Abstract
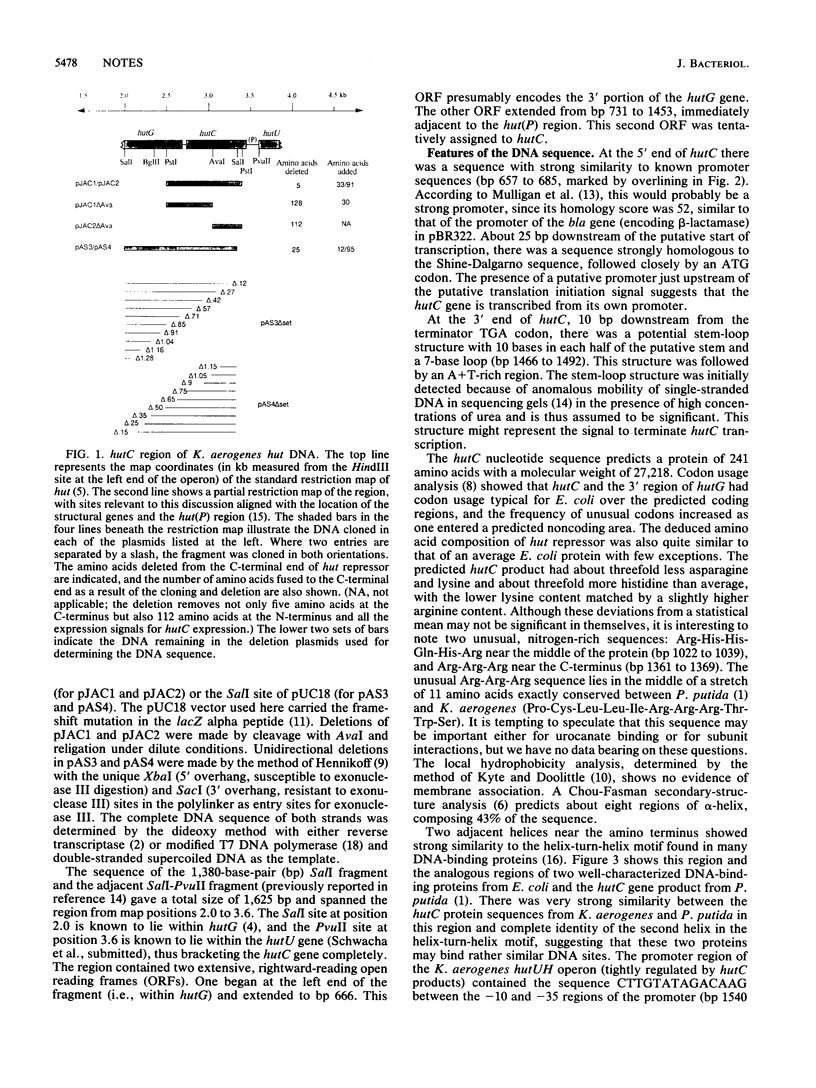
The hutC gene of Klebsiella aerogenes encodes a repressor that regulates expression of the histidine utilization (hut) operons. The DNA sequence of a region known to contain hutC was determined and shown to contain two long rightward-reading open reading frames (ORFs). One of these ORFs was identified as the 3' portion of the hutG gene. The other ORF was the hutC gene. The repressor predicted from the hutC sequence contained a helix-turn-helix motif strongly similar to that seen in other DNA-binding proteins, such as lac repressor and the catabolite gene activator protein. This motif was located in the N-terminal portion of the protein, and this portion of the protein seemed to be sufficient to allow repression of the hutUH operon but insufficient to allow interaction with the inducer. The presence of a promoterlike sequence and a ribosome-binding site immediately upstream of the hutC gene explained the earlier observation that hutC can be transcribed independently of the other hut operon genes. The predicted amino acid sequence of hut repressor strongly resembled that of the corresponding protein from Pseudomonas putida (S. L. Allison and A. T. Phillips, J. Bacteriol. 172:5470-5476, 1990). An unexpected, leftward-reading ORF extending from about the middle of hutC into the preceding (hutG) gene was also detected. The deduced amino acid sequence of this leftward ORF was quite distinct from that of an unexpected ORF of similar size found immediately downstream of the P. putida hutC gene. The nonstandard codon usage of this leftward ORF and the expression of repressor activity from plasmids with deletions in this region made it unlikely that this ORF was necessary for repressor activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison S. L., Phillips A. T. Nucleotide sequence of the gene encoding the repressor for the histidine utilization genes of Pseudomonas putida. J Bacteriol. 1990 Sep;172(9):5470–5476. doi: 10.1128/jb.172.9.5470-5476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenberg M., Magasanik B. A study in evolution: the histidine utilization genes of enteric bacteria. J Mol Biol. 1979 Nov 25;135(1):23–37. doi: 10.1016/0022-2836(79)90338-3. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Bender R. A. Genetic and physical maps of Klebsiella aerogenes genes for histidine utilization (hut). Mol Gen Genet. 1984;193(1):99–103. doi: 10.1007/BF00327421. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Eades L. J., Janssen K. A., Lomax M. I., Bender R. A. A restriction enzyme cleavage map of the histidine utilization (hut) genes of Klebsiella aerogenes and deletions lacking regions of hut DNA. Mol Gen Genet. 1984;193(1):92–98. doi: 10.1007/BF00327420. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Structural and functional role of leucine residues in proteins. J Mol Biol. 1973 Mar 5;74(3):263–281. doi: 10.1016/0022-2836(73)90372-0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Magasanik B. Gene order of the histidine utilization (hut) operons in Klebsiella aerogenes. J Bacteriol. 1975 Jun;122(3):1025–1031. doi: 10.1128/jb.122.3.1025-1031.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lobet Y., Peacock M. G., Cieplak W., Jr Frame-shift mutation in the lacZ gene of certain commercially available pUC18 plasmids. Nucleic Acids Res. 1989 Jun 26;17(12):4897–4897. doi: 10.1093/nar/17.12.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop A. J., Baldauf S. A., Hudspeth M. E., Bender R. A. Bidirectional promoter in the hut(P) region of the histidine utilization (hut) operons from Klebsiella aerogenes. J Bacteriol. 1988 May;170(5):2240–2246. doi: 10.1128/jb.170.5.2240-2246.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop A. J., Boylan S. A., Bender R. A. Regulation of hutUH operon expression by the catabolite gene activator protein-cyclic AMP complex in Klebsiella aerogenes. J Bacteriol. 1984 Sep;159(3):934–939. doi: 10.1128/jb.159.3.934-939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the pyruvate dehydrogenase component. Eur J Biochem. 1983 Jun 1;133(1):155–162. doi: 10.1111/j.1432-1033.1983.tb07441.x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]



