Abstract
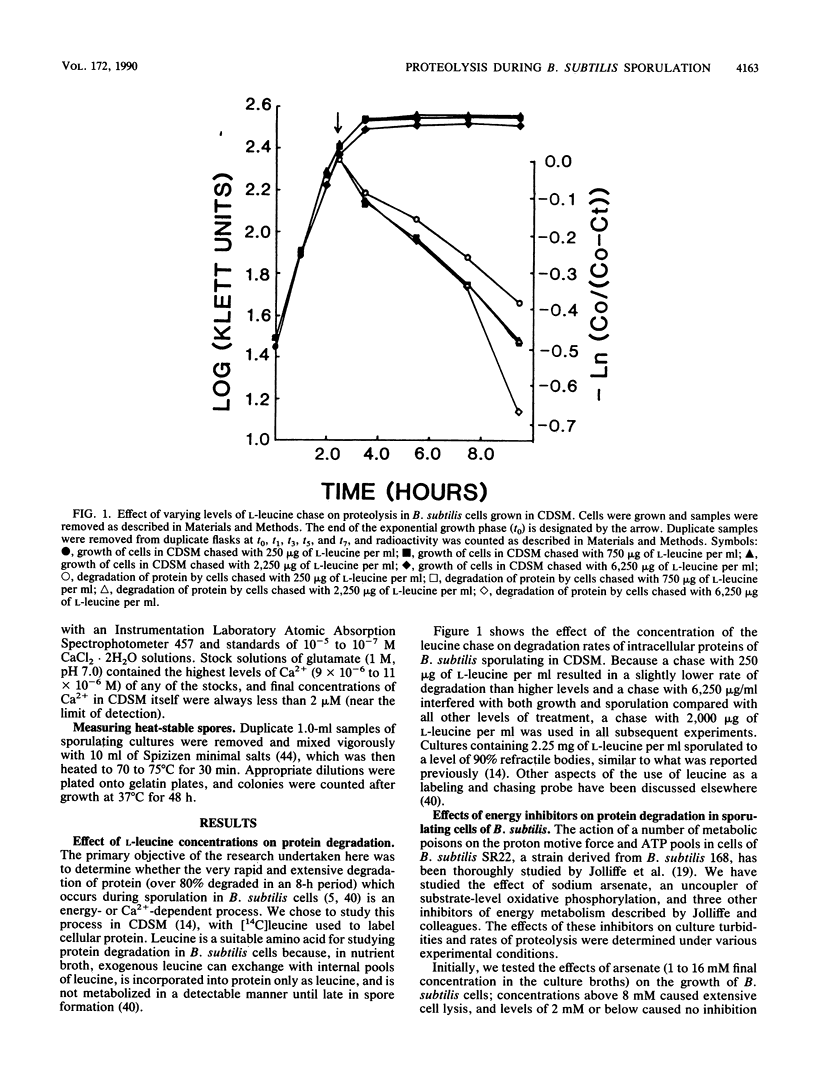
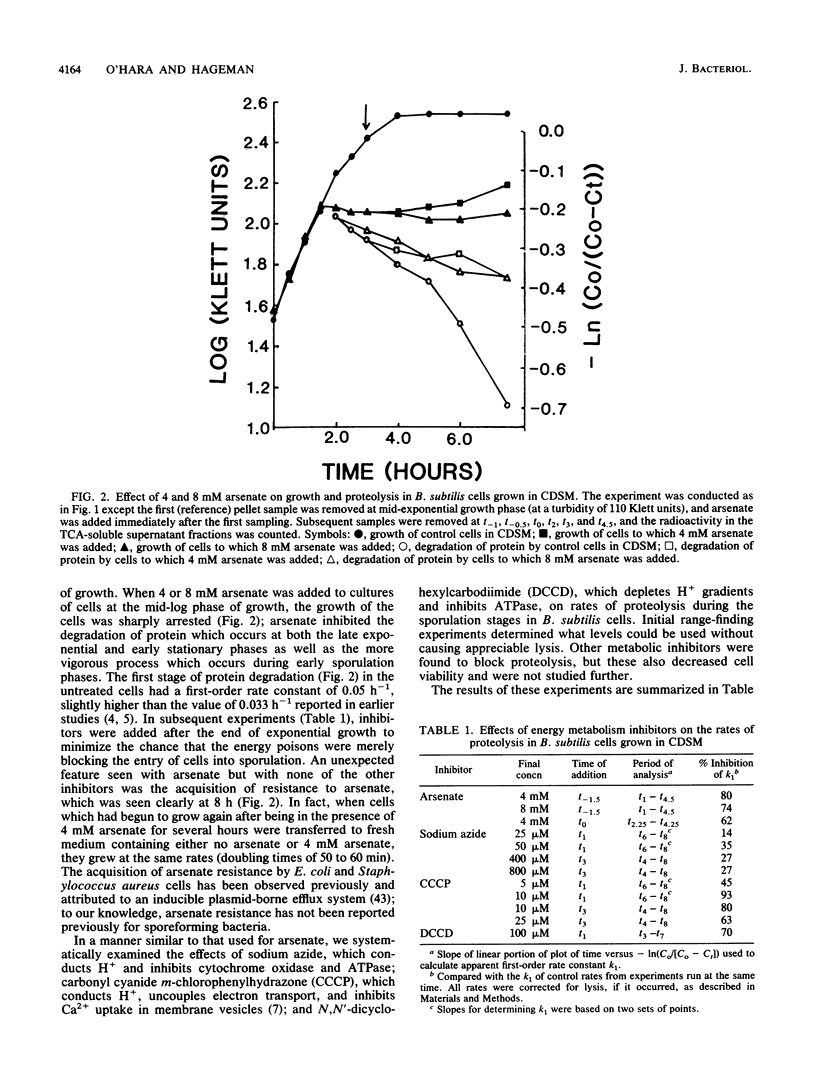
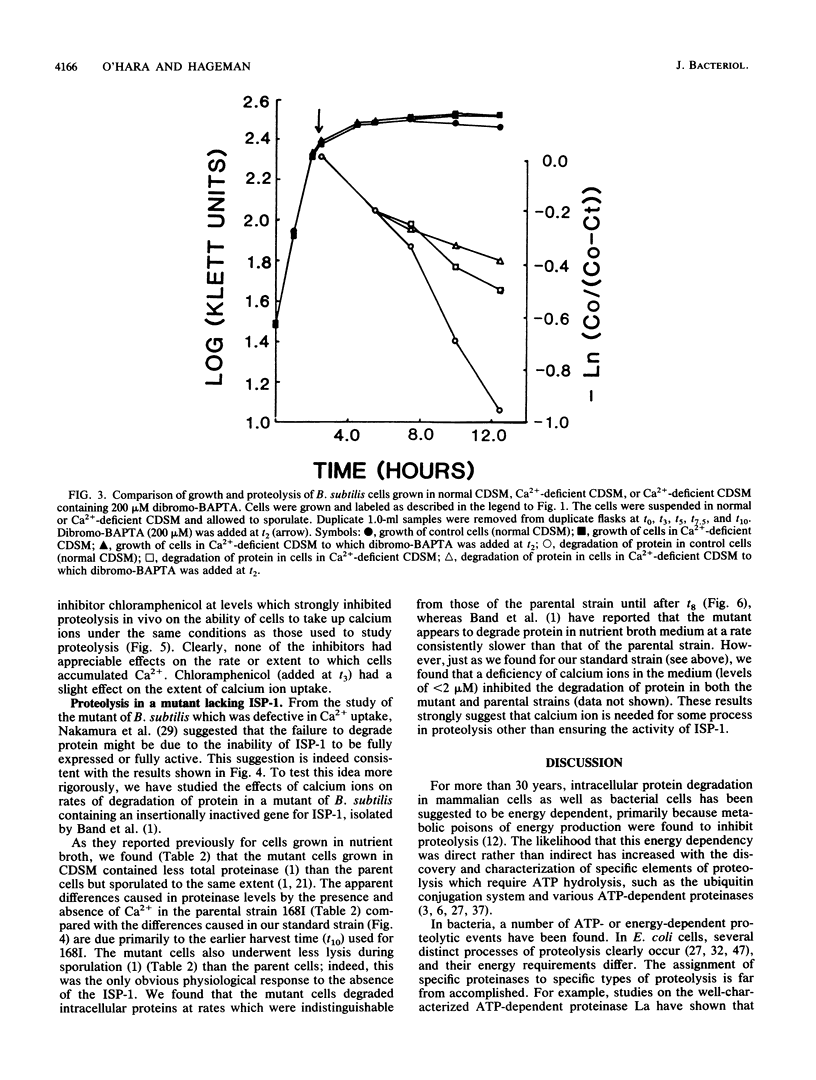
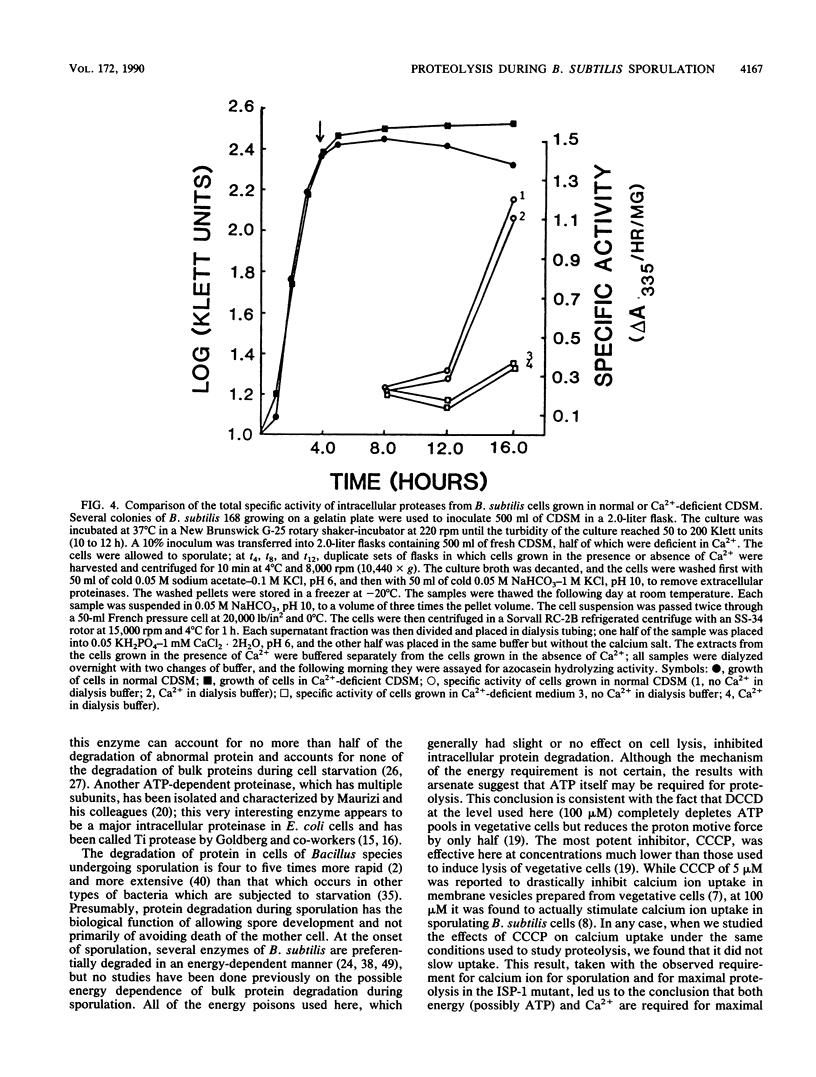
Bacterial cells degrade intracellular proteins at elevated rates during starvation and can selectively degrade proteins by energy-dependent processes. Sporulating bacteria can degrade protein with apparent first-order rate constants of over 0.20 h-1. We have shown, with an optimized [14C]leucine-labeling and chasing procedure, in a chemically defined sporulation medium, that intracellular protein degradation in sporulating cells of Bacillus subtilis 168 (trpC2) is apparently energy dependent. Sodium arsenate, sodium azide, carbonyl cyanide m-chlorophenylhydrozone, and N,N'-dicyclohexylcarbodiimide, at levels which did not induce appreciable lysis (less than or equal to 10%) over 10-h periods of sporulation, inhibited intracellular proteolysis by 13 to 93%. Exponentially growing cells acquired arsenate resistance. In contrast to earlier reports, we found that chloramphenicol (100 micrograms/ml) strongly inhibited proteolysis (68%) even when added 6 h into the sporulation process. Restricting the calcium ion concentration (less than 2 microM) in the medium had no effect on rates or extent of vegetative growth, strongly inhibited sporulation (98%), and inhibited rates of proteolysis by 60% or more. Inhibitors of energy metabolism, at the same levels which inhibited proteolysis, did not affect the rate or degree of uptake of Ca2+ by cells, which suggested that the Ca2+ and metabolic energy requirements of proteolysis were independent. Restricting the Ca2+ concentration in the medium reduced by threefold the specific activity in cells of the major intracellular serine proteinase after 12 h of sporulation. Finally, cells of a mutant of B. subtilis bearing an insertionally inactivated gene for the Ca2(+)-dependent intracellular proteinase-1 degraded protein in chemically defined sporulation medium at a rate indistinguishable from that of the wild-type cells for periods of 8 h.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band L., Henner D. J., Ruppen M. Construction and properties of an intracellular serine protease mutant of Bacillus subtilis. J Bacteriol. 1987 Jan;169(1):444–446. doi: 10.1128/jb.169.1.444-446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W. 18 Oxygen probes of protein turnover, amino acid transport, and protein synthesis in Bacillus licheniformis. J Biol Chem. 1972 Aug 10;247(15):4893–4899. [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Bond R. W., Field A. S., Switzer R. L. Nutritional regulation of degradation of aspartate transcarbamylase and of bulk protein in exponentially growing Bacillus subtilis cells. J Bacteriol. 1983 Jan;153(1):253–258. doi: 10.1128/jb.153.1.253-258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett T. J., Shankweiler G. W., Hageman J. H. Activation of intracellular serine proteinase in Bacillus subtilis cells during sporulation. J Bacteriol. 1986 Jan;165(1):139–145. doi: 10.1128/jb.165.1.139-145.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- Ford S. R., Switzer R. L. Stimulation of enzyme synthesis by sublethal concentrations of chloramphenicol is not mediated by ribonucleotide pools. Antimicrob Agents Chemother. 1975 May;7(5):564–570. doi: 10.1128/aac.7.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry I. J., Villa L., Kuehn G. D., Hageman J. H. Calmodulin-like protein from Bacillus subtilis. Biochem Biophys Res Commun. 1986 Jan 14;134(1):212–217. doi: 10.1016/0006-291x(86)90549-8. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Greene R. A., Slepecky R. A. Minimal requirements for commitment to sporulation in Bacillus megaterium. J Bacteriol. 1972 Aug;111(2):557–565. doi: 10.1128/jb.111.2.557-565.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. H., Shankweiler G. W., Wall P. R., Franich K., McCowan G. W., Cauble S. M., Grajeda J., Quinones C. Single, chemically defined sporulation medium for Bacillus subtilis: growth, sporulation, and extracellular protease production. J Bacteriol. 1984 Oct;160(1):438–441. doi: 10.1128/jb.160.1.438-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Park W. J., Chung C. H., Goldberg A. L. Escherichia coli contains a soluble ATP-dependent protease (Ti) distinct from protease La. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5550–5554. doi: 10.1073/pnas.84.16.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Woo K. M., Goldberg A. L., Chung C. H. Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J Biol Chem. 1988 Jun 25;263(18):8727–8734. [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Inouye M. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6829–6833. doi: 10.1073/pnas.80.22.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y., Yonemitsu K., Matsui K., Fukunaga K., Miyamoto E. Calmodulin-like activity in the soluble fraction of Escherichia coli. Biochem Biophys Res Commun. 1981 Feb 12;98(3):656–660. doi: 10.1016/0006-291x(81)91164-5. [DOI] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Katayama-Fujimura Y., Gottesman S., Maurizi M. R. A multiple-component, ATP-dependent protease from Escherichia coli. J Biol Chem. 1987 Apr 5;262(10):4477–4485. [PubMed] [Google Scholar]
- Koide Y., Nakamura A., Uozumi T., Beppu T. Cloning and sequencing of the major intracellular serine protease gene of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):110–116. doi: 10.1128/jb.167.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Brabson J. S., Switzer R. L. Immunochemical studies of the inactivation of aspartate transcarbamylase by stationary phase Bacillus subtilis cells. Evidence for selective, energy-dependent degradation. J Biol Chem. 1978 Aug 25;253(16):5585–5593. [PubMed] [Google Scholar]
- Maurizi M. R., Switzer R. L. Proteolysis in bacterial sporulation. Curr Top Cell Regul. 1980;16:163–224. doi: 10.1016/b978-0-12-152816-4.50010-8. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R., Trisler P., Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985 Dec;164(3):1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Neway J. O., Switzer R. L. Degradation of ornithine transcarbamylase in sporulating Bacillus subtilis cells. J Bacteriol. 1983 Aug;155(2):522–530. doi: 10.1128/jb.155.2.522-530.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Synthesis and inactivation of carbamyl phosphate synthetase isozymes of Bacillus subtilis during growth and sporulation. J Bacteriol. 1979 Dec;140(3):769–773. doi: 10.1128/jb.140.3.769-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson A., Bergman B. Calmodulin in heterocystous cyanobacteria: biochemical and immunological evidence. FEMS Microbiol Lett. 1989 Jul 1;51(1):95–99. doi: 10.1016/0378-1097(89)90084-0. [DOI] [PubMed] [Google Scholar]
- Reeve C. A., Bockman A. T., Matin A. Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella typhimurium. J Bacteriol. 1984 Mar;157(3):758–763. doi: 10.1128/jb.157.3.758-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Regulation of intracellular protein turnover: covalent modification as a mechanism of marking proteins for degradation. Curr Top Cell Regul. 1986;28:291–337. doi: 10.1016/b978-0-12-152828-7.50010-x. [DOI] [PubMed] [Google Scholar]
- Ruppen M. E., Switzer R. L. Degradation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase in vivo. J Biol Chem. 1983 Mar 10;258(5):2843–2851. [PubMed] [Google Scholar]
- Sekar V., Hageman J. H. Protein turnover and proteolysis during sporulation of Bacillus subtilis. Folia Microbiol (Praha) 1987;32(6):465–480. doi: 10.1007/BF02877199. [DOI] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of reduced energy production on protein degradation, guanosine tetraphosphate, and RNA synthesis in Escherichia coli. J Biol Chem. 1978 Apr 25;253(8):2705–2711. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of starvation for potassium and other inorganic ions on protein degradation and ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1223–1233. doi: 10.1128/jb.143.3.1223-1233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D. G., Cortes J., Hale R. S., Leadlay P. F. Cloning, characterization, and heterologous expression of the Saccharopolyspora erythraea (Streptomyces erythraeus) gene encoding an EF-hand calcium-binding protein. J Bacteriol. 1989 Oct;171(10):5614–5619. doi: 10.1128/jb.171.10.5614-5619.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waindle L. M., Switzer R. L. Inactivation of aspartic transcarbamylase in sporulating Bacillus subtilis: demonstration of a requirement for metabolic energy. J Bacteriol. 1973 May;114(2):517–527. doi: 10.1128/jb.114.2.517-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij W., Bulthuis R., Postma E., Konings W. N. Calcium transport in membrane vesicles of Bacillus subtilis. J Bacteriol. 1985 Dec;164(3):1294–1300. doi: 10.1128/jb.164.3.1294-1300.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



