Abstract
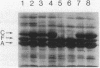
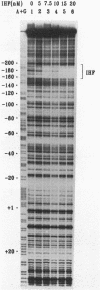
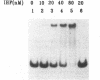
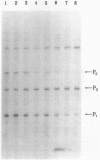
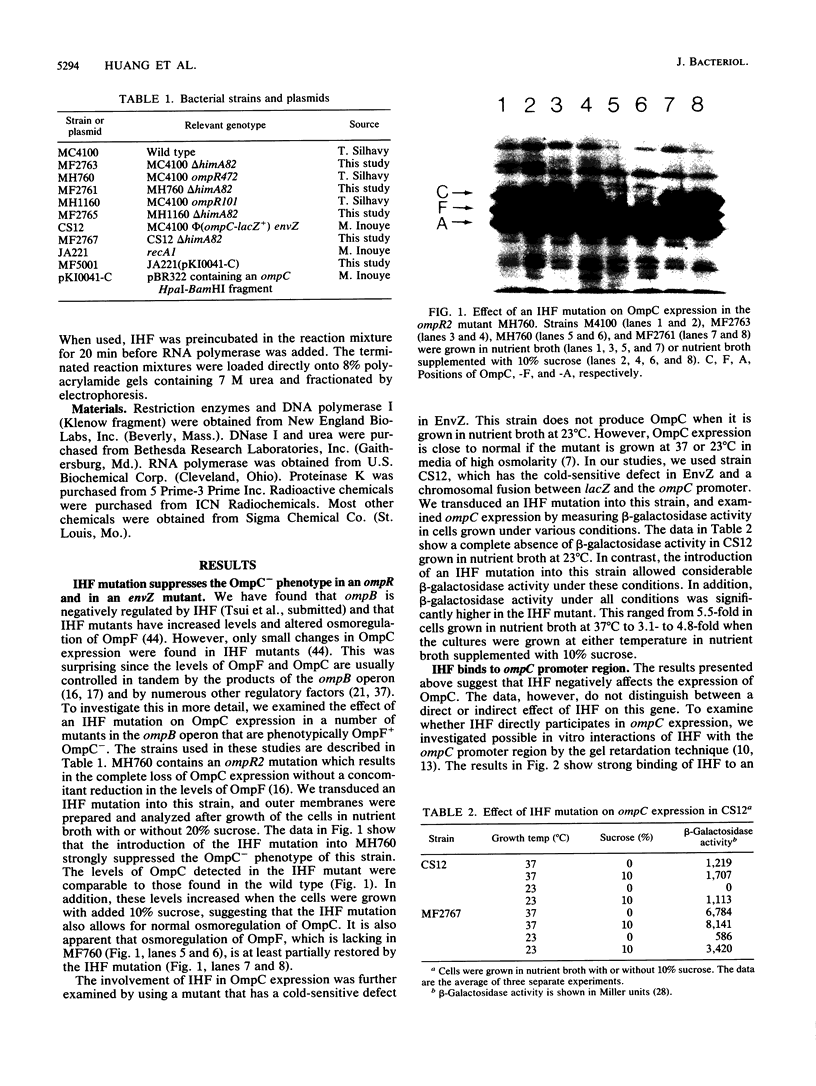
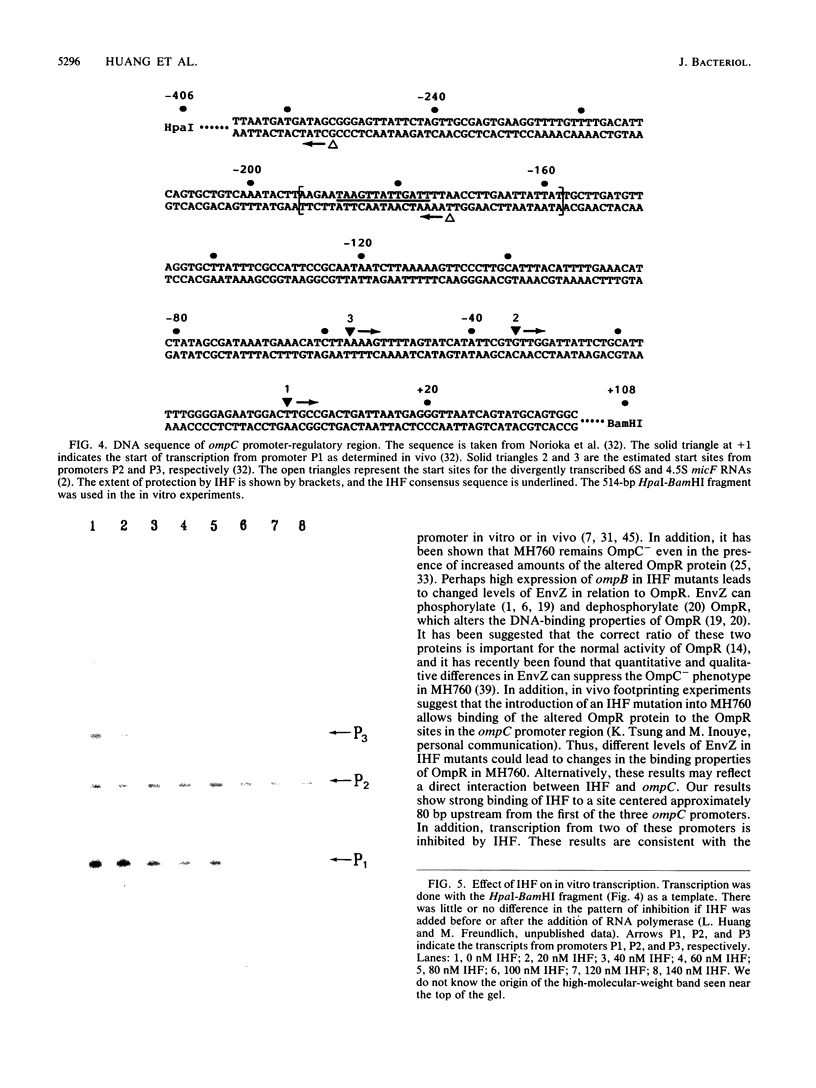
Integration host factor (IHF) of Escherichia coli is a DNA-binding protein involved in gene expression and other cellular functions in E. coli and some of its bacteriophages and plasmids. We report here that IHF is a direct negative effector of the ompC operon of E. coli. IHF binds to ompC DNA and protects a region of 35 base pairs located upstream from the ompC promoters. The addition of IHF to a purified in vitro transcription system inhibited transcription from two of the three ompC promoters. In vivo experiments suggest that the in vitro results are physiologically relevant. IHF mutants show increased expression of OmpC. In addition, the OmpC- phenotype of certain strains is completely suppressed by a mutation in IHF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mizuno T., Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989 May 25;264(15):8563–8567. [PubMed] [Google Scholar]
- Andersen J., Delihas N., Ikenaka K., Green P. J., Pines O., Ilercil O., Inouye M. The isolation and characterization of RNA coded by the micF gene in Escherichia coli. Nucleic Acids Res. 1987 Mar 11;15(5):2089–2101. doi: 10.1093/nar/15.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Higgins C. F. Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol. 1987 Aug;169(8):3840–3843. doi: 10.1128/jb.169.8.3840-3843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Inouye M. Environmentally regulated gene expression for membrane proteins in Escherichia coli. Annu Rev Cell Biol. 1988;4:21–42. doi: 10.1146/annurev.cb.04.110188.000321. [DOI] [PubMed] [Google Scholar]
- Friden P., Tsui P., Okamoto K., Freundlich M. Interaction of cyclic AMP receptor protein with the ilvB biosynthetic operon in E. coli. Nucleic Acids Res. 1984 Nov 12;12(21):8145–8160. doi: 10.1093/nar/12.21.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Voelkel K., Sternglanz R., Freundlich M. Reduced expression of the isoleucine and valine enzymes in integration host factor mutants of Escherichia coli. J Mol Biol. 1984 Feb 5;172(4):573–579. doi: 10.1016/s0022-2836(84)80024-8. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Ellis E. M., Graeme-Cook K. A., Higgins C. F. OmpR and EnvZ are pleiotropic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella typhimurium. Mol Gen Genet. 1987 Apr;207(1):120–129. doi: 10.1007/BF00331499. [DOI] [PubMed] [Google Scholar]
- Griffo G., Oppenheim A. B., Gottesman M. E. Repression of the lambda pcin promoter by integrative host factor. J Mol Biol. 1989 Sep 5;209(1):55–64. doi: 10.1016/0022-2836(89)90169-1. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Collier D. A., Kilpatrick M. W., Krause H. M. Supercoiling and integration host factor change the DNA conformation and alter the flow of convergent transcription in phage Mu. J Biol Chem. 1989 Feb 15;264(5):3035–3042. [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause H. M., Higgins N. P. Positive and negative regulation of the Mu operator by Mu repressor and Escherichia coli integration host factor. J Biol Chem. 1986 Mar 15;261(8):3744–3752. [PubMed] [Google Scholar]
- Kur J., Hasan N., Szybalski W. Physical and biological consequences of interactions between integration host factor (IHF) and coliphage lambda late p'R promoter and its mutants. Gene. 1989 Sep 1;81(1):1–15. doi: 10.1016/0378-1119(89)90331-4. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Liljeström P., Luokkamäki M., Palva E. T. Isolation and characterization of a substitution mutation in the ompR gene of Salmonella typhimurium LT2. J Bacteriol. 1987 Jan;169(1):438–441. doi: 10.1128/jb.169.1.438-441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Kirk M., Echols H. SOS induction and autoregulation of the himA gene for site-specific recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6754–6758. doi: 10.1073/pnas.78.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I. Multilevel regulation of bacteriophage lambda lysogeny by the E. coli himA gene. Cell. 1981 Jul;25(1):269–276. doi: 10.1016/0092-8674(81)90252-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kato M., Jo Y. L., Mizushima S. Interaction of OmpR, a positive regulator, with the osmoregulated ompC and ompF genes of Escherichia coli. Studies with wild-type and mutant OmpR proteins. J Biol Chem. 1988 Jan 15;263(2):1008–1012. [PubMed] [Google Scholar]
- Norioka S., Ramakrishnan G., Ikenaka K., Inouye M. Interaction of a transcriptional activator, OmpR, with reciprocally osmoregulated genes, ompF and ompC, of Escherichia coli. J Biol Chem. 1986 Dec 25;261(36):17113–17119. [PubMed] [Google Scholar]
- Pirhonen M., Saarilahti H. T., Kurkela S., Palva E. T. In vivo cloning ad characterization of mutations of the regulatory locus ompR of Escherichia coli K12. Mol Gen Genet. 1986 Jun;203(3):520–523. doi: 10.1007/BF00422079. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G., Ikenaka K., Inouye M. Uncoupling of osmoregulation of the Escherichia coli K-12 ompF gene from ompB-dependent transcription. J Bacteriol. 1985 Jul;163(1):82–87. doi: 10.1128/jb.163.1.82-87.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Stenzel T. T., Patel P., Bastia D. The integration host factor of Escherichia coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell. 1987 Jun 5;49(5):709–717. doi: 10.1016/0092-8674(87)90547-2. [DOI] [PubMed] [Google Scholar]
- Surette M. G., Lavoie B. D., Chaconas G. Action at a distance in Mu DNA transposition: an enhancer-like element is the site of action of supercoiling relief activity by integration host factor (IHF). EMBO J. 1989 Nov;8(11):3483–3489. doi: 10.1002/j.1460-2075.1989.tb08513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Mosig G. Integration host factor (IHF) represses a Chlamydomonas chloroplast promoter in E. coli. Nucleic Acids Res. 1988 Apr 25;16(8):3313–3326. doi: 10.1093/nar/16.8.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P., Freundlich M. Integration host factor binds specifically to sites in the ilvGMEDA operon in Escherichia coli. J Mol Biol. 1988 Oct 5;203(3):817–820. doi: 10.1016/0022-2836(88)90212-4. [DOI] [PubMed] [Google Scholar]
- Tsui P., Helu V., Freundlich M. Altered osmoregulation of ompF in integration host factor mutants of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4950–4953. doi: 10.1128/jb.170.10.4950-4953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Identification of the DNA-binding domain of the OmpR protein required for transcriptional activation of the ompF and ompC genes of Escherichia coli by in vivo DNA footprinting. J Biol Chem. 1989 Jun 15;264(17):10104–10109. [PubMed] [Google Scholar]