Santos A M, Junqueira V B C, Augusto O. Biochem J. 1996;314:869–876. doi: 10.1042/bj3140869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg N, Joseph J, Kalyanaraman B. Arch Biochem Biophys. 1994;314:153–158. doi: 10.1006/abbi.1994.1423. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard K A, Jr, Groszek L, Smalley D M, Sessa W C, Wu M, Villalon P, Wolin M S, Stemmerman M B. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 14.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 15.Vásquez-Vivar J M, Martásek P, Masters B S S, Hogg N, Kalyanaraman B, Pritchard K A., Jr Circulation Suppl. 1997;96:I–233. [Google Scholar]
- 16.Griffith O W, Stuehr D J. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 17.Masters B S S. Annu Rev Nutr. 1994;14:131–145. doi: 10.1146/annurev.nu.14.070194.001023. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Soud H M, Stuehr D J. Proc Natl Acad Sci USA. 1993;90:10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Soud H M, Yoho L L, Stuehr D J. J Biol Chem. 1994;269:32047–32050. [PubMed] [Google Scholar]
- 20.Abu-Soud H M, Gachhui R, Raushel F M, Stuehr D J. J Biol Chem. 1997;272:17349–17353. doi: 10.1074/jbc.272.28.17349. [DOI] [PubMed] [Google Scholar]
- 21.Heinzel B, Klatt J M, Böhme E, Mayer B. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt H H H W, Hofmann H, Schindler U, Shutenko Z A, Cunningham D D, Feelisch M. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCord J M, Fridovich I. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 24.Chen P F, Tsai A L, Berka V, Wu K K. J Biol Chem. 1997;272:6114–6118. doi: 10.1074/jbc.272.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huhmer A F, Nishida C R, Ortiz de Montellano P R, Schoneich C. Chem Res Toxicol. 1997;10:618–626. doi: 10.1021/tx960188t. [DOI] [PubMed] [Google Scholar]
- 26.Sheta E A, McMillan K, Masters B S S. J Biol Chem. 1994;269:15147–15153. [PubMed] [Google Scholar]
- 27.Vásquez-Vivar, J., Martásek, P., Hogg, N., Karoui, H., Masters, B. S. S., Pritchard, K. A., Jr., & Kalyanaraman, B. (1998) Methods Enzymol., in press. [DOI] [PubMed]
- 28.Roubaud V, Sankarapandi S, Kuppusamy P, Tordo P, Zweier J L. Anal Biochem. 1997;247:404–411. doi: 10.1006/abio.1997.2067. [DOI] [PubMed] [Google Scholar]
- 29.Laursen J B, Harrison D G. Circulation. 1997;95:14–16. doi: 10.1161/01.cir.95.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Wever R M F, Lüscher T F, Cosentino F, Rabelink T J. Circulation. 1998;97:108–112. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- 31.Vásquez-Vivar J, Hogg N, Pritchard K A, Jr, Martásek P, Kalyanaraman B. FEBS Lett. 1997;403:127–130. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 32.Vásquez-Vivar J, Mártasek P, Hogg N, Masters B S S, Pritchard K A, Jr, Kalyanaraman B. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 33.Frejaville C, Karoui H, Tuccio B, Le Moigne F, Culcasi M, Pietri I, Lauricella R, Tordo P. J Med Chem. 1995;38:258–265. doi: 10.1021/jm00002a007. [DOI] [PubMed] [Google Scholar]
- 34.Martásek P, Liu Q, Liu J, Roman L J, Gross S, Sessa W C, Masters B S S. Biochem Biophys Res Commun. 1996;219:359–365. doi: 10.1006/bbrc.1996.0238. [DOI] [PubMed] [Google Scholar]
- 35.Duling D R. J Magn Reson B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 36.Gerber N C, Rodriguez-Crespo I, Nishida C R, Ortiz de Montellano P R. J Biol Chem. 1997;272:6285–6290. doi: 10.1074/jbc.272.10.6285. [DOI] [PubMed] [Google Scholar]
- 37.Guengerich F P. J Biol Chem. 1991;266:10019–10022. [PubMed] [Google Scholar]
- 38.Berka V, Chen P-F, Tsai A-L. J Biol Chem. 1996;271:33293–33300. doi: 10.1074/jbc.271.52.33293. [DOI] [PubMed] [Google Scholar]
- 39.Tierney D L, Martásek P, Doan P E, Masters B S S, Hoffman B M. J Am Chem Soc. 1998;120:2983–2984. [Google Scholar]
- 40.Gorren A C F, List B M, Schrammel A, Pitters E, Hemmens B, Werner E R, Schmidt K, Mayer B. Biochemistry. 1996;35:16735–16745. doi: 10.1021/bi961931j. [DOI] [PubMed] [Google Scholar]
- 41.Hellermann G R, Solomonson L P. J Biol Chem. 1997;272:12030–12034. doi: 10.1074/jbc.272.18.12030. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Crespo I, Gerber N C, Ortiz de Montellano P R. J Biol Chem. 1996;271:11462–11467. doi: 10.1074/jbc.271.19.11462. [DOI] [PubMed] [Google Scholar]
- 43.List B M, Klosch B, Volker C, Gorren A C F, Sessa W, Werner E R, Kukovetz W R, Schmidt K, Mayer B. Biochemistry. 1997;323:159–165. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Soud H M, Presta A, Mayer B, Stuehr D J. Biochemistry. 1997;36:10811–10816. doi: 10.1021/bi971414g. [DOI] [PubMed] [Google Scholar]
- 45.Miller R T, Martásek P, Roman L, Nishimura J S, Masters B S S. Biochemistry. 1997;36:15277–15284. doi: 10.1021/bi972022c. [DOI] [PubMed] [Google Scholar]
- 46.Hobbs A J, Fukuto J M, Ignarro L J. Proc Natl Acad Sci USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer B, Klatt P, Werner E R, Schmidt K. J Biol Chem. 1995;270:655–659. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- 48.Cosentino F, Katusic Z S. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- 49.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Lüscher T, Rabelink T. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieper G M. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]