Abstract
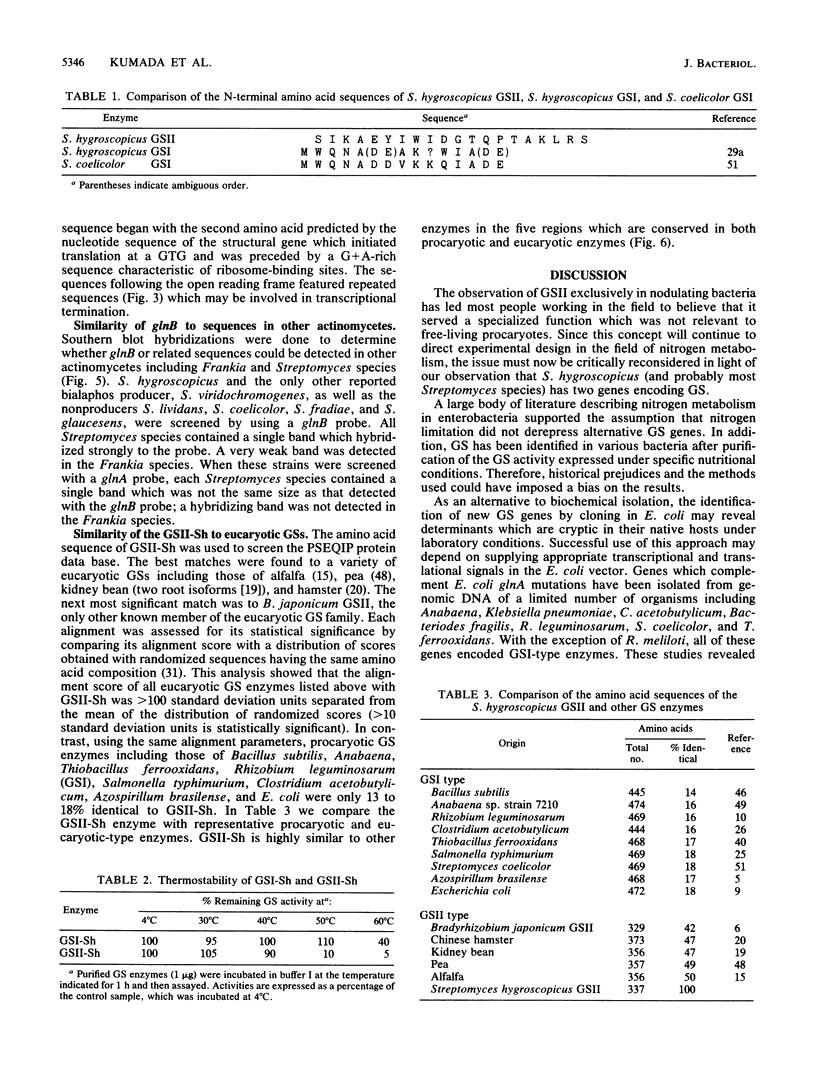
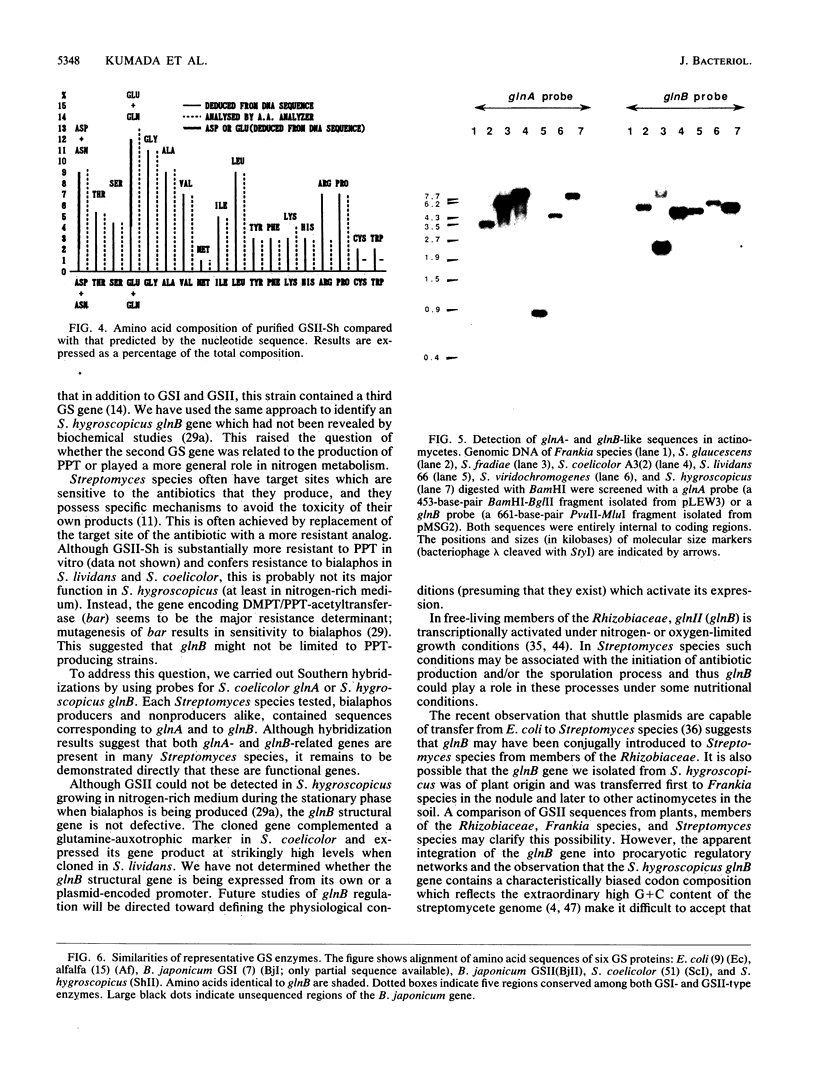
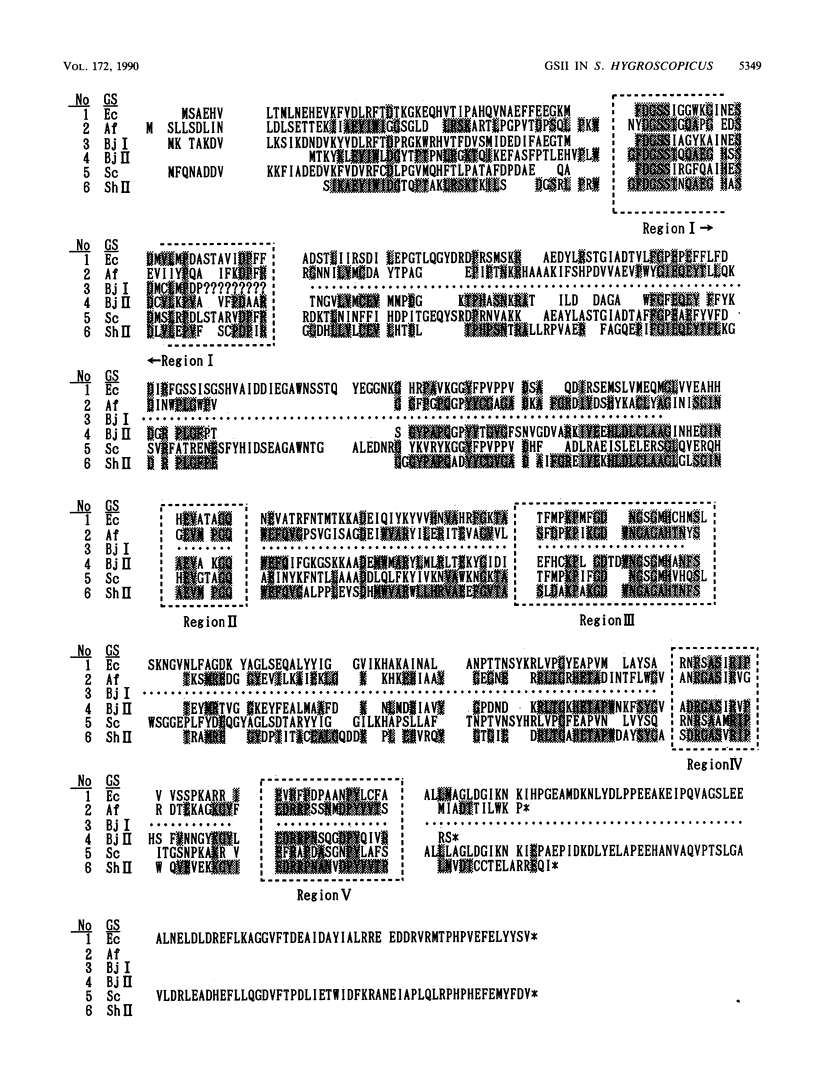
Streptomyces hygroscopicus, which produces the glutamine synthetase inhibitor phosphinothricin, possesses at least two genes (glnA and glnB) encoding distinct glutamine synthetase isoforms (GSI and GSII). The glnB gene was cloned from S. hygroscopicus DNA by complementation in an Escherichia coli glutamine auxotrophic mutant (glnA). glnB was subcloned in Streptomyces plasmids by insertion into pIJ486 (pMSG3) and pIJ702 (pMSG5). Both constructions conferred resistance to the tripeptide form of phosphinothricin (bialaphos) and were able to complement a glutamine auxotrophic marker in S. coelicolor. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of S. lividans(pMSG5) revealed a highly overexpressed 40-kilodalton protein. When GS was purified from this strain, it was indistinguishable in apparent molecular mass from the 40-kilodalton protein. The nucleic acid sequence of the cloned region contained an open reading frame which encoded a protein whose size, amino acid composition, and N-terminal sequence corresponded to those of the purified GS. glnB had a high G + C content and codon usage typical of streptomycete genes. A comparison of its predicted amino acid sequence with the protein data bases revealed that it encoded a GSII-type enzyme which had previously been found only in various eucaryotes (47 to 50% identity) and nodulating bacteria such as Bradyrhizobium spp. (42% identity). glnB had only 13 to 18% identity with eubacterial GSI enzymes. Southern blot hybridization experiments showed that sequences similar to glnB were present in all of the five other Streptomyces species tested, as well as Frankia species. These results do not support the previous suggestion that GSII-type enzymes found in members of the family Rhizobiaceae represent a unique example of interkingdom gene transfer associated with symbiosis in the nodule. Instead they imply that the presence of more than one gene encoding GS may be more common among soil microorganisms than previously appreciated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E., Gugel K. H., Hägele K., Hagenmaier H., Jessipow S., König W. A., Zähner H. Stoffwechselprodukte von Mikroorganismen. 98. Phosphinothricin und Phosphinothricyl-Alanyl-Alanin. Helv Chim Acta. 1972 Jan 31;55(1):224–239. doi: 10.1002/hlca.19720550126. [DOI] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bozouklian H., Elmerich C. Nucleotide sequence of the Azospirillum brasilense Sp7 glutamine synthetase structural gene. Biochimie. 1986 Oct-Nov;68(10-11):1181–1187. doi: 10.1016/s0300-9084(86)80062-1. [DOI] [PubMed] [Google Scholar]
- Carlson T. A., Guerinot M. L., Chelm B. K. Characterization of the gene encoding glutamine synthetase I (glnA) from Bradyrhizobium japonicum. J Bacteriol. 1985 May;162(2):698–703. doi: 10.1128/jb.162.2.698-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Martin G. B., Chelm B. K. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Dec;169(12):5861–5866. doi: 10.1128/jb.169.12.5861-5866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G., Villafranca J. J. Amino acid sequence of Escherichia coli glutamine synthetase deduced from the DNA nucleotide sequence. J Biol Chem. 1986 Aug 15;261(23):10587–10591. [PubMed] [Google Scholar]
- Colonna-Romano S., Riccio A., Guida M., Defez R., Lamberti A., Iaccarino M., Arnold W., Priefer U., Pühler A. Tight linkage of glnA and a putative regulatory gene in Rhizobium leguminosarum. Nucleic Acids Res. 1987 Mar 11;15(5):1951–1964. doi: 10.1093/nar/15.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Donn G., Tischer E., Smith J. A., Goodman H. M. Herbicide-resistant alfalfa cells: an example of gene amplification in plants. J Mol Appl Genet. 1984;2(6):621–635. [PubMed] [Google Scholar]
- Edmands J., Noridge N. A., Benson D. R. The actinorhizal root-nodule symbiont Frankia sp. strain CpI1 has two glutamine synthetases. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6126–6130. doi: 10.1073/pnas.84.17.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Identification of two glutamine synthetases in Agrobacterium. J Bacteriol. 1980 Feb;141(2):996–998. doi: 10.1128/jb.141.2.996-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hill R. T., Parker J. R., Goodman H. J., Jones D. T., Woods D. R. Molecular analysis of a novel glutamine synthetase of the anaerobe Bacteroides fragilis. J Gen Microbiol. 1989 Dec;135(12):3271–3279. doi: 10.1099/00221287-135-12-3271. [DOI] [PubMed] [Google Scholar]
- Imai S., Seto H., Sasaki T., Tsuruoka T., Ogawa H., Satoh A., Inouye S., Niida T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 6. Production of N-acetyl-demethylphosphinothricin and N-acetylbialaphos by blocked mutants of Streptomyces hygroscopicus SF-1293 and their roles in the biosynthesis of bialaphos. J Antibiot (Tokyo) 1985 May;38(5):687–690. doi: 10.7164/antibiotics.38.687. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Kayne P. S., Almassy R. J., Grunstein M., Eisenberg D. Sequence of glutamine synthetase from Salmonella typhimurium and implications for the protein structure. Gene. 1986;46(2-3):297–300. doi: 10.1016/0378-1119(86)90415-4. [DOI] [PubMed] [Google Scholar]
- Janssen P. J., Jones W. A., Jones D. T., Woods D. R. Molecular analysis and regulation of the glnA gene of the gram-positive anaerobe Clostridium acetobutylicum. J Bacteriol. 1988 Jan;170(1):400–408. doi: 10.1128/jb.170.1.400-408.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kumada Y., Anzai H., Takano E., Murakami T., Hara O., Itoh R., Imai S., Satoh A., Nagaoka K. The bialaphos resistance gene (bar) plays a role in both self-defense and bialaphos biosynthesis in Streptomyces hygroscopicus. J Antibiot (Tokyo) 1988 Dec;41(12):1838–1845. doi: 10.7164/antibiotics.41.1838. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. B., Chapman K. A., Chelm B. K. Role of the Bradyrhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J Bacteriol. 1988 Dec;170(12):5452–5459. doi: 10.1128/jb.170.12.5452-5459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Petter R., Thompson C. Intergeneric conjugation between Escherichia coli and Streptomyces species. J Bacteriol. 1989 Jun;171(6):3583–3585. doi: 10.1128/jb.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Nojiri C., Toyama H., Hayashi E., Yamada Y., Nagaoka K. Pock forming plasmids from antibiotic-producing Streptomyces. J Antibiot (Tokyo) 1983 Apr;36(4):429–434. doi: 10.7164/antibiotics.36.429. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Imai S., Satoh A., Kojima M. An improved method for the preparation of Streptomycetes and Micromonospora protoplasts. J Antibiot (Tokyo) 1983 Feb;36(2):184–186. doi: 10.7164/antibiotics.36.184. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Darrow R. A., Keister D. L. Effect of oxygen tension on nitrogenase and on glutamine synthetases I and II in Rhizobium jaonicum 61A76. Biochem Biophys Res Commun. 1978 Mar 15;81(1):224–231. doi: 10.1016/0006-291x(78)91653-4. [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Jones W. A., O'Neill E. G., Woods D. R. Nucleotide sequence of the glutamine synthetase gene and its controlling region from the acidophilic autotroph Thiobacillus ferrooxidans. Gene. 1987;53(2-3):211–217. doi: 10.1016/0378-1119(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Shatters R. G., Somerville J. E., Kahn M. L. Regulation of glutamine synthetase II activity in Rhizobium meliloti 104A14. J Bacteriol. 1989 Sep;171(9):5087–5094. doi: 10.1128/jb.171.9.5087-5094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M. A., Aronson A. I., Brown S. W., Schreier H. J., Sonenhein A. L. Sequence of the Bacillus subtilis glutamine synthetase gene region. Gene. 1988 Nov 30;71(2):257–265. doi: 10.1016/0378-1119(88)90042-x. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Wray L. V., Jr, Fisher S. H. Cloning and nucleotide sequence of the Streptomyces coelicolor gene encoding glutamine synthetase. Gene. 1988 Nov 30;71(2):247–256. doi: 10.1016/0378-1119(88)90041-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Rossbach S., Schneider M., Ratet P., Messmer S., Szeto W. W., Ausubel F. M., Schell J. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J Bacteriol. 1989 Mar;171(3):1673–1682. doi: 10.1128/jb.171.3.1673-1682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]