Abstract
Human protein Z (PZ) is a 62,000-Mr, vitamin K-dependent plasma protein whose structure is similar to coagulation factors VII, IX, X, protein C, and protein S, but whose function is not known. The procoagulant activity of factor Xa in a one-stage plasma coagulation assay is reduced when factor Xa is first incubated with PZ. This apparent inhibitory effect is time dependent, requires the presence of calcium ions and procoagulant phospholipids (rabbit brain cephalin), and appears predominantly related to the incubation period of PZ with cephalin. In serum the initial rate of inhibition of factor Xa with calcium ions and cephalin also is enhanced in the presence PZ. A PZ-dependent protease inhibitor (ZPI) has been isolated from plasma. ZPI is a 72,000-Mr single-chain protein with an N-terminal amino acid sequence of LAPSPQSPEXXA (X = indeterminate) and an estimated concentration in citrate-treated plasma of 1.0–1.6 μg/ml. In systems using purified components, the factor Xa inhibition produced by ZPI is rapid (>95% within 1 min by coagulation assay) and requires the presence of PZ, calcium ions, and cephalin. The inhibitory process appears to involve the formation of a factor Xa–PZ–ZPI complex at the phospholipid surface.
Vitamin K is required for the posttranslational formation of γ-carboxyglutamic acid (Gla), which is present in a number of plasma proteins that are involved in coagulation: prothrombin, factors VII, IX, and X, protein C, and protein S (1, 2). Gla-mediated calcium ion binding in these proteins is necessary for their association with phospholipid surfaces and is critical for their hemostatic function (3). In 1977, Prowse and Esnouf (4) identified an additional vitamin K-dependent protein circulating in bovine plasma and named it protein Z (PZ). Initially thought to represent a single-chain form of bovine factor X, bovine PZ was later identified as a discrete Gla-containing protein (5, 6). The human counterpart of bovine PZ was isolated in 1984 (7).
Human PZ is a 62,000-Mr glycoprotein that has a plasma half-life of ≈2.5 days (8). Plasma PZ levels in blood donors span a broad range with a mean concentration of 2.9 ± 1.0 μg/ml in EDTA-anticoagulated samples (corresponding to ≈2.6 μg/ml in citrated plasma) (8). The N-terminal half of PZ is very homologous to those of factors VII, IX, and X, and contains a Gla-domain, two epidermal growth factor-like domains, and a region that connects to a homologue of the catalytic domains present in the serine protease zymogens. In the C-terminal domain of PZ, however, the region around the typical “activation” site is absent and the His and Ser residues of the catalytic triad are lacking (the Asp residue is conserved) (9, 10).
McDonald et al. (11) recently reported that the kinetics of the binding of human and bovine PZ to phosphatidylcholine/phosphatidylserine (75%/25%) vesicles is different from that of the other vitamin K-dependent coagulation factors. The kassn (10−5 s−1⋅M−1) and kdssn (s−1) rate constants are 1.95 and 0.0063 for bovine PZ and 3.36 and 0.057 for human PZ. In comparison, the values of these constants for bovine prothrombin are 176.0 and 1.9, respectively. Thus, the association and dissociation rate constants for bovine and human PZ are dramatically slower than those of prothrombin, and the dissociation of bovine PZ from phospholipids is significantly slower than that of human PZ.
Previous studies in our laboratory had shown that the incubation of PZ with factor Xa, phospholipids, and Ca2+ ions prolonged the clotting time of subsequently added factor X-deficient plasma (G.J.B., unpublished data). The results of recent experiments reexamining this apparent PZ-mediated inhibition of factor Xa procoagulant activity show that PZ affects factor Xa function in part by enhancing the inhibition of factor Xa produced by another plasma protein we tentatively have termed the PZ-dependent protease inhibitor (ZPI).
MATERIALS AND METHODS
Materials.
SDS, Hepes, Mes, Trizma base, diisopropyl fluorophosphate (DFP), Triton X-100, Tween 20, EDTA, polyethylene glycol (Mr, 8,000), S Fast Flow, BSA, and rabbit brain cephalin were from Sigma. Mono-Q, Mono-S, and heparin-Sepharose were purchased from Pharmacia. Low molecular weight standards for PAGE were from Bio-Rad, and protein A-agarose was from Repligen. Spectrazyme Xa (MeO-CO-d-cyclohexylglycyl-Gly-Arg-pNA⋅AcOH) was from American Diagnostica (Greenwich, CT).
Plasma/Serum.
Factor X-deficient plasma was purchased from George King Biomedical (Overland Park, KS). To produce serum, fresh blood was allowed to clot for 1 hr at 37°C, the clot was rimmed, and serum was collected after centrifugation (10,000 × g, 20 min). Barium-adsorbed serum was produced by adding sodium oxalate (10 mM final) and two subsequent adsorptions with barium sulfate (100 mg/ml, 4°C, 30 min). By mAb sandwich immunoassay (8), the barium-adsorbed serum contained 0.10 μg/ml PZ. PZ-immunodepleted serum was made by passing serum over a column of anti-PZ mAb-Affigel-10 as described previously (8). PZ-immunodepleted serum contained <0.03 μg/ml PZ.
Proteins.
α1-Antitrypsin was purchased from Sigma, α2-antiplasmin and thrombin were from American Diagnostica, protein C inhibitor was from Enzyme Research Laboratories (South Bend, IN), and antithrombin III was from Chromogenix (Molndal, Sweden). α2-Macroglobulin was a gift from A. Schwartz (Washington University, St. Louis), and heparin cofactor II was a gift from D. Tollefsen (Washington University). Inter-α-trypsin inhibitor was purified as described previously (12). Prothrombin and factor X were purified and factor Xa was produced from purified factor X by using insolubilized X-coagulant protein from Russell’s viper venom as described previously (13). Additional factor Xa was purchased from Enzyme Research Laboratories.
PZ was isolated from citrated fresh-frozen plasma (Missouri–Illinois Regional Red Cross) by using a four-step purification procedure that included barium citrate adsorption–elution, ammonium sulfate fractionation, mAb anti-PZ immunoaffinity chromatography, and Mono-Q anion-exchange chromatography. Thrombin-cleaved PZ (PZT) was produced by incubating 1 mg/ml PZ with 300 units/ml thrombin in 0.1 M NaCl/0.05 M Tris⋅HCl, pH 8.0 for 3 hr at 37°C. The solution was treated with DFP (5 mM final) before removing the thrombin by passage through a small column of CG-50 in the same buffer (7). The N-terminal amino acid sequence of PZT (Mr ≈ 56,000) (7) after SDS/PAGE, transfer to a poly(vinylidene difluoride)-Plus membrane (Micron Separations, Westboro, MA), and gas-phase sequencing (Applied Biosystems) by the Protein Chemistry Laboratory (Washington University) is RYKGGSPXISQPXL (X = indeterminate). This amino acid sequence is identical to the sequence of PZ beginning at residue 44 of the mature protein. Thus, thrombin appears to cleave PZ after Arg-43, thereby separating the Gla-domain from the remainder of the molecule.
One-Stage, Factor Xa-Induced Coagulation Assay.
Fifty microliters of cephalin (75 μM), 50 μl of CaCl2 (25 mM), 50 μl of PZ or PZT (160 nM), and 50 μl of factor Xa (0.5 nM) were incubated at 37°C in the sample cup of a fibrometer (BBL). After 2 min, 50 μl of factor X-deficient plasma was added and the clotting time was measured. In certain experiments the PZ or factor Xa was added at various times during the preincubation period and the cephalin or PZ reagents were added to the reaction with the factor X-deficient plasma (100 μl of 1:1 mixtures). Apparent factor Xa activity is determined by comparing the clotting time with a standard curve constructed by using various concentrations of factor Xa in the absence of PZ.
Factor Xa Coagulation Assay.
Fifty microliters of cephalin (75 μM), 50 μl of CaCl2 (25 mM), and 50 μl of buffer containing 0.1 M NaCl, 0.05 M Hepes (pH 7.4), and BSA (1 mg/ml) (HSA) were incubated at 37°C. After 30 sec, 50 μl of the sample diluted in HSA with 1 mM EDTA was added, followed immediately by 50 μl of factor X-deficient plasma. Apparent factor Xa activity is determined by comparing the clotting time with a factor Xa standard curve.
Factor Xa Amidolytic Assay.
Mixtures (100 μl) containing various concentrations of cephalin, PZ, ZPI, factor Xa, and Ca2+ in HSA buffer were incubated at 22°C in the wells of a microtiter plate. After the specified period of time, 50 μl of Spectrazyme Xa (0.5 mM) was added and the initial rate of substrate cleavage (A405/min) was determined in a Vmax microtiter plate reader (Molecular Devices). In experiments studying the time course of factor Xa inhibition by PZ/ZPI, the solution containing the Spectrazyme Xa (0.5 mM) also contained 15 mM EDTA and 0.3 M Tris⋅HCl, pH 8.3. Factor Xa activity is determined by comparing the initial rate of substrate cleavage with a standard curve produced with various concentrations of factor Xa in the same buffer conditions.
Two-Stage Factor Xa Inhibition Assay.
To measure ZPI functional activity, 10 μl of cephalin (75 μM), 10 μl of CaCl2 (25 mM), 10 μl of PZ (200 nM), 10 μl of the sample to be tested diluted in HSA, and 10 μl of factor Xa (2.5 nM) were incubated in the sample cup of a fibrometer at 37°C. After 60 sec, 50 μl of HSA, 50 μl of cephalin (75 μM), 50 μl of CaCl2 (25 mM), and 50 μl of factor X-deficient plasma were added in succession and the clotting time was measured. ZPI activity is determined by comparing the clotting time with a standard curve produced by using various concentrations of purified ZPI. The activity of 1 μg purified ZPI was assigned arbitrarily a value of 1 unit.
Purification of ZPI.
Human citrated fresh-frozen plasma (2.3 liters) from the Missouri–Illinois Regional Red Cross was thawed at 37°C and transferred to the cold room.
Barium citrate adsorption and ammonium sulfate fractionation (4°C).
Two hundred and thirty milliliters of 1.0 M BaCl2 was added dropwise over 45 min, and the mixture was stirred an additional 15 min. The barium citrate precipitate was removed by centrifugation at 3,000 × g for 20 min and supernatant plasma was collected. Ammonium sulfate was added to 45% saturation and the mixture was stirred for 30 min before the precipitate was removed by centrifugation at 10,000 × g for 20 min. Sufficient ammonium sulfate was added to bring the supernatant to 75% saturation and the mixture was stirred for 30 min before centrifugation at 10,000 × g for 20 min. The protein precipitate was dissolved in 0.1 M NaCl/0.05 M Tris⋅HCl, pH 7.5, treated with DFP (1 mM), and dialyzed overnight against the same buffer.
Polyethylene glycol (PEG) fractionation (22°C).
Sufficient 50% (wt/vol) PEG (Mr, 8,000) was added dropwise to the sample to produce a PEG concentration of 7.5% and the mixture was stirred for 30 min before the precipitate was removed by centrifugation at 10,000 × g for 20 min. PEG (50% wt/vol) was added dropwise to the supernatant solution to produce a PEG concentration of 18.5%, and the mixture was stirred for 30 min before centrifugation at 10,000 × g for 20 min. The protein precipitate was dissolved in 0.1 M NaCl/0.020 M Mes, pH 6.15, and treated with DFP (1 mM).
S Fast Flow cation-exchange chromatography (4°C).
The sample was applied at flow rate of 150 ml/hr to a 5 × 47-cm column of S Fast Flow equilibrated in 0.1 M NaCl/0.02 M Mes, pH 6.15. The column was washed with 1.5 liters of the same buffer and then eluted with a linear gradient to 0.5 M NaCl/0.02 M Mes, pH 6.15, over 8 liters. Fractions containing ZPI activity, which eluted at ≈0.25 M NaCl, were combined and the pool was concentrated to 25 ml (YM 10, Amicon) and treated with DFP (5 mM).
Mono-Q anion-exchange chromatography (22°C).
The concentrated sample was diluted 2.5-fold with 0.02 M Mes, pH 6.15, and applied at a flow rate of 1.5 ml/min to a 10-ml Mono-Q column equilibrated in 0.1 M NaCl/0.02 M Mes, pH 6.15, containing 0.1% Tween-20. The column was washed with 15 ml of the same buffer and then eluted with a linear gradient to 0.5 M NaCl in the same buffer over 100 ml. Fractions containing ZPI activity, which eluted at ≈0.18 M NaCl, were combined and treated with DFP (5 mM).
Heparin-Sepharose affinity chromatography (22°C).
The sample was diluted 2-fold with 0.02 M Mes, pH 6.15, and applied at a flow rate of 1 ml/min to a 5-ml heparin-Sepharose column equilibrated in 0.1 M NaCl/0.02 M Mes, pH 6.15, containing 0.1% Tween-20. The column was washed with 10 ml of the same buffer and then eluted with a linear gradient to 0.6 M NaCl in the same buffer over 50 ml. Fractions containing ZPI activity, which eluted at ≈0.25 M NaCl, were pooled and treated with DFP (5 mM).
Mono-S cation-exchange chromatography (22°C).
The sample was diluted 3-fold with 0.02 M Mes, pH 6.15, and applied at a flow rate of 0.5 ml/min to a 1-ml Mono-S column equilibrated in 0.1 M NaCl/0.02 M Mes, pH 6.15, containing 0.01% Tween-20. The column was washed with 2 ml of the same buffer and then eluted with a linear gradient to 0.5 M NaCl in the same buffer over 20 ml. Fractions containing ZPI activity, which eluted at ≈0.25 M NaCl, were pooled, and the purified ZPI was stored at −70°C in small aliquots. The molar concentration of ZPI was estimated assuming a ZPI concentration of 1.0 mg/ml produces an absorbance at 280 nm (A280) of 1.0 and that the molecular weight is 72,000.
Other Methods.
SDS/PAGE was performed by using the method of Laemmli (14). Rabbit polyclonal anti-ZPI antibodies were developed as described previously by using purified ZPI as immunogen (15). Preimmune and immune IgG were isolated by using protein A-agarose. N-terminal amino acid sequence analysis of purified ZPI was performed by the Protein Chemistry Laboratory (Washington University) by using a gas-phase sequenator (Applied Biosystems). Two separate analyses were performed with 0.3 nmol of ZPI and gave identical results. The phospholipid content of the rabbit brain cephalin was determined as inorganic phosphate (16).
RESULTS
Reduction in Factor Xa Procoagulant Activity in the Presence of PZ, Ca2+, and Phospholipids.
Initial studies investigating the potential function of human PZ showed that the apparent procoagulant activity of factor Xa in a one-stage plasma coagulation assay was reduced when the factor Xa was first incubated with PZ (Table 1). The inhibitory effect was time-dependent, required the presence of calcium ions and procoagulant phospholipids (rabbit brain cephalin), and appeared predominantly related to the period of preincubation of PZ with phospholipids (Table 1). Thrombin treatment of PZ, which cleaves PZ at Arg-43 and separates the Gla domain from the remainder of the molecule (see Materials and Methods), abolished the inhibitory effect. These results suggest that an interaction between factor Xa and PZ may occur at the phospholipid surface. Consistent with this notion, the rate of inhibition of factor Xa by antithrombin III was slowed by PZ in the presence of cephalin and Ca2+ (t1/2 = 35 min vs. 15 min) (Fig. 1).
Table 1.
Apparent inhibition of factor Xa produced by incubation with PZ, cephalin and calcium ions before one-stage assay
| Incubation period, sec
|
Apparent inhibition, % | |
|---|---|---|
| Factor Xa | PZ | |
| 120 | 0 | 0 |
| 120 | 15 | 50 |
| 120 | 30 | 61 |
| 120 | 60 | 72 |
| 120 | 90 | 76 |
| 120 | 120 | 78 |
| 120 | PZT 120 | 0 |
| 15 | 120 | 70 |
| 30 | 120 | 71 |
| 60 | 120 | 73 |
| 90 | 120 | 76 |
| 120 | 120 | 78 |
| 120 | 120 − cephalin | 0 |
| 120 | 120 − Ca2+ | 0 |
PZT refers to thrombin-treated PZ (see Materials and Methods).
Figure 1.
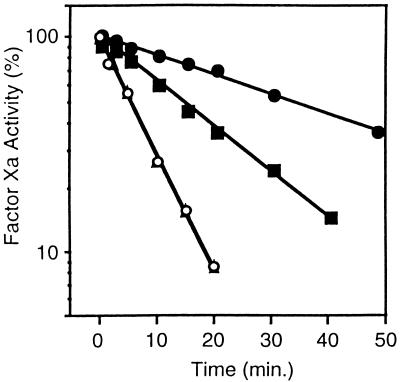
Effect of PZ on the inhibition of factor Xa by antithrombin III. Reaction mixtures containing factor Xa (5 nM), CaCl2 (4 mM), with or without PZ (40 nM), and with or without cephalin (15 μM) were incubated 5 min at 22°C before the addition of antithrombin III (3.4 μM). At the indicated times thereafter, samples were removed, diluted in HSA with 1 mM EDTA, and assayed for factor Xa activity by coagulation assay. (▴), Without cephalin, without PZ; (○), without cephalin, with PZ; (■), with cephalin, without PZ; (•), with cephalin, with PZ.
To further evaluate the effect of PZ on factor Xa inactivation, the time course of the loss of factor Xa (5 nM) coagulant activity in PZ immunodepleted serum (25% vol/vol) with and without added PZ (40 nM) was determined in the presence of cephalin (15 μM) and CaCl2 (4 mM). The rate of factor Xa inhibition in serum was enhanced substantially in the presence of PZ (not shown, see below), suggesting that serum contains a PZ-dependent factor Xa inhibitor(s). In systems containing purified proteins, PZ does not enhance the inhibition of factor Xa by α1-antitrypsin, protein C inhibitor, α2-antiplasmin, heparin cofactor II, inter-α-trypsin inhibitor, or α2-macroglobulin (not shown).
Isolation of ZPI.
A two-stage coagulation assay designed to measure PZ-dependent factor Xa inhibition was used to isolate a ZPI from plasma (see Materials and Methods and Table 2). The ZPI activity of the starting plasma could not be measured because of thrombin generation and fibrin formation during the first stage of the two-stage factor Xa inhibition assay. Nevertheless, assuming a 50–75% recovery of ZPI after ammonium sulfate fractionation of plasma (Table 2), we estimate the plasma concentration of ZPI to be 1.0–1.6 μg/ml (14–22 nM).
Table 2.
ZPI purification
| Step | Volume, ml | Protein, mg | Activity, units | Specific activity, units/mg | Purification, -fold | Yield, % |
|---|---|---|---|---|---|---|
| Plasma | 2,300 | 144,740 | —* | — | — | — |
| Barium adsorption, (NH4)2SO4 fractionation | 1,430 | 59,730 | 1,820 | 0.031 | 1.0 | 100 |
| PEG fractionation | 257 | 25,950 | 1,720 | 0.066 | 2.2 | 94 |
| S-Fast Flow, concentration | 25 | 191 | 1,365 | 7.15 | 231 | 75 |
| Mono-Q | 10 | 50.2 | 1,056 | 21.0 | 677 | 58 |
| Heparin-Sepharose | 5.8 | 1.32 | 837 | 634 | 20,450 | 46 |
| Mono-S | 1.9 | 0.59 | 590 | 1,000 | 32,800 | 32 |
Protein was determined assuming A280 1.0 = 1.0 mg/ml. Activity is expressed in abitrary units with 1.0 unit = 1 μg of purified ZPI.
Activity of plasma could not be determined because of thrombin generation in first stage of functional assay.
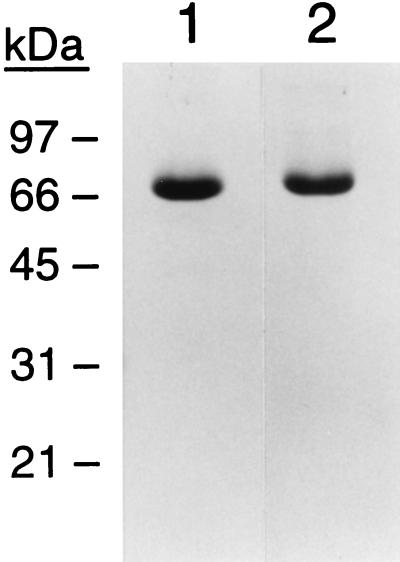
By SDS/PAGE analysis, the isolated ZPI appears >90% pure and migrates as a single-chain protein with an apparent molecular mass of 72 kDa (Fig. 2). Preliminary characterization of the purified protein shows that ZPI activity is abolished by treatment with SDS (1%), urea (8 M), and 2-mercaptoethanol (5%, vol/vol) but is stable in Tween-20 (2%) and Triton X-100 (2%). ZPI is also unaffected by methylamine treatment under conditions that completely inactivate α2-macroglobulin. The N-terminal amino acid sequence of ZPI is LAPSPQSPEXXA. This sequence does not match or show significant homology with the sequences accessible in publicly available protein or DNA databases. Thus, ZPI may represent a previously unidentified gene product.
Figure 2.
SDS/PAGE of purified ZPI. ZPI (5 μg) was analyzed with (lane 2) or without (lane 1) reduction with 5% 2-mercaptoethanol. Protein was stained with Coomassie brilliant blue. The position of molecular mass standards in kDa is shown on the left.
PZ-Dependent Inhibition of Factor Xa by ZPI.
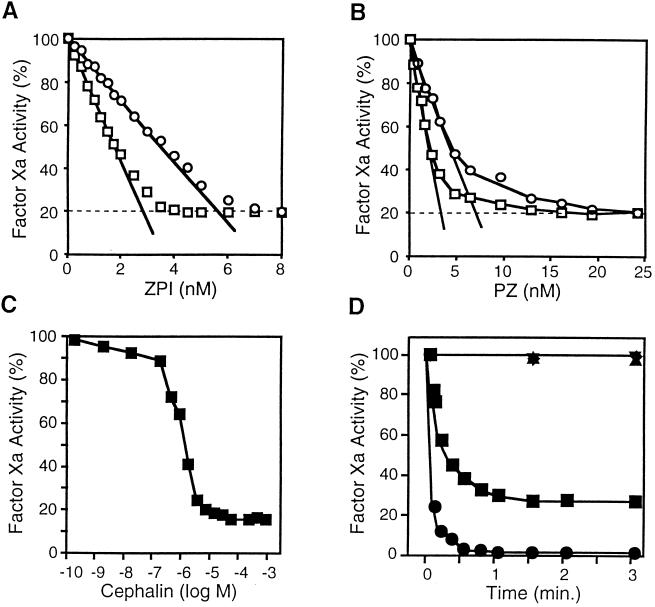
To further investigate the factor Xa–ZPI interaction, mixtures containing factor Xa, CaCl2, cephalin, and PZ were incubated with increasing concentrations of ZPI for 15 min (22°C) (Fig. 3A). The remaining factor Xa activity then was determined in an amidolytic assay (Materials and Methods) after the addition of Spectrazyme Xa. The results suggest a high-affinity interaction between ZPI and factor Xa, but a significant amount (≈20%) of factor Xa amidolytic activity persists even at relatively high concentrations of ZPI. Maximal ZPI-mediated factor Xa inhibition occurs at an apparent ZPI/factor Xa stoichiometry of 1.2:1. The PZ dose/response of ZPI-mediated factor Xa inhibition was evaluated in similar reactions (Fig. 3B). Again an apparent stoichiometric relationship between PZ and factor Xa was demonstrated with a molar ratio of 1.4:1 (PZ/factor Xa). Optimal PZ-dependent inhibition of factor Xa by ZPI occurs at concentration of mixed rabbit brain phospholipids (cephalin) of ≥15 μM (Fig. 3C). The inhibition of factor Xa produced by ZPI is rapid after the incubation of factor Xa with PZ, Ca2+, and cephalin (Fig. 3D). Maximal factor Xa inhibition as assessed by amidolytic assay (70%) and coagulation assay (97%) is reached within <1 min. When the amidolytic assay (Fig. 3D) or coagulation assay (not shown) is used, no factor Xa inhibition occurs if PZ, phospholipids, or Ca2+ (1 mM EDTA) is omitted from the reactions. The inhibitory process does not appear to involve a covalent interaction between the proteins, as a complex of factor Xa and ZPI (or PZ) is not demonstrable by SDS/PAGE (not shown).
Figure 3.
PZ-dependent inhibition of factor Xa by ZPI. (A) ZPI dose/response. Reaction mixtures containing factor Xa (2.5 or 5.0 nM), CaCl2 (4 mM), cephalin (15 μM), and PZ (40 nM) were incubated with increasing concentrations of ZPI for 15 min at 22°C before remaining factor Xa activity was determined by amidolytic assay. The molar concentration of ZPI was estimated assuming 1.0 mg/ml ZPI produces an A280 of 1.0. (□), Factor Xa, 2.5 nM; (○), factor Xa, 5.0 nM. (B) PZ dose/response. Reaction mixtures containing factor Xa (2.5 or 5.0 nM), CaCl2 (4 mM), cephalin (15 μM), and ZPI (10 nM) were incubated with increasing concentrations of PZ for 15 min at 22°C before remaining factor Xa activity was determined by amidolytic assay. (□), Factor Xa, 2.5 nM; (○), factor Xa, 5.0 nM. (C) Cephalin dose/response. Reaction mixtures containing factor Xa (2.5 nM), CaCl2 (4 mM), PZ (40 nM), and ZPI (10 nM) were incubated with increasing concentrations of cephalin for 15 min at 22°C before remaining factor Xa activity was determined by amidolytic assay. (D) Time course of factor Xa inhibition by PZ/ZPI. Reaction mixtures containing factor Xa (5.0 nM), with or without CaCl2 (4 mM), with or without cephalin (15 μM), and with or without PZ (40 nM) were incubated 5 min at 22°C before the addition of ZPI (10 nM). At the specified times thereafter remaining factor Xa activity was determined by coagulation assay or amidolytic assay. Coagulation assay: (•), with all reactants. Amidolytic assay: (■), with all reactants; (▾), without CaCl2; (▴), without cephalin; (⧫), without PZ.
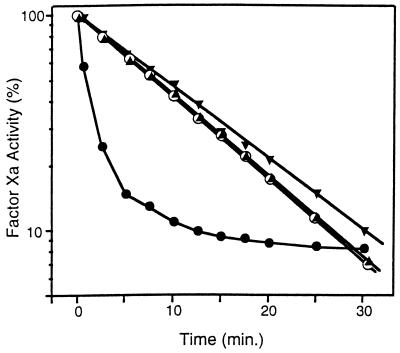
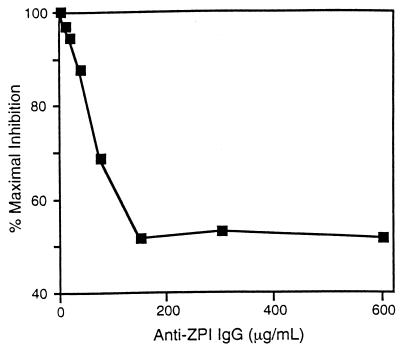
The effect of PZ and ZPI on the inactivation of factor Xa in serum is shown in Fig. 4. In the presence of Ca2+ (4 mM) and phospholipids (15 μM), the half-life of factor Xa (5 nM) activity in barium sulfate-adsorbed, PZ-deficient serum (25%, vol/vol) is 9 min. The initial rate of factor Xa inhibition is enhanced by the addition of PZ (40 nM), with less than 50% of the initial factor Xa activity remaining at 1 min. Factor Xa inhibition produced by serum in the presence of PZ, however, appears to reach a plateau such that, at 30 min and later time points (not shown), the residual factor Xa activity is greater in the reaction mixture containing PZ than in the reaction mixture not supplemented with PZ (see Discussion). The increased rate of factor Xa inhibition produced by serum in the presence of PZ depends on ZPI and is abrogated completely by treatment of the serum with anti-ZPI antibodies (Fig. 4). In contrast, the decrease in apparent factor Xa activity produced by its incubation with PZ, phospholipids, and Ca2+ before the addition of substrate plasma in the one-stage, factor Xa-induced coagulation assay was reduced by only ≈50% when the plasma was treated with anti-ZPI antibodies (Fig. 5).
Figure 4.
Effect of PZ and anti-ZPI antibodies on factor Xa inhibition in serum. Factor Xa (5 nM), CaCl2 (4 mM), and cephalin (15 μM) with or without PZ (40 nM) were incubated 5 min at 22°C before the addition of barium-adsorbed serum (25%, vol/vol), which had been treated previously for 30 min with rabbit preimmune or immune anti-ZPI IgG (300 μg/ml). At the specified times thereafter, samples of the reaction mixtures were diluted in HSA with 1 mM EDTA and assayed for factor Xa activity by coagulation assay. (•), With PZ and preimmune IgG; (▾), with PZ and anti-ZPI IgG; (○), without PZ and with preimmune IgG; (▴), without PZ and with anti-ZPI IgG.
Figure 5.
Effect of anti-ZPI antibodies on factor Xa-induced coagulation of plasma. Reaction mixtures (200 μl) containing factor Xa (0.125 nM), CaCl2 (5 mM), and cephalin (18.75 μM), with or without PZ (50 nM), were incubated in the sample cup of a fibrometer. After 2 min at 37°C, 50 μl of factor X-deficient plasma that had been treated with rabbit preimmune IgG (600 μg/ml) or various concentrations of immune anti-ZPI IgG for 30 min was added and the clotting time was measured. Apparent factor Xa inhibition (76%) produced by the inclusion of PZ during the preincubation period and using plasma treated with preimmune IgG is listed as maximal PZ-dependent inhibition (100% on the ordinate). The concentration of anti-ZPI IgG used to treat the plasma is listed on the abscissa. Note that the range of “% Maximal Inhibition” listed on the ordinate is plotted from 40 to 100.
DISCUSSION
Despite the isolation of PZ several years ago, its physiologic function has remained uncertain. The experiments presented here show that PZ slows the inhibition of factor Xa by antithrombin III in the presence of phospholipids and Ca2+, but also plays an important role in the inhibition of factor Xa by another plasma protein that we have termed protein Z-dependent protease inhibitor, ZPI. PZ and/or its apparent interaction with factor Xa, however, may serve other functions. In this regard, it is important to note that treatment of the substrate plasma in the one-stage, factor Xa-induced coagulation assay with anti-ZPI antibodies reduced the apparent inhibitory effect of the preincubation of PZ with phospholipids, Ca2+, and factor Xa by only ≈50% (Fig. 5). The remaining anticoagulant effect of PZ could be related to its interference with the binding of other proteins to phospholipids (e.g., prothrombin), the slow dissociation of factor Xa from a putative factor Xa–PZ–phospholipid-Ca2+ complex, or the presence of additional PZ-dependent coagulation inhibitors in plasma. A similar ZPI-independent effect of PZ was not detected in the studies of the factor Xa inhibition in serum (Fig. 4) in which samples were diluted and calcium ions chelated (EDTA) before factor Xa functional assay. The relatively slow association of PZ with phospholipids (11) is consistent with the time-dependent inhibitory effect of PZ during its incubation with phospholipids and Ca2+ in the one-stage assay, factor Xa-induced coagulation assay, and presumably also explains the absence of clotting time prolongation when PZ is added instead with the substrate plasma to the reaction (Table 1).
The inhibition of factor Xa by presumed physiologic concentrations of ZPI requires the presence of phospholipids, Ca2+, and PZ and appears to involve a stoichiometric complex of factor Xa, PZ, and ZPI at the phospholipid surface. The extent of factor Xa inhibition produced by ZPI, however, is considerably less when remaining factor Xa activity is measured by using a low molecular weight chromogenic substrate (Spectrazyme Xa) than when remaining factor Xa activity is measured by coagulation assay. The cause of this discrepancy is not clear. One explanation could be the presence in the factor Xa preparations of degraded forms of factor Xa that retain activity against the chromogenic substrate but do not bind phospholipid and thus are not inhibited by ZPI and lack procoagulant activity. The disparity between the inhibitory effect measured by amidolytic assay and coagulation assay, however, was seen consistently with several factor Xa preparations whose amidolytic activity was bound by barium sulfate (>97%) and that, by SDS/PAGE analysis, contained various proportions of the α and β forms of factor Xa (α:β = 1:1 to 1:4). Moreover, the chromogenic activity of the factor Xa preparations was inhibited >99% by tissue factor pathway inhibitor, suggesting that the residual amidolytic activity remaining after the interaction of factor Xa with ZPI is not a result of a contaminating protease (data not shown).
Preliminary studies show that PZ/ZPI does not inhibit factor VIIa, factor IXa, activated protein C, or thrombin. The inhibitory specificity of PZ/ZPI, however, has not been fully explored, and it is conceivable that PZ/ZPI or ZPI alone may regulate additional proteolytic enzymes, besides factor Xa, that are involved in coagulation, fibrinolysis, or the inflammatory response.
Although PZ dramatically enhances the inhibition of factor Xa produced by ZPI, the overall effect of PZ on coagulation remains to be determined. PZ with Ca2+ and procoagulant phospholipids also dampens the inhibition of factor Xa by antithrombin III (Fig. 1), and the PZ-dependent inhibition of factor Xa produced by ZPI is incomplete. Inhibition of factor Xa in serum initially is enhanced by the presence of PZ, but it reaches a limiting plateau of persistent factor Xa activity (Fig. 4). It is conceivable that this reservoir of factor Xa may play a role in hemostasis. Ongoing studies investigating the phenotype of PZ gene-deleted mice (Z. F. Yin and G.J.B., unpublished data) may provide further insight into the physiological function of PZ.
Acknowledgments
We thank Drs. Alan Schwartz and Douglas Tollefsen for providing purified α2-macroglobulin and heparin cofactor II, respectively, and Ms. Terri Lewis for preparing this manuscript. This research was supported in part by a grant from the National Institutes of Health (HL34462) and by a grant from the Monsanto Chemical Corporation of St. Louis.
ABBREVIATIONS
- PZ
human protein Z
- ZPI
PZ-dependent protease inhibitor
- DFP
diisopropyl fluorophosphate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Stenflo J, Fernlund P, Egan W, Roepstorff P. Proc Natl Acad Sci USA. 1974;71:2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelsestuen G L, Zytkovicz T H, Howard J B. J Biol Chem. 1974;249:6347–6350. [PubMed] [Google Scholar]
- 3.Esmon C T, Suttie J W, Jackson C M. J Biol Chem. 1975;250:4094–4099. [PubMed] [Google Scholar]
- 4.Prowse C V, Esnouf M P. Biochem Soc Trans. 1977;5:255–256. doi: 10.1042/bst0050255. [DOI] [PubMed] [Google Scholar]
- 5.Mattock P, Esnouf M P. Nat New Biol. 1973;242:90–92. doi: 10.1038/newbio242090a0. [DOI] [PubMed] [Google Scholar]
- 6.Petersen T E, Thogersen H C, Sottrup-Jensen L, Magnusson S, Jornvall H. FEBS Lett. 1980;114:278–282. doi: 10.1016/0014-5793(80)81133-1. [DOI] [PubMed] [Google Scholar]
- 7.Broze G J, Jr, Miletich J P. J Clin Invest. 1984;73:933–938. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miletich J P, Broze G J., Jr Blood. 1987;69:1580–1586. [PubMed] [Google Scholar]
- 9.Sejima H, Hayashi T, Deyashiki Y, Nishioka J, Suzuki K. Biochem Biophys Res Commun. 1990;171:661–668. doi: 10.1016/0006-291x(90)91197-z. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose A, Takeya H, Espling E, Iwanaga S, Kisiel W, Davie E W. Biochem Biophys Res Commun. 1990;172:1139–1144. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 11.McDonald J F, Shah A M, Schwalbe R A, Kisiel W, Dahlback B, Nelsestuen G L. Biochemistry. 1997;36:5120–5127. doi: 10.1021/bi9626160. [DOI] [PubMed] [Google Scholar]
- 12.Pratt C W, Pizzo S V. Biochemistry. 1987;26:2855–2863. doi: 10.1021/bi00384a029. [DOI] [PubMed] [Google Scholar]
- 13.Broze G J, Jr, Warren L A, Novotny W F, Higuchi D A, Girard J J, Miletich J P. Blood. 1988;71:335–343. [PubMed] [Google Scholar]
- 14.Laemmli M K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Vaitukaitis J L. Methods Enzymol. 1981;73:46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- 16.Ames B W, Dubin D T. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]