Abstract
Cocaine and other psychostimulants act by blocking the dopamine transporter. Binding of the cocaine analog, [3H]2-β-carbomethoxy-3-β-(4-fluorophenyl) tropane (CFT) to the dopamine transporter is sensitive to polar sulfhydryl-specific derivatives of methanethiosulfonate (MTS). These reagents preferentially react with water-accessible, reduced cysteines. The human dopamine transporter has 13 cysteines. Their topology is not completely determined. We sought to identify those cysteine residues the modification of which affects CFT binding and to determine the topology of these reactive cysteines. We mutated each of the cysteines, one at a time and in various combinations, to residues that preserved binding and transport, and we tested the sensitivity of each of the mutant transporters to the reagents. One construct, X5C, had five mutated cysteines (C90A, C135A, C306A, C319F, and C342A). Using a membrane preparation in which both extracellular and intracellular cysteines could be accessible, we found that CFT binding in X5C, as compared with wild-type transporter, was two orders of magnitude less sensitive to MTS ethylammonium (MTSEA). The wild-type cysteines were substituted back into X5C, one at a time, and these constructs were tested in cells and in membranes. Cys-90 and Cys-306 appear to be extracellular, and Cys-135 and Cys-342 appear to be intracellular. Each of these residues is predicted to be in extramembranous loops. The binding of cocaine increases the rate of reaction of MTSEA and MTS ethyltrimethylammonium with the extracellular Cys-90 and therefore acts by inducing a conformational change. Cocaine decreases the rate of reaction of MTSEA with Cys-135 and Cys-342, acting either directly or indirectly on these intracellular residues.
The concentration of dopamine in and around the synapse is reduced by reuptake. The dopamine transporter (DAT), the protein which carries out this reuptake at the plasma membrane, has been cloned (1–5). DAT, like other related transporters (6–8), requires extracellular Na+ and Cl− and couples substrate translocation to the movement of these ions (9).
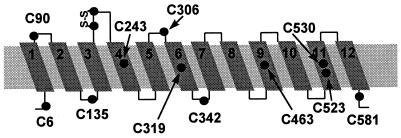
The human DAT cDNA encodes a primary sequence of 620 amino acids. Hydrophobicity analysis of the sequence predicted the presence of 12 membrane-spanning segments, which were presumed to be α-helices (3, 4). In this predicted topology, the N and C termini were both cytoplasmic (Fig. 1), several consensus N-glycosylation sites were extracellular, and several consensus phosphorylation sites were intracellular. Studies with antibodies against defined epitopes also were consistent with this proposed topology (10, 11). Different topologies for the amino-terminal third of the homologous γ-aminobutyric acid (GABA) and glycine transporters, however, also were proposed (12, 13).
Figure 1.
Predicted topology of DAT showing 12 putative membrane-spanning segments. The predicted positions of the 13 endogenous cysteines are shown. The cysteines in the second extracellular loop between M3 and M4 are thought to be disulfide bonded in the dopamine transporter and the serotonin transporter (see text).
The dopamine transporter is the major molecular target responsible for the rewarding properties and abuse potential of several psychostimulants, including cocaine, amphetamine, and methamphetamine (14, 15). Despite significant effort, the structural bases of the binding and transport of dopamine remain poorly understood. In particular, the molecular relationships between the cocaine-binding site, the binding sites of other inhibitors of dopamine transport, and the dopamine-binding site remain controversial. Mutation of Asp-79 in the first putative membrane-spanning segment dramatically reduced both dopamine transport and the binding affinity of the cocaine analogue, [3H]2-β-carbomethoxy-3-β-(4-fluorophenyl) tropane (CFT) (16). In contrast, mutation to glycine of two serines in the seventh putative membrane-spanning segment inhibited transport whereas [3H]CFT binding was affected less (16). Mutations in the seventh putative membrane-spanning segment increased 1-methyl-4-phenylpyridinium (MPP+) transport substantially more than dopamine transport. These mutations also had little impact on [3H]CFT binding (17).
Cysteine substitution and covalent modification has been used to study structure–function relationships and the dynamics of protein function in a variety of membrane proteins (18–27). Sulfhydryl reagents including N-ethylmaleimide (NEM) and mercurial compounds inhibited dopamine transport and the binding of radiolabeled inhibitors (28–36). In addition, various metal ions also have been found to affect dopamine uptake and inhibitor binding, and some of these effects also may be mediated by interaction of the metal ions with sulfhydryls in the transporter (31, 33). Cocaine and dopamine both protected against reaction with NEM (28, 29, 31, 36, 37).
These findings suggest the presence of at least one sulfhydryl the modification of which alters the structure and function of DAT. There are 13 cysteines in human DAT (Fig. 1). Two of the cysteines in the putative second extracellular loop are conserved completely within related transporters; in DAT, mutation of either of these cysteines to alanine abolished transport as well as the binding of [3H]CFT (38). Furthermore, very little transporter was expressed on the cell surface when either of these cysteines was mutated. They are likely to be disulfide-bonded. In the homologous serotonin transporter (SERT), the aligned cysteines also are likely disulfide-bonded (39). Disulfide-bonded cysteines are unavailable for reaction with NEM, mercurial compounds, or methanethiosulfonate (MTS) derivatives.
Based on the originally proposed topology, five cysteines are in putative membrane-spanning segments (Cys-243, Cys-319, Cys-463, Cys-523, and Cys-530). Four cysteines are in putative extracellular loops (Cys-180 and Cys-189, which are likely to be disulfide-bonded, and Cys-90 and Cys-306) and four cysteines (Cys-6, Cys-135, Cys-342, and Cys-581) are predicted to be intracellular.
Here, we report the identification of the cysteine residues, the modification of which affects CFT binding, and the determination of the topology of these reactive cysteines. Cys-90 and Cys-306 appear to be extracellular, and Cys-135 and Cys-342 appear to be intracellular. Cocaine alters the rate of reaction of the MTS reagents with several of these cysteines, acting either directly or indirectly on these residues in putative extracellular and intracellular loops. Furthermore, the pattern of reactivity of the endogenous cysteines with impermeant sulfhydryl reagents is consistent with the originally proposed topology (Fig. 1).
MATERIALS AND METHODS
Site-Directed Mutagenesis.
Cysteine mutations were generated by the Altered Sites Mutagenesis System (Promega) or by the Chameleon Mutagenesis Kit (Stratagene). Mutations were confirmed by DNA sequencing.
Stable Transfection of DAT.
HEK 293 cells in DMEM/F12 (1:1) with 10% bovine calf serum (HyClone) were maintained at 37°C and 5% CO2. For stable transfection, 35-mm dishes of HEK 293 cells at 70–80% confluence were transfected with 2 μg of wild-type (wt) or mutant DAT cDNA in the bicistronic expression vector pcin4 (a gift from S. Rees, Glaxo) (40) by using 9 μl of lipofectamine (GIBCO) and 1 ml of OPTIMEM (GIBCO). Five hours after transfection, the solution was removed and fresh media added. Twenty-four hours after transfection the cells were split to a 100-mm dish and 700 μg/ml geneticin was added to select for a stably transfected pool of cells.
Harvesting Cells and Membrane Preparation.
Stably transfected HEK 293 cells were washed with PBS (154 mM NaCl/11 mM Na2HPO4/2.7 mM KCl/1 mM MgCl2/0.1 mM CaCl2, pH 7.4) and dissociated in PBS. Cells were pelleted at 1,000 × g for 5 min at 4°C, and resuspended in PBS for binding or treatment with MTS reagents. For membrane preparation, the pellets were resuspended in PBS containing 1 mM EDTA and disrupted on ice with a Polytron homogenizer at a setting of 6 for 12 s. The membranes were collected by centrifugation at 40,000 × g for 15 min at 4°C. The pellet was resuspended in PBS and stored at −80°C until use.
[3H]CFT Binding.
For saturation binding, the appropriate number of intact cells or membranes were resuspended in PBS. Duplicate determinations of total and of nonspecific binding were made at six different concentrations of [3H]CFT (New England Nuclear) between 1 and 100 nM with 50 μl of cell suspension in a final volume of 75 μl. The mixture was incubated at 4°C for 2 hr and then filtered by using a Brandel (Bethesda) cell harvester through Whatman 934AH glass fiber filters soaked in 0.2% polyethyleneimine. The filter was washed three times with 1 ml of 10 mM Tris⋅HCl and 120 mM NaCl (pH 7.4) at 4°C. Specific [3H]CFT binding was defined as total binding less nonspecific binding in the presence of 100 μM cocaine.
Reactions with MTS Reagents.
Intact cells or membranes from one-half to two confluent 10-cm plates, depending on levels of expression of the various mutants, were resuspended in 400 μl of PBS. Aliquots (50 μl) of cell suspension or membrane preparation were incubated with freshly prepared MTSEA, MTSethyltrimethylammonium (MTSET), or MTSethylsulfonate (MTSES) (Toronto Research Chemicals, Toronto) at the stated concentrations at room temperature for 2 min. Cells or membranes were then diluted threefold with PBS, and 50 μl aliquots of this diluted preparation were then directly used to assay for [3H]CFT (10nM) binding as described above. In the experiments shown in Figs. 5 and 6, the MTS reagents were removed by filtration and washing rather than by dilution. No significant differences in the effect of reaction were observed with these two methods.
Figure 5.
The effects of cocaine and dopamine on the reactivity of MTSEA with the endogenous cysteines individually restored into the X5C background. Membranes were incubated in PBS for 20 min at room temperature in the presence or absence of (hatched bars) 3 μM cocaine or (open bars) 1 mM dopamine. MTSEA was added, in the continued presence or absence of cocaine or dopamine, for 2 min at the following concentrations: 2.5 mM, X5C; 1 mM, X-A90C; 0.5 mM, X-A135C; 0.25 mM, HDAT; 0.1 mM, X-A306C; and 0.06 mM, X-A342C. To facilitate determination of a change in the rate of reaction, the concentrations of MTSEA were chosen to produce, in the absence of ligand, ≈70% of the maximal effect. Cells were washed with PBS by filtration through 96-well multiscreen plates containing GF/B filters (Millipore). [3H]CFT binding to the washed cells was performed in a final volume of 75 μl. The means and SEM of 4–6 independent experiments each performed with triplicate determinations are shown. Filled bars represent the effect of MTSEA alone.
Figure 6.
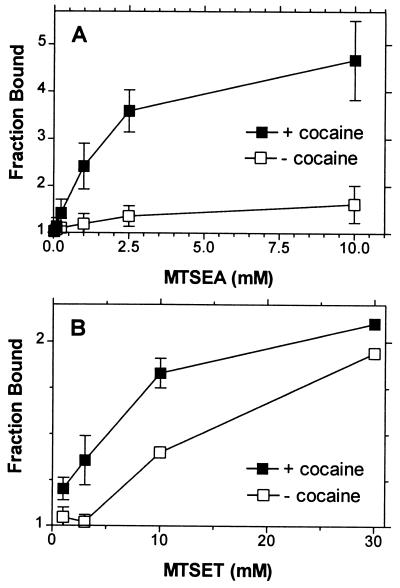
The potentiation of [3H]CFT (10 nM) binding to membranes prepared from HEK 293 cells stably transfected with X-A90C resulting from reaction of MTSEA (A) or MTSET (B) at varying concentrations in the (■) presence of 3 μM cocaine or (□) in its absence. The means and SEM of two independent experiments each performed with triplicate determinations are shown.
RESULTS
Effects of MTSEA on [3H]CFT binding to wt DAT.
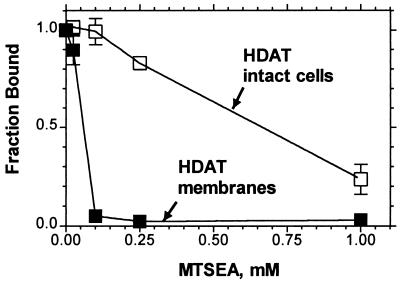
MTSEA irreversibly inhibited [3H]CFT binding to intact cells stably expressing wt DAT (Fig. 2). The rate of reaction of MTSEA was much faster in membranes prepared from these cells (Fig. 2). The rate constants were ≈110 M−1 s−1 in membranes and 10 M−1 s−1 in cells. Because MTSEA is membrane permeant (41, 42), it can react with cytoplasmic cysteines in intact cells, albeit at a slower rate than with directly accessible cysteines (see Discussion).Thus MTSEA was likely reacting with one or more cytoplasmic cysteines in DAT.
Figure 2.
The inhibition of specific [3H]CFT binding (10 nM) to (□) intact dissociated HEK 293 cells stably transfected with wt DAT or (■) membranes prepared from these cells, resulting from a 2-min application of varying concentrations of MTSEA. The means and SEM of 3–6 independent experiments each performed with triplicate determinations are shown.
Effects of MTSEA on [3H]CFT binding to mutant DATs.
We mutated to serine or alanine each of the endogenous cysteines (except Cys-180 and Cys-189, which are likely disulfide-bonded), individually and in a large number of combinations to identify the accessible cysteines responsible for the inhibition of binding. We succeeded in substituting each of these endogenous cysteines in DAT, one at a time. Each mutant bound [3H]CFT with approximately normal affinity and with ≈30–200% the Bmax of wt DAT (data not shown). None of the mutants with a single cysteine removed, however, was resistant to the effects of MTSEA on ligand binding (data not shown).
We tested mutants with combinations of mutated cysteines, several of which had significantly reduced levels of expression. By mutating Cys-319 to Phe§ rather than Ser or Ala, we constructed a mutant DAT in which five endogenous cysteines were simultaneously replaced by other residues. This transporter, which we refer to as “X5C,” contains the mutations C90A, C135A, C306A, C319F, and C342A. When stably expressed in HEK 293 cells, X5C expressed at levels ≈30–50% that of wt DAT. Furthermore, wt DAT and X5C had nearly identical affinities for [3H]CFT (31 ± 5 nM (n = 3) and 38 ± 6 nM (n = 3), respectively). Moreover, in cells expressing X5C, a 5-min incubation with 20 nM [3H]dopamine resulted in ≈50% of the uptake seen in cells expressing wt DAT (data not shown).
In membranes prepared from cells expressing X5C, MTSEA had a significantly reduced effect on [3H]CFT binding (Fig. 3A). Although binding was still inhibited at high concentrations of MTSEA, the rate of reaction was two orders of magnitude slower than with wt. In a background of X5C, each of the remaining endogenous cysteines (Cys-6, Cys-243, Cys-463, Cys-523, Cys-530, or Cys-581) was mutated, one at a time, to alanine or serine. Binding to each of the resulting mutants (each with six cysteines removed) was inhibited by high concentrations of MTSEA approximately as much as was binding to X5C (data not shown), suggesting that more than one residual cysteine reacts with MTSEA but relatively slowly.¶ The simultaneous mutation of additional endogenous cysteines resulted in a transporter with substantially reduced expression (data not shown).
Figure 3.
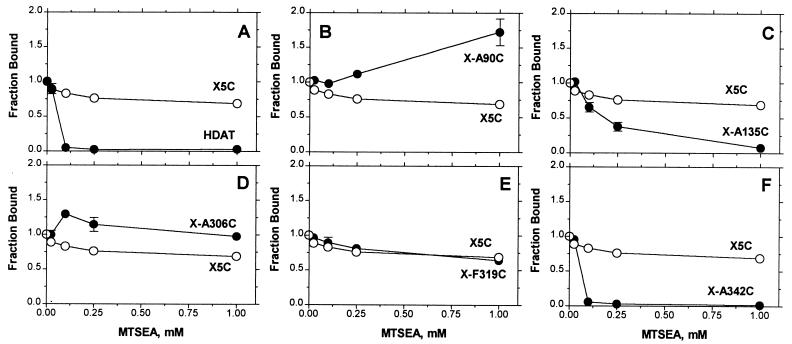
The inhibition of specific [3H]CFT binding (10nM) to membranes prepared from HEK 293 cells stably transfected with (•) wt (A), X-A90C (B), X-A135C (C), X-A306C (D), X-F319C (E), X-A342C (F), or with (○) X5C, resulting from a 2-min application of varying concentrations of MTSEA. The means and SEM of 3–6 independent experiments each performed with triplicate determinations are shown.
We sought to determine the effect of reaction with MTSEA of each of the five endogenous cysteines, which had been removed in X5C. Therefore, in the X5C background, we restored, one at a time, each cysteine. These mutants, referred to as X-A90C, X-A135C, X-A306C, X-F319C, and X-A342C, were stably expressed in HEK 293 cells. We determined the effects of increasing concentrations of MTSEA in membranes and in cells on the subsequent binding of [3H]CFT. The difference between the effect of reaction in each mutant and that seen in X5C was inferred to be the effect of the reaction of MTSEA with the cysteine, which had been reintroduced into X5C.
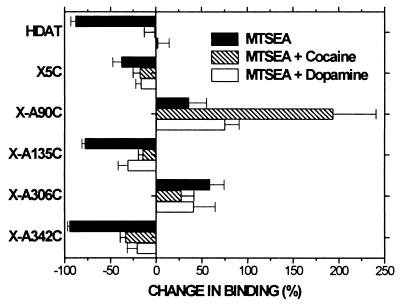
Reaction of MTSEA with X-A90C (Fig. 3B) and with X-A306C (Fig. 3D) potentiated [3H]CFT binding. This potentiation resulted from a decrease in the Kd for [3H]CFT (data not shown). Reaction of MTSEA adds SCH2CH2NH3+ to the cysteine sulfhydryl, thereby creating a lysine-like side chain; mutation of Cys-306 to Lys also decreased the Kd for [3H]CFT and for [3H]mazindol (data not shown). X-A90C and X-A306C reacted with MTSEA with similar rates in intact cells and in membranes (cell data not shown).
Reaction of MTSEA with X-A135C (Fig. 3C) and with X-A342C (Fig. 3F) inhibited [3H]CFT binding. In membranes, the rate of reaction of MTSEA with X-A342C was greater than that with X-A135C. Both of these mutants reacted faster with MTSEA in membranes than in cells (cell data not shown), consistent with a cytoplasmic location of Cys-135 and Cys-342. [3H]CFT binding to X-F319C was not affected by treatment with MTSEA (Fig. 3E).
Effects of MTSET and MTSES on [3H]CFT binding to mutant DATs.
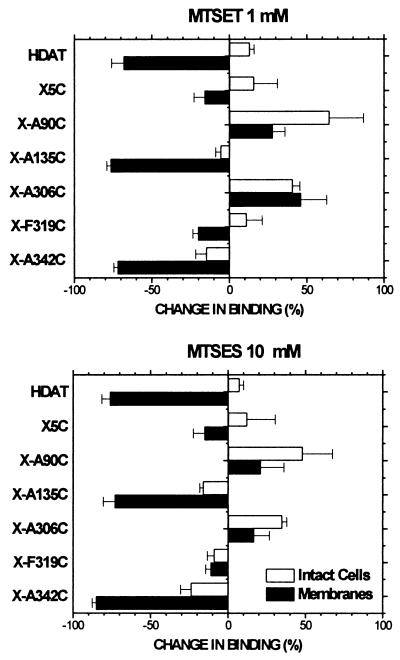
Reaction of the relatively membrane-impermeant sulfhydryl reagents MTSET (at 1 mM) and MTSES (at 10 mM) with X-A90C and X-A306C potentiated [3H]CFT binding, and the extent of reaction resulting from a 2-min application of the reagents was similar in cells and membranes (Fig. 4). A 2-min application of MTSET and MTSES to X-A135C and X-A342C, however, resulted in a much greater extent of reaction in membranes than in intact cells (Fig. 4).
Figure 4.
The percentage change of specific [3H]CFT binding (10 nM) to (filled bars) membranes prepared from or (open bars) intact dissociated HEK 293 cells stably transfected with the indicated DAT mutants resulting from a 2-min application of (Upper) 1 mM MTSET or (Lower) 10 mM MTSES. These concentrations were chosen to normalize for the different reactivities of the reagents with simple sulfhydryls in solution. The means and SEM of 5–11 independent experiments each performed with triplicate determinations are shown.
Effects of cocaine and dopamine on the reaction of MTSEA.
After determining that the MTS reagents reacted with Cys-90, Cys-135, Cys-306, and Cys-342, we determined the effects of dopamine and of cocaine on these reactions. In a membrane preparation, the reaction of MTSEA with Cys-135 and with Cys-342 was significantly retarded by the presence of either 3 μM cocaine or of 1 mM dopamine (Fig. 5).
In contrast, in the presence of 3 μM cocaine, a 2-min reaction of Cys-90 with MTSEA produced a greater potentiation of binding at all concentrations (Fig. 6). This effect was much smaller in the presence of 1 mM dopamine (Fig. 5). The rate of reaction of Cys-90 with MTSET also was faster in the presence of cocaine (Fig. 6).
DISCUSSION
We found that reaction of the MTS reagents with Cys-90, Cys-135, Cys-306, and Cys-342 in the human dopamine transporter altered the binding of the cocaine analog CFT. Thus, consistent with their predicted location in extracellular and intracellular loops, these four endogenous cysteines are exposed on the water-accessible surface of DAT. This inference is based on the observation that the MTS reagents react 1 billion times faster with the ionized thiolate form of cysteine (RS−) than with unionized thiol form (RSH) (43) and only water-accessible cysteines are likely to ionize to a significant extent. Moreover, the reagents are quite polar (44, 45). Cys-90, Cys-135, and Cys-342 are highly conserved in related neurotransmitter transporters. In contrast, Cys-306 is unique to DAT, as is Cys-319 in putative M6, which does not appear to be accessible to the MTS reagents.‖
By comparing the effects of the various MTS reagents in cells and in membranes, we can infer the topology of DAT. In a membrane preparation, the MTS reagents should have equal access to cysteines accessible on the extracellular and cytoplasmic sides of DAT.** Furthermore, in cells, the reagents should have access to extracellular cysteines similar to that observed in membranes. MTSEA is membrane permeant (41, 42). It is a weak base and presumably can cross the membrane in the unprotonated state. MTSET is a quaternary ammonium with a fixed charge and MTSES is fully ionized at neutral pH. Both are relatively membrane-impermeant (41, 42). Therefore, a residue which is water-accessible from the cytoplasmic side but not from the extracellular side of the membrane should not react at an appreciable rate with extracellularly applied MTSET or MTSES even though it might react with MTSEA.
The rates of reaction of Cys-90 and Cys-306 with MTSEA, MTSET, and MTSES were similar in cells and in membranes (Figs. 3 and 4), consistent with an extracellular localization of these residues.‡‡ In contrast, Cys-135 and Cys-342 reacted with MTSEA more rapidly in membranes than in cells. Moreover, the effects of a 2-min application of the impermeant reagents on X-A135C and X-A342C were dramatically less in intact cells compared with membranes (Fig. 4). These findings are consistent with the cytoplasmic localization of these cysteines.
According to the originally proposed topology, Cys-90 is located at the extracellular end of the first membrane-spanning segment (M1), Cys-135 is in the intracellular loop between M2 and M3, Cys-306 is in an extracellular loop between M5 and M6, and Cys-342 is in an intracellular loop between M6 and M7 (Fig. 1). Our results are entirely consistent with this topology but not with a revised topology for the GABA and glycine transporters that placed the residue aligned with Cys-90 in an intracellular loop and the residue aligned with Cys-135 in an extracellular loop (12, 13).
Related experiments in SERT and in the GABA transporter also were consistent with the accessibility of extracellularly applied MTSET to the cysteine that aligns with DAT Cys-90, further supporting the original topology (39, 46). Moreover, in intact cells, cysteine inserted into each of the putative extracellular loops of SERT reacted with N-biotinylaminoethyl-MTS, whereas the endogenous cytoplasmic cysteines did not react (47), again consistent with the original topology and with our findings.
Although GABA and glycine transporters with glycosylation sites added to the intracellular loop between M2 and M3, the loop which contains DAT Cys-135, were glycosylated, these transporters were almost completely nonfunctional (12, 13). The most likely explanation for the discrepancy between these results and our findings is that the topology of these nonfunctional glycosylation site mutants may have been disrupted, and the small amount of transport function may have resulted from a small amount of nonglycosylated transporter with normal topology.
In cells, MTSEA likely gains access to Cys-135 and Cys-342 by passing through the membrane and reacting from the cytoplasmic side. The slower rate of reaction compared with that in membranes is consistent with only a small fraction of the total MTSEA being unprotonated and with the cytoplasmic reducing environment scavenging some of the MTSEA that does cross the membrane. It is surprising, however, that the relatively impermeant MTSET and MTSES reacted at all with Cys-135 and with Cys-342 in intact cells. It is conceivable that the MTS reagents gained access to the cytoplasmic residues by passing through the transporter itself and then reacted with these cysteines as the reagents emerged into the cytoplasm. Sodium is required for dopamine transport, so the similar reactivities of Cys-135 and Cys-342 in sodium-free high-potassium buffer (data not shown) argue that the MTS reagents do not traverse the transporter in a sodium-dependent manner.
The structure and orientation of the loop residues are unclear, and it is likely that these “loops” have significant structure and play important functional roles in binding and transport. It is possible that the critical intracellular cysteines in DAT, Cys-135 and Cys-342, reenter the transport pathway where they may be accessible for reaction from the extracellular side, albeit at a slower rate of reaction than from the cytoplasmic side. In the GABA transporter, extracellular loop residues have been suggested to form part of the substrate-binding site (48). Based on the protease sensitivity of GABA transporter-prolactin chimeras, extracellular pore loop structures also have been proposed to extend into the membrane sufficiently to expose the fused prolactin to cytoplasmic proteolysis (49). The limited reaction of Cys-135 and Cys-342 in intact cells may, however, reflect a small percentage of “intact” cells that became leaky to the reagents during dissociation of the cells.
The cytoplasmic loop between M2 and M3 which contains Cys-135 also contains the conserved sequence GXXXRXG. The M2-M3 loop in the bacterial Tn10 Tetracycline antiporter, and related bacterial transporters, contains the related highly conserved sequence GXXXXRXG. This loop in the Tn10 antiporter has been suggested to form part of the gating mechanism in this transporter (50). The related sequences raise the possibility of a structurally and functionally similar role for this loop in DAT and related neurotransmitter transporters.
The reactivities of Cys-135 and Cys-342 with MTSEA were dramatically reduced in the presence of either cocaine or dopamine. Protection could result from the ligand obstructing access of MTSEA to the reactive cysteine or from a ligand-induced conformational change that reduces the accessibility of the reactive cysteine to the reagent. If protection resulted from direct steric block, then bound ligand must prevent access to the cytoplasmic loops bearing these cysteines, even in a membrane preparation. This would require that the M2-M3 and M6-M7 cytoplasmic loops contribute to the binding sites for dopamine and cocaine. It seems unlikely, however, that Cys-135 and Cys-342 directly interact with bound cocaine or dopamine, given that their mutation to alanine had no significant effect on CFT binding or dopamine uptake. Alternatively, the binding of cocaine and dopamine may indirectly alter the conformation and, thereby, reduce the accessibility of these cytoplasmic loop cysteines. In this scenario, the MTS-modified cysteine side chain might indirectly lower the affinity of the binding site for cocaine (and its analogue CFT) through a reciprocal allosteric effect, thereby resulting in a loss of binding.
In X-A90C the potentiation of CFT binding induced by MTSEA was substantially increased in the presence of 3 μM cocaine (Figs. 5 and 6). Part of this increased potentiation resulted from partial protection by cocaine against the residual effects of MTSEA in X5C (and hence in X-A90C) and not solely from increased reactivity of Cys-90 in the presence of cocaine. MTSET, however, did not significantly affect the binding of CFT to X5C, but, nonetheless, the reactivity of MTSET with Cys-90 also was significantly enhanced in the presence of cocaine (Fig. 6B). Thus, the binding of cocaine resulted in a conformational change which increased the accessibility of Cys-90.
The greater effect of cocaine than dopamine on the accessibility of Cys-90 likely accounts for reports that cocaine protected wt DAT better than did dopamine against the effects of NEM (36, 37). Thus, the ability of cocaine to increase the accessibility of Cys-90 to NEM, and the resulting potentiation, could have led to higher binding and the inference that cocaine protected better than did dopamine.
In SERT, reaction of Cys-109, the residue aligned with DAT Cys-90, inhibited serotonin transport (39). The rate of reaction of MTSET with SERT Cys-109 substantially increased when lithium was substituted for sodium, a finding which we did not observe with X-A90C in DAT when using CFT binding as the readout for reaction (data not shown). Moreover, the presence of cocaine failed to alter the reactivity of SERT Cys-109 (G. Rudnick, personal communication). In GABA transporter, the reactivity of MTSET with Cys-74, the residue aligned with DAT Cys-90, was altered by mutation of a residue in M1 (46). These findings suggest that the accessibility of the cysteine in this position varies significantly during different functional states of these transporters. Furthermore, differences in the ionic regulation of the accessibility of this residue in SERT and DAT may relate to the differences in ionic dependence and stoichiometry between these two transporters (51).
In another curious potential parallel, a cysteine inserted into the loop between M1 and M2 in the Tn10 transporter also underwent dramatic changes in accessibility upon mutation of residues in the M2-M3 loop, suggesting a conformational interaction between these two domains, separated by the M2 membrane-spanning segment (50). In addition, in the lactose permease, substrate alters the rate of disulfide cross-linking between cysteines in the M1-M2 loop and the M7-M8 loop, suggesting that conformational changes in these domains accompany substrate binding and/or transport (H. R. Kaback, personal communication).
The effects on binding of reaction of a cysteine with a MTS reagent could be a result of steric block, electrostatic interaction, or indirect structural changes. Although reaction with Cys-135 and Cys-342 inhibited binding, reaction with Cys-90 and Cys-306 increased the affinity of the transporter for CFT and mazindol and thereby potentiated binding. Reaction of MTSEA adds SCH2CH2NH3+ to the cysteine sulfhydryl, thereby creating a lysine-like side chain. Curiously, the norepinephrine transporter, which has a higher affinity for mazindol than does DAT, contains a Lys at the position aligned with DAT Cys-306 (52). Mutation of Cys-306 in DAT to lysine increased the affinity of DAT for CFT and mazindol but did not increase the affinity of tricyclic antidepressants for DAT (data not shown). Given that potentiation resulted from the reaction of either the positively charged MTSEA or MTSET, or the negatively charged MTSES with Cys-90 and with Cys-306, the increased affinity apparently does not result from an electrostatic interaction but rather from a structural perturbation which favors a conformation of the transporter having a higher affinity for cocaine and mazindol.
In summary, we have created a mutant transporter, X5C, in which five endogenous cysteines have been replaced by other residues. This transporter expressed at reasonable levels and bound CFT in a near-normal manner. In preliminary studies, dopamine uptake by X5C appeared near-normal as well, and we are currently characterizing uptake and the effects of the MTS reagents thereon. Binding to X5C is two orders of magnitude less sensitive to MTSEA and nearly insensitive to MTSET and MTSES. Thus, this mutant appears to be a suitable background for the application of the SCAM (25, 27, 45) to map the binding site and transport pathway of DAT.
Acknowledgments
We thank Drs. Mark Sonders and Susan Amara for the human DAT cDNA, Thomas Livelli for the HEK 293 cells, Gursh Khatra and Susan Amara for the C135A mutation, Thomas Caughey and Lei Shi for making a number of the mutants used in these studies, and Jiayun Chen for invaluable assistance with preliminary work on DAT. We thank Myles Akabas, Arthur Karlin, and Mark Sonders for valuable discussion and for comments on this manuscript. This work was supported in part by the Nathan and Suzanne Cohen Foundation, by a Grant-in-Aid from the American Heart Association, and by National Institutes of Health Grants MH57324, MH54137, DA11495, and GM07182.
ABBREVIATIONS
- HDAT
human dopamine transporter
- wt
wild-type
- SCAM
substituted-cysteine accessibility method
- CFT
2-β-carbomethoxy-3-β-(4-fluorophenyl) tropane
- MTS
methanethiosulfonate
- MTSEA
MTSethylammonium
- MTSET
MTSethyltrimethylammonium
- MTSES
MTSethylsulfonate
- SERT
serotonin transporter
- GABA
γ-aminobutyric acid
- M1-M12
first through twelfth membrane-spanning segment
Footnotes
In the aligned sequences of every other NaCl-coupled transporter, the residue corresponding to DAT Cys-319 is a Phe. We found that Phe was a much better tolerated substitution at this position than was Ser, as C319S, expressed at low levels.
The slow rate of reaction of the residual cysteines likely results from limited access caused by steric constraints and/or intermittent accessibility due to dynamic structural changes in the transporter. Another theoretical possibility is that modification of a cysteine in another protein endogenously expressed in HEK 293 cells indirectly interferes with cocaine binding to DAT.
It is possible that reaction could take place without an alteration in function, although this seems unlikely for a residue in a membrane-spanning segment.
Although it is possible that membrane vesicles reseal to some extent, the dramatically faster reaction of MTSEA and MTSET with X-A135C and X-A342C in membranes than in intact cells suggests that this is not a significant factor.
If a significant amount of CFT binding were to intracellular transporter, the extracellular Cys-90 and Cys-306 would not have been similarly accessible in cells and in membranes to the impermeant MTSET and MTSES. Thus, CFT does not appear to bind to a significant extent to intracellular transporter.
References
- 1.Shimada S, Kitayama S, Lin C L, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 2.Kilty J E, Lorang D, Amara S G. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- 3.Vandenbergh D J, Persico A M, Uhl G R. Brain Res Mol Brain Res. 1992;15:161–166. doi: 10.1016/0169-328x(92)90165-8. [DOI] [PubMed] [Google Scholar]
- 4.Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, Caron M G. Mol Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- 5.Usdin T B, Mezey E, Chen C, Brownstein M J, Hoffman B J. Proc Natl Acad Sci USA. 1991;88:11168–11171. doi: 10.1073/pnas.88.24.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amara S G, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 7.Kilty J E, Amara S G. Curr Opin Biotechnol. 1992;3:675–682. doi: 10.1016/0958-1669(92)90015-b. [DOI] [PubMed] [Google Scholar]
- 8.Kanner B I. J Exp Biol. 1994;196:237–249. doi: 10.1242/jeb.196.1.237. [DOI] [PubMed] [Google Scholar]
- 9.Giros B, Caron M G. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan R A, Kuhar M J. J Biol Chem. 1996;271:21672–21680. doi: 10.1074/jbc.271.35.21672. [DOI] [PubMed] [Google Scholar]
- 11.Bruss M, Hammermann R, Brimijoin S, Bonisch H. J Biol Chem. 1995;270:9197–9201. doi: 10.1074/jbc.270.16.9197. [DOI] [PubMed] [Google Scholar]
- 12.Olivares L, Aragon C, Gimenez C, Zafra F. J Biol Chem. 1997;272:1211–1217. doi: 10.1074/jbc.272.2.1211. [DOI] [PubMed] [Google Scholar]
- 13.Bennett E R, Kanner B I. J Biol Chem. 1997;272:1203–1210. doi: 10.1074/jbc.272.2.1203. [DOI] [PubMed] [Google Scholar]
- 14.Ritz M C, Lamb R J, Goldberg S R, Kuhar M J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 15.Kuhar M J, Ritz M C, Boja J W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 16.Kitayama S, Shimada S, Xu H, Markham L, Donovan D M, Uhl G R. Proc Natl Acad Sci USA. 1992;89:7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitayama S, Wang J B, Uhl G R. Synapse. 1993;15:58–62. doi: 10.1002/syn.890150107. [DOI] [PubMed] [Google Scholar]
- 18.Todd A P, Cong J, Levinthal F, Levinthal C, Hubbell W L. Proteins. 1989;6:294–305. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- 19.Jakes K S, Abrams C K, Finkelstein A, Slatin S L. J Biol Chem. 1990;265:6984–6991. [PubMed] [Google Scholar]
- 20.Altenbach C, Marti T, Khorana H G, Hubbell W L. Science. 1990;248:1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- 21.Careaga C L, Falke J J. Biophys J. 1992;62:209–216. doi: 10.1016/S0006-3495(92)81806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pakula A A, Simon M I. Proc Natl Acad Sci USA. 1992;89:4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung K, Jung H, Wu J, Prive G G, Kaback H R. Biochemistry. 1993;32:12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- 24.Akabas M H, Stauffer D A, Xu M, Karlin A. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- 25.Chen J G, Sachpatzidis A, Rudnick G. J Biol Chem. 1997;272:28321–28327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- 26.Yan R T, Maloney P C. Proc Natl Acad Sci USA. 1995;92:5973–5976. doi: 10.1073/pnas.92.13.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javitch J A, Fu D, Chen J, Karlin A. Neuron. 1995;14:825–831. doi: 10.1016/0896-6273(95)90226-0. [DOI] [PubMed] [Google Scholar]
- 28.Reith M E, Selmeci G. Naunyn–Schmiedebergs Arch Pharmacol. 1992;345:309–318. doi: 10.1007/BF00168692. [DOI] [PubMed] [Google Scholar]
- 29.Saadouni S, Refahi-Lyamani F, Costentin J, Bonnet J J. Eur J Pharmacol. 1994;268:187–197. doi: 10.1016/0922-4106(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 30.Refahi-Lyamani F, Saadouni S, Costentin J, Bonnet J J. Naunyn–Schmiedebergs Arch Pharmacol. 1995;351:136–145. doi: 10.1007/BF00169327. [DOI] [PubMed] [Google Scholar]
- 31.Richfield E K. Mol Pharmacol. 1993;43:100–108. [PubMed] [Google Scholar]
- 32.Cao C J, Young M M, Wong J B, Mahran L G, Eldefrawi M E. Membr Biochem. 1989;8:207–220. doi: 10.3109/09687688909026815. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet J J, Benmansour S, Amejdki-Chab N, Costentin J. Eur J Pharmacol. 1994;266:87–97. doi: 10.1016/0922-4106(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 34.Schweri M M. Synapse. 1994;16:188–194. doi: 10.1002/syn.890160304. [DOI] [PubMed] [Google Scholar]
- 35.Schweri M M. Neuropharmacology. 1990;29:901–908. doi: 10.1016/0028-3908(90)90140-m. [DOI] [PubMed] [Google Scholar]
- 36.Johnson K M, Bergmann J S, Kozikowski A P. Eur J Pharmacol. 1992;227:411–415. doi: 10.1016/0922-4106(92)90159-s. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Coffey L L, Reith M E. Naunyn–Schmiedebergs Arch Pharmacol. 1997;355:64–73. doi: 10.1007/pl00004919. [DOI] [PubMed] [Google Scholar]
- 38.Wang J B, Moriwaki A, Uhl G R. J Neurochem. 1995;64:1416–1419. doi: 10.1046/j.1471-4159.1995.64031416.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen J G, Liu-Chen S, Rudnick G. Biochemistry. 1997;36:1479–1486. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 40.Rees S, Coote J, Stables J, Goodson S, Harris S, Lee M G. BioTechniques. 1996;20:102–110. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- 41.Holmgren M, Liu Y, Xu Y, Yellen G. Neuropharmacology. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 42.Olami Y, Rimon A, Gerchman Y, Rothman A, Padan E. J Biol Chem. 1997;272:1761–1768. doi: 10.1074/jbc.272.3.1761. [DOI] [PubMed] [Google Scholar]
- 43.Roberts D D, Lewis S D, Ballou D P, Olson S T, Shafer J A. Biochemistry. 1986;25:5595–5601. doi: 10.1021/bi00367a038. [DOI] [PubMed] [Google Scholar]
- 44.Stauffer D A, Karlin A. Biochemistry. 1994;33:6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- 45.Karlin A, Akabas M H. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 46.Yu N, Cao Y, Mager S, Lester H A. FEBS Lett. 1998;426:174–178. doi: 10.1016/s0014-5793(98)00333-0. [DOI] [PubMed] [Google Scholar]
- 47.Chen J G, Liu-Chen S, Rudnick G. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 48.Tamura S, Nelson H, Tamura A, Nelson N. J Biol Chem. 1995;270:28712–28715. doi: 10.1074/jbc.270.48.28712. [DOI] [PubMed] [Google Scholar]
- 49.Clark J A. J Biol Chem. 1997;272:14695–14704. doi: 10.1074/jbc.272.23.14695. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi A, Kimura T, Sawai T. FEBS Lett. 1993;322:201–204. doi: 10.1016/0014-5793(93)81568-k. [DOI] [PubMed] [Google Scholar]
- 51.Gu H, Wall S C, Rudnick G. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- 52.Pacholczyk T, Blakely R D, Amara S G. Nature (London) 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]