Abstract
African–American men have a higher dietary intake of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), which is the most abundant heterocyclic amine in cooked meats and is carcinogenic in rat prostate through the formation of DNA adducts. To determine the clinical and demographic factors associated with PhIP-DNA adduct levels, the biologically effective dose of PhIP in human prostate, we immunohistochemically measured PhIP-DNA adducts in a study of 162 Caucasian and 102 African–American men who underwent radical prostatectomy for prostate cancer. A strong correlation between PhIP-DNA adduct levels in prostate tumor and adjacent non-tumor cells was observed (ρ = 0.62; p < 0.0001); however, non-tumor cells had significantly higher adduct levels compared with tumor (0.167 optical density (OD) units ± 0.043 vs. 0.104 OD ± 0.027; p < 0.0001). Race was not associated with PhIP-DNA adduct levels in either tumor or non-tumor cells, but race-specific associations were observed. In prostate tumor and non-tumor cells, tumor volume had the strongest association with PhIP-DNA adducts in Caucasians, whereas in African–Americans prostate volume was most strongly associated with adduct levels in tumor cells and advanced Gleason grade had the strongest association in non-tumor cells. In race interaction models, while the only statistically significant interaction was between African–American race and advanced Gleason grade in non-tumor cells (β = 0.029; p = 0.02), in tumor cells we observed opposite effects by race (positive for African–Americans, negative for Caucasians) for older age and high PSA levels at diagnosis. In conclusion, while PhIP-DNA adduct levels in prostate cells do not vary significantly by race, our results suggest that PhIP exposure may have stronger effects on prostate tumor differentiation in African–American men.
Keywords: 2-amino-1-methyl-6-phenylimidazo(45-b)pyridine, African–Americans, immunohistochemistry, neoplasms, DNA damage
Dietary sources of potential prostate carcinogens include fat and meat.1 One possible link between a diet high in meat consumption and prostate cancer is the cooking of meat at high temperatures and the subsequent formation of heterocyclic amines (HA), which are potent carcinogens in animals.2,3 A direct correlation between HA exposure and DNA adduct formation in the prostate is supported by animal studies and in vitro studies of human tissues. Rats given food-derived 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), the most abundant HA in human diets, for 52 weeks had PhIP-DNA adducts in all prostate lobes and subsequently developed prostate cancer.3 The tumorigenic potential of PhIP-laden diets in rats has also been shown by significantly elevated mutation frequencies in all prostate lobes after only 4 weeks4 and a significant prostate tumor incidence within 20 weeks.5 Nude mice administered an intragastric injection of PhIP showed positive staining for PhIP-DNA adducts in 70–95% of both epithelial and stromal cells in human prostate xenografts.6 Several in vitro studies of human prostatic tissue incubated in HA laden milieu have demonstrated detectable PhIP-DNA adducts in prostate cells afterwards,7-9 but one study that examined human prostate specimens that were not experimentally exposed to PhIP found only 2 of 24 specimens had detectable PhIP-DNA adducts.10 In vitro experiments of human prostate epithelial cells have shown that increased doses of PhIP result in increased DNA damage as measured by the comet assay.11
Metabolic activation of PhIP, which is necessary for DNA adduct formation, is thought to occur primarily in the liver via a two-step process in which N-oxidation of the PhIP compound is catalyzed by cytochrome P4501A2 (CYP1A2). Subsequent acetylation or sulfation is catalyzed by acetyltransferases (NAT) or sulfotransferases (SULT), respectively, which generate N-acetoxy or N-sulfonyloxy esters, electrophiles that are much more reactive with DNA.12,13 Recent studies have shown that this two-step bioactivation of PhIP may also occur in the prostate. PhIP-DNA adducts formed at levels 30–600 times higher when human prostate tissues were incubated with N-hydroxy PhIP compared with PhIP.8 Several studies have demonstrated expression of CYP1A2 in prostate cells8,14,15 (previously thought to be confined to the liver), which supports the O-acetylation step of PhIP also occurring in the prostate. In addition, Nelson et al. showed that loss of expression of an important gatekeeper in the prostate carcinogenic pathway, GSTP1, enhances susceptibility to carcinogenic insult by N-OH-PhIP in prostate.9
The U.S. population lifetime time-weighted average of total HA consumed has been estimated to be ~9 ng/kg/day, with PhIP comprising about two thirds of this intake.16 Mean HA intakes are greatest for African–American males, who were estimated to consume ~2- to 3-fold more PhIP than their white male counterparts.16 Biomarker studies support the findings from dietary questionnaires in that African–American men have been shown to excrete a higher level of HA metabolites.17,18 In addition to having a greater HA exposure, African–American men may be at greater biologic risk due to higher activity levels of the human sulfotransferase 1A1 (SULT1A1) enzyme that is involved in the bioactivation of N-hydroxy metabolite of PhIP.13 Although race-specific prostate cancer risk associated with SULT1A genotypes are comparable, African–American men with the highest human SULT1A1 activity levels are at a 2-fold greater risk for prostate cancer compared with Whites at comparable SULT1A1 activity levels,19 which suggests that additional factors interacting with SULT1A1 may increase prostate cancer risk in African–Americans.
Despite evidence that PhIP-DNA adducts may play an important role in prostate carcinogenesis, population level studies of PhIP-DNA adducts in the prostate do not exist. Such studies are necessary to determine whether HA intake results in a biologically effective dose measurable in the prostate and whether PhIP-DNA adducts might explain racial differences in prostate cancer risk and outcomes. In the present study, we describe the distribution of PhIP-DNA adduct levels in tumor and non-tumor prostate cells of prostate cancer cases who underwent radical prostatectomy, investigate potential associations between clinical and histologic characteristics and PhIP-DNA adduct levels, and test whether race is a modifying factor for these associations.
Material and methods
Study Sample
The study population consisted of men who were part of the Henry Ford Health System. Details concerning the ascertainment and recruitment of study cases can be found in a previous publication.20 The present study includes 264 men who underwent radical prostatectomy for prostate cancer. Slides were cut from a specimen block that contained both tumor and non-tumor cells and subject to immunohistochemical studies for PhIP-DNA adduct determination. The analytic sample was 39% African–American and had a mean age at diagnosis of 60.9 ± 6.9 years with an average of 3 ± 2.5 months between diagnosis and surgery.
Pathology
Hematoxylin-eosin stained slides of study cases were reviewed by the study uropathologist (ATS) to confirm the diagnosis and identify a paraffin block with sufficient tumor and non-tumor prostatic tissue for staining. Using a microtome, 5 consecutive sections (5 μm thick) were cut from the tissue block of each patient sample. One slide was hematoxylin and eosin stained and examined by the study uropathologist who circled separate areas of tumor and non-tumor cell populations to be used for adduct scoring. Tumors were given a TNM staging score and characterized by histologic grade (i.e., primary and secondary Gleason scores), lobe involvement, volume, resection margins, vascular invasion, perineural invasion, extraprostatic extension, and seminal vesicle and lymph node involvement. Prostate volumes were also calculated based on the dimensions of the gland measured after surgical removal.
Immunohistochemistry
Immunohistochemistry studies were performed as described by Takahashi et al.21 and Zhu et al.22,23 Briefly, the paraffin-embedded sections were baked at 59°C for 1 hr, deparaffinized in xylene, and rehydrated in serial alcohol. Endogenous peroxidase activity was blocked using 0.3% H2O2 in methanol for 20 min. After treatment using RNase and proteinase K, the sections were blocked using 3% BSA and normal goat serum. The primary anti-PhIP-DNA adduct polyclonal antibody was provided by Dr. Shirai at Nagoya City University Medical School, Nagoya, Japan.3 The polyclonal antibody was incubated with the sections at 4°C overnight in a humid chamber at a dilution of 1:750. The biotinylated secondary antibody was incubated with the sections at room temperature for 30 min, at a dilution of 1:200. The antibody complex was detected using an avidin-biotin-peroxidase complex solution and visualized using 3,3′-diaminobenzidine (Zymed Laboratories, San Francisco, CA). The staining specificity was confirmed with positive control samples that were run with every experimental batch using the primary antibody preabsorbed with 2 or 20 μg/mL DNA extract from MCF-7 cells and treated with 150 μM N-hydroxy-PhIP. A cytospin sample of MCF-7 cells without PhIP treatment served as a negative exposure control. In addition, slides of prostate tumor from a non-study subject were inserted into each batch of staining in which the primary anti-PhIP-DNA adduct polyclonal antibody was omitted (negative control) and the specimen was treated with both antibodies (positive control). The scores of the positive controls also served as calibration references between staining batches. Staining was quantified by absorbance image analysis in optical density (OD) units using a Cell Analysis System 200 microscope as described previously.24
Statistical analyses
PhIP-DNA adduct levels in prostatic epithelial tumor and non-tumor were tested for normality using the Shapiro-Wilk statistic. Paired t-tests were used to discern whether the difference in adduct levels between tumor and non-tumor cells deviated significantly from zero. Multiple linear regression models were used to calculate group specific means and standard errors and test for associations between predictor and outcome variables. Potential batch effects in the PhIP-DNA adduct assay were taken into account by computing a batch correction factor that was the difference between the adduct level of the positive control slide in a single batch and the mean adduct level of the positive control slides across all batches. The batch-adjusted adduct level was the crude adduct level minus the batch correction factor. This approach was used in our previous studies involving PAH-DNA adducts.24 Statistical significance was assessed at the Type-I error level of 0.05 and all tests were two-sided.
Results
The distributions of PhIP-DNA adduct levels in 264 paired tumor and non-tumor prostate specimens are shown in Figure 1. Both the tumor and non-tumor PhIP-DNA adduct distributions were highly symmetrical based on a Shapiro-Wilk goodness-of-fit statistic. PhIP-DNA adduct levels in tumor and non-tumor prostate cells fell into 2 separate normal distributions with the mean level of adducts in non-tumor cells 0.063 OD units significantly higher than that in tumor cells (O.167 ± 0.043 OD vs. 0.104 ± 0.027 OD; p < 0.0001). A strong correlation between adduct levels in tumor and non-tumor cells was observed (ρ = 0.62; p < 0.0001) with the absolute difference in adduct levels between the 2 cell types constant across all levels based on a slope of one from a linear regression model (Fig. 2).
FIGURE 1.
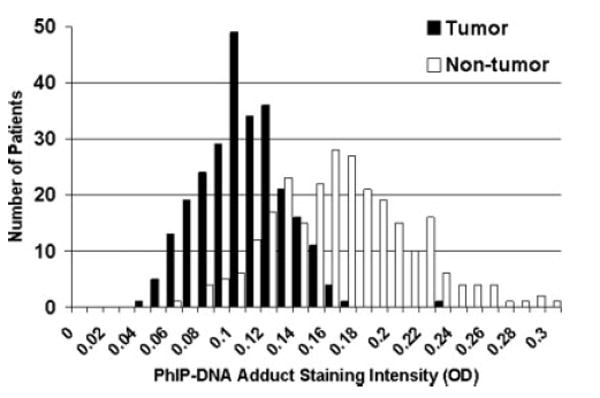
PhIP-DNA adduct staining intensity frequency distribution in 264 prostate cancer patients based on optical density scores in tumor and adjacent non-tumor prostate cells.
FIGURE 2.
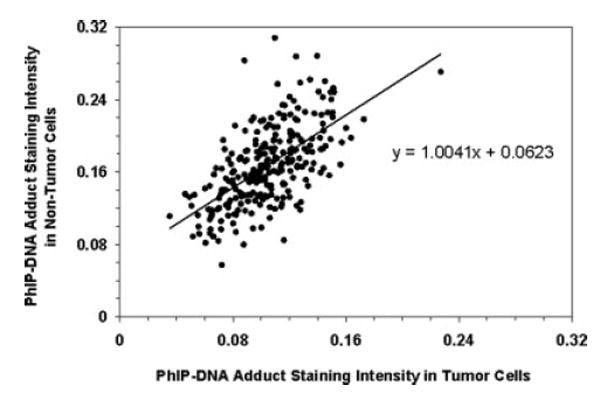
Correlation between PhIP-DNA adduct optical density scores in tumor and adjacent non-tumor prostate cells in 264 prostate cancer patients.
Table I depicts the mean PhIP-DNA adduct levels in prostate tumor cells for all subjects and within each racial group. In all subjects, PhIP-DNA adduct levels were higher in tumors that involved less than 20% of the prostate and in smaller prostates. Examining these 2 factors together, the mean adduct level in smaller prostates with low tumor volume was 23% higher than the mean adduct level in larger prostates with high tumor volume (0.116 vs. 0.094 OD). While the direction of these associations was consistent between Caucasians and African–Americans, a statistically stronger association with adduct levels and tumor volume was observed in Caucasians whereas prostate volume had a greater statistical association with adduct level in African–Americans. A weak (p = 0.1) positive association between advanced Gleason grade and adduct level was also observed exclusively in African–Americans. In non-tumor cells (Table II), tumor volume was inversely associated with PhIP-DNA adduct level in Caucasians with a weaker, but similar, association observed in African–Americans. In tumor cells, prostate volume was not significantly associated with adduct level in either race, and only in African–Americans was an association between advanced Gleason grade and adduct level observed (p = 0.005).
TABLE I.
MEAN PhIP-DNA ADDUCT LEVELS IN PROSTATE TUMOR CELLS EXPRESSED AS ABSORBANCE UNITS FOR ALL SUBJECTS AND STRATIFIED BY RACE
| All subjects (n = 264) | Caucasians (n = 162) | African–Americans (n = 102) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | p value | n | Mean ± SE | p value | n | Mean ± SE | p value | |
| Age at prostatectomy | |||||||||
| <60 | 114 | 0.105 ± 0.003 | 0.91 | 66 | 0.105 ± 0.003 | 0.36 | 48 | 0.103 ± 0.004 | 0.34 |
| 60+ | 150 | 0.104 ± 0.002 | 96 | 0.102 ± 0.003 | 54 | 0.109 ± 0.004 | |||
| PSA at diagnosis | |||||||||
| Low (<4 ng/mL) | 45 | 0.105 ± 0.004 | 0.88 | 34 | 0.106 ± 0.004 | 0.38 | 11 | 0.104 ± 0.008 | 0.53 |
| Moderate (4–10 ng/mL) | 176 | 0.105 ± 0.002 | 98 | 0.104 ± 0.003 | 78 | 0.105 ± 0.003 | |||
| High (>10 ng/mL) | 43 | 0.103 ± 0.004 | 30 | 0.097 ± 0.005 | 13 | 0.114 ± 0.008 | |||
| Tumor volume | |||||||||
| Low (<20% of gland) | 136 | 0.110 ± 0.002 | 0.0006 | 82 | 0.109 ± 0.003 | 0.003 | 54 | 0.111 ± 0.004 | 0.07 |
| High (≥20% of gland) | 128 | 0.099 ± 0.002 | 80 | 0.097 ± 0.003 | 48 | 0.101 ± 0.004 | |||
| Prostate volume | |||||||||
| Low (< 65 cc) | 133 | 0.108 ± 0.002 | 0.02 | 78 | 0.105 ± 0.003 | 0.35 | 55 | 0.112 ± 0.004 | 0.02 |
| High (≥65 cc) | 131 | 0.101 ± 0.002 | 84 | 0.101 ± 0.003 | 47 | 0.099 ± 0.004 | |||
| Tumor stage | |||||||||
| 2 | 204 | 0.105 ± 0.002 | 0.54 | 125 | 0.103 ± 0.003 | 0.76 | 79 | 0.107 ± 0.003 | 0.56 |
| 3 or 4 | 60 | 0.103 ± 0.003 | 37 | 0.102 ± 0.004 | 23 | 0.103 ± 0.004 | |||
| Advanced Gleason Grade1 | |||||||||
| No | 200 | 0.103 ± 0.002 | 0.31 | 122 | 0.103 ± 0.002 | 0.97 | 78 | 0.104 ± 0.004 | 0.10 |
| Yes | 64 | 0.107 ± 0.003 | 40 | 0.103 ± 0.004 | 24 | 0.114 ± 0.006 | |||
No = Gleason sum 6 or less or Gleason sum 7 and primary Gleason grade 3 or lower; Yes = Gleason sum 8 or higher or Gleason sum 7 and primary Gleason grade 4 or higher.
TABLE II.
MEAN PhIP-DNA ADDUCT LEVELS IN PROSTATE NON-TUMOR CELLS EXPRESSED AS ABSORBANCE UNITS FOR ALL SUBJECTS AND STRATIFIED BY RACE
| All subjects (n = 264) | Caucasians (n = 162) | African–Americans (n = 102) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | p value | n | Mean ± SE | p value | n | Mean ± SE | p value | |
| Age at prostatectomy | |||||||||
| <60 | 114 | 0.167 ± 0.004 | 0.99 | 66 | 0.169 ± 0.005 | 0.53 | 48 | 0.164 ± 0.007 | 0.47 |
| 60+ | 150 | 0.167 ± 0.004 | 96 | 0.165 ± 0.004 | 54 | 0.171 ± 0.006 | |||
| PSA at diagnosis | |||||||||
| Low (<4 ng/mL) | 45 | 0.105 ± 0.004 | 0.88 | 34 | 0.161 ± 0.007 | 0.56 | 11 | 0.153 ± 0.014 | 0.37 |
| Moderate (4–10 ng/mL) | 176 | 0.105 ± 0.002 | 98 | 0.170 ± 0.004 | 78 | 0.168 ± 0.005 | |||
| High (>10 ng/mL) | 43 | 0.103 ± 0.004 | 30 | 0.162 ± 0.008 | 13 | 0.179 ± 0.013 | |||
| Tumor Volume | |||||||||
| Low (<20% of gland) | 136 | 0.173 ± 0.004 | 0.02 | 82 | 0.175 ± 0.005 | 0.02 | 54 | 0.171 ± 0.006 | 0.52 |
| High (≥20% of gland) | 128 | 0.161 ± 0.004 | 80 | 0.159 ± 0.005 | 48 | 0.165 ± 0.007 | |||
| Prostate volume | |||||||||
| Low (<65 cc) | 133 | 0.171 ± 0.004 | 0.11 | 78 | 0.172 ± 0.005 | 0.11 | 55 | 0.170 ± 0.006 | 0.59 |
| High (≥65 cc) | 131 | 0.163 ± 0.004 | 84 | 0.161 ± 0.005 | 47 | 0.165 ± 0.007 | |||
| Tumor stage | |||||||||
| 2 | 204 | 0.166 ± 0.003 | 0.65 | 125 | 0.167 ± 0.004 | 0.81 | 79 | 0.166 ± 0.005 | 0.31 |
| 3 or 4 | 60 | 0.169 ± 0.006 | 37 | 0.165 ± 0.007 | 23 | 0.177 ± 0.010 | |||
| Advanced Gleason Grade1 | |||||||||
| No | 200 | 0.165 ± 0.003 | 0.09 | 122 | 0.167 ± 0.004 | 0.94 | 78 | 0.161 ± 0.005 | 0.005 |
| Yes | 64 | 0.175 ± 0.005 | 40 | 0.166 ± 0.007 | 24 | 0.190 ± 0.009 | |||
No = Gleason sum 6 or less or Gleason sum 7 and primary Gleason grade 3 or lower; Yes 5 Gleason sum 8 or higher or Gleason sum 7 and primary Gleason grade 4 or higher.
We next used multiple linear regression analyses to estimate race-specific beta coefficients for the independent associations of the clinical parameters of interest with PhIP-DNA adduct level in prostate tumor and non-tumor cells (Table III). Tumor volume was inversely associated with PhIP-DNA adduct level in both tumor and non-tumor cells in both races, but this association reached greater statistical significance in the Caucasian sample. In African–Americans, an inverse association between tumor volume and adduct level in tumor and non-tumor cells was also observed, but it was not as strong as in Caucasians. In tumor cells, prostate volume had a stronger inverse association with adduct levels in African–Americans. Age, PSA level and advanced Gleason grade were all positively associated with adduct level in tumor and non-tumor cells in African–Americans, with advanced Gleason grade in non-tumor cells having the largest and most statistically significant β coefficient (β = 0.029 ± 0.012; p = 0.02). The African–American race by advanced Gleason grade interaction term in non-tumor cells was also the largest and most statistically significant (β = 0.029 ± 0.013; p=0.02). In tumor cells, other notable interactions with African–American race were observed for older (60+) age (β = 0.012 ± 0.017; p = 0.08) and the highest PSA level category (>10 ng/mL) at diagnosis (β = 0.019 ± 0.012; p = 0.12).
TABLE III.
RACE-SPECIFIC EFFECTS OF CLINICAL FACTORS ON MEAN PhIP-DNA ADDUCT LEVELS IN PROSTATE TUMOR AND NON-TUMOR CELLS1
| Caucasians (n = 162) | African–Americans (n = 102) | AA race × risk factor interaction | ||||
|---|---|---|---|---|---|---|
| B ± SE | p value | β ± SE | p value | β ± SE | p value | |
| Older Age at prostatectomy (≥60) | ||||||
| Tumor | −0.003 ± 0.004 | 0.43 | 0.009 ± 0.005 | 0.10 | 0.012 ± 0.007 | 0.08 |
| Non-tumor | −0.002 ± 0.007 | 0.77 | 0.006 ± 0.009 | 0.52 | 0.013 ± 0.011 | 0.24 |
| PSA at diagnosis Tumor | ||||||
| Tumor | ||||||
| Moderate (4–10 ng/mL) | −0.0006 ± 0.005 | 0.90 | 0.007 ± 0.009 | 0.42 | 0.006 ± 0.010 | 0.55 |
| High (>10 ng/mL) | −0.005 ± 0.007 | 0.49 | 0.018 ± 0.012 | 0.11 | 0.019 ± 0.012 | 0.12 |
| Non-tumor | ||||||
| Moderate (4–10 ng/mL) | 0.010 ± 0.008 | 0.22 | 0.016 ± 0.015 | 0.29 | 0.010 ± 0.016 | 0.55 |
| High (>10 ng/mL) | 0.009 ± 0.011 | 0.40 | 0.019 ± 0.020 | 0.35 | 0.023 ± 0.021 | 0.26 |
| High tumor volume (≥20%) | ||||||
| Tumor | −0.014 ± 0.004 | 0.002 | −0.012 ± 0.005 | 0.03 | 0.004 ± 0.007 | 0.57 |
| Non-tumor | −0.021 ± 0.007 | 0.003 | −0.011 ± 0.009 | 0.24 | 0.014 ± 0.011 | 0.20 |
| High Prostate Volume (≥65 cc) | ||||||
| Tumor | −0.005 ± 0.004 | 0.22 | −0.018 ± 0.005 | 0.001 | −0.008 ± 0.007 | 0.25 |
| Non-tumor | −0.014 ± 0.007 | 0.04 | −0.009 ± 0.009 | 0.33 | 0.007 ± 0.011 | 0.54 |
| Advanced tumor stage (3 or 4) | ||||||
| Tumor | 0.004 ± 0.005 | 0.51 | −0.012 ± 0.007 | 0.11 | −0.004 ± 0.008 | 0.61 |
| Non-tumor | 0.0008 ± 0.009 | 0.93 | −0.001 ± 0.012 | 0.94 | 0.013 ± 0.013 | 0.30 |
| Advanced Gleason Grade2 | ||||||
| Tumor | 0.003 ± 0.005 | 0.57 | 0.013 ± 0.007 | 0.06 | 0.010 ± 0.008 | 0.19 |
| Non-tumor | 0.003 ± 0.008 | 0.70 | 0.029 ± 0.012 | 0.02 | 0.029 ± 0.013 | 0.02 |
All beta estimates adjusted for the other five clinical factors.
Gleason sum 8 or higher or Gleason sum 7 and primary Gleason grade 4 or higher.
In a related study, we found PhIP-DNA adduct levels were strongly correlated with grilled meat consumption and in particular grilled red meat consumption.25 Racial differences in grilled meat consumption in our study population were mixed. For specific meats, Caucasian men had significantly higher consumption of steak (p = 0.035), hamburger (p < 0.001), and chicken without skin (p < 0.001), while African–American men had significantly higher consumption of chicken with skin (p = 0.006). Overall grilled meat consumption was slightly higher in Caucasians (p = 0.045) as was grilled red meat consumption (p = 0.037).
Figure 3a-c shows representative tumor and non-tumor prostate cells from tumors of primary Gleason Grade 3 and 4. By visual inspection, one can see the dark staining for PhIP-DNA adducts in non-tumor prostate cells (Panel A), the relative absence of staining for PhIP-DNA adducts in tumor cells of primary Gleason 3 (Panel B) and mixed light and dark staining for PhIP-DNA adducts in tumor cells of primary Gleason Grade 4 (Panel C).
FIGURE 3.
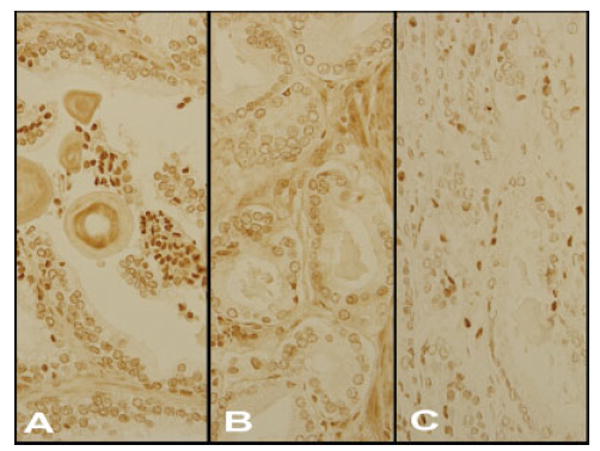
PhIP-DNA adduct staining in prostate epithelial non-tumor (Panel A) and tumor cells of Gleason primary Grade 3 (Panel B) and 4 (Panel C). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
In an unselected sample of men who underwent radical prostatectomy for treatment of their prostate cancer, variations in PhIP-DNA adduct levels were measurable in their tumor and non-tumor prostate cells. Previous in vitro studies have shown that human prostate tissue can metabolically activate “cooked meat” carcinogens, including PhIP.8,26 PhIP-DNA adduct levels were significantly lower in tumor cells compared with that in non-tumor cells consistent with what we previously observed in this same population for PAH-DNA adducts.24 This difference might be caused by higher cell turnover in tumor cells. It might also be due to differences in metabolism15 or DNA repair27 between normal and malignant cells. Tumor cells are mostly cloned from one mutated cell, and therefore more homogeneous than non-tumor cells. Microarray studies have demonstrated that hundreds of genes are differentially expressed between prostate tumor and non-tumor cells.28
PhIP-DNA adduct levels were the same in African–Americans and Caucasians, even after taking into account potential confounders such as tumor stage or grade. In a parallel study, we found grilled red meat consumption was strongly associated with PhIP-DNA adduct level in prostate tumor cells and moderately associated with PhIP-DNA adduct level in prostate non-tumor cells.25 While analysis of the grilled meat consumption variables found slightly higher consumption of both grilled red meat and all grilled meat in Caucasians, we found no racial differences in overall PhIP-DNA adduct levels in either tumor or adjacent non-tumor prostate cells. However, we did find that PhIP-DNA adduct levels had differential associations with some clinical and pathologic factors by race. An African–American race by advanced Gleason grade interaction term was significant in non-tumor cells (p = 0.02) with a similar trend of positive association between adduct level and higher Gleason grade exclusive to African–Americans observed in tumor cells. Older age and high pre-diagnosis PSA level were also two factors with moderate positive associations with adduct levels in tumor cells of African–Americans, but had no associations with adduct levels in Caucasians.
A growing body of evidence supports the notion that prostate carcinogenesis is biologically different in African–American men. Black patients who underwent radical prostatectomy in one study exhibited a higher incidence of transition zone cancer foci and higher serum PSA levels in patients with locally advanced prostate cancer.29 In a more recent study, African–American men with organ confined disease and moderate PSA levels had higher overall tumor volumes than white men with comparable clinical characteristics.30 In general, African–American race has been associated with higher PSA levels at prostate cancer diagnosis.29,31-33 Having both a high PSA level and a poorly differentiated prostate tumor is more likely in African–Americans than Caucasians.33 While underlying biology may be in part responsible for these observed racial differences in PSA level at prostate cancer diagnosis, a recent study of healthy African–American men found that high dietary PhIP intake was correlated with elevated PSA levels.34 The associations between high PSA level and Gleason grade and PhIP exposure observed in African–Americans is consistent with the suggestive positive associations we found in our study between PhIP-DNA adduct levels in both tumor and non-tumor cells and high PSA levels and advanced Gleason that was exclusive to African–American men. In terms of metabolizing PhIP, African–American men with the highest human SULT1A1 activity levels, an enzyme which is involved in the bioactivation of N-hydroxy metabolite of PhIP,13 are at a 2-fold greater risk for prostate cancer compared with Whites at comparable SULT1A1 levels.19 African–Americans are also known to have higher enzymatic activity levels of CYP1A2 and N-acetyltransferase,35 two enzymes important in the O-acetylation and N-oxidation of PhIP, respectively. Interestingly, CYP1A2 appears to be strongly inducible by smoking in whites, but not in African–Americans,36 which has potentially important implications in the race-specific effects of PhIP exposure on cancer risk.
Our study was not designed to directly test whether PhIP-DNA adducts increase risk for prostate cancer, but rather to describe the distribution of and determine what factors influence PhIP-DNA adduct levels in prostate cells of men with prostate cancer. While previous studies have suggested that African–Americans are both exposed to higher levels of PhIP and excrete higher levels of PhIP metabolites, we found no evidence for a racial difference in PhIP-DNA adducts in either non-tumor or tumor prostate cells. Our results do suggest, however, that prostate tumor differentiation may be more strongly linked with PhIP exposure in African–Americans, which implies that the PhIP-induced carcinogenesis pathway in the prostate may biologically differ by race. Future studies of the PhIP metabolism pathway in prostate cancer should consider how race-specific factors may influence the importance of PhIP with regard to the ethnic variation in this disease.
Acknowledgments
We thank study participants and the interviewers, abstracters, data managers, data programmers and lab technicians who worked on this study.
Grant sponsor: National Institutes of Health; Grant numbers: RO1 ES011126 and RO1 ES011126-S1.
References
- 1.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23:72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21:387–95. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 3.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, Sugimura T, Ito N. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–8. [PubMed] [Google Scholar]
- 4.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–84. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 5.Shirai T, Kato K, Futakuchi M, Takahashi S, Suzuki S, Imaida K, Asamoto M. Organ differences in the enhancing potential of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine on carcinogenicity in the prostate, colon and pancreas. Mutat Res. 2002;506/507:129–36. doi: 10.1016/s0027-5107(02)00159-8. [DOI] [PubMed] [Google Scholar]
- 6.Cui L, Takahashi S, Tada M, Kato K, Yamada Y, Kohri K, Shirai T. Immunohistochemical detection of carcinogen-DNA adducts in normal human prostate tissues transplanted into the subcutis of athymic nude mice: results with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 3,2′-dimethyl-4-aminobiphenyl (DMAB) and relation to cytochrome P450s and N-acetyltransferase activity. Jpn J Cancer Res. 2000;91:52–8. doi: 10.1111/j.1349-7006.2000.tb00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CY, Debiec-Rychter M, Schut HA, Morse P, Jones RF, Archer C, King CM, Haas GP. N-Acetyltransferase expression and DNA binding of N-hydroxyheterocyclic amines in human prostate epithelium. Carcinogenesis. 1999;20:1591–5. doi: 10.1093/carcin/20.8.1591. [DOI] [PubMed] [Google Scholar]
- 8.Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis. 2000;21:1683–9. doi: 10.1093/carcin/21.9.1683. [DOI] [PubMed] [Google Scholar]
- 9.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–9. [PubMed] [Google Scholar]
- 10.Di Paolo OA, Teitel CH, Nowell S, Coles BF, Kadlubar FF. Expression of cytochromes P450 and glutathione S-transferases in human prostate, and the potential for activation of heterocyclic amine carcinogens via acetyl-coA- Int J Cancer. 2005;117:8–13. doi: 10.1002/ijc.21152. [DOI] [PubMed] [Google Scholar]
- 11.Kooiman GG, Martin FL, Williams JA, Grover PL, Phillips DH, Muir GH. The influence of dietary and environmental factors on prostate cancer risk. Prostate Cancer Prostatic Dis. 2000;3:256–8. doi: 10.1038/sj.pcan.4500489. [DOI] [PubMed] [Google Scholar]
- 12.Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, Ilett KF, Kadlubar FF. N-and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyl-transferases expressed in COS-1 cells. Biochem Biophys Res Commun. 1992;185:839–44. doi: 10.1016/0006-291x(92)91703-s. [DOI] [PubMed] [Google Scholar]
- 13.Chou HC, Lang NP, Kadlubar FF. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s) Cancer Res. 1995;55:525–9. [PubMed] [Google Scholar]
- 14.Ragavan N, Hewitt R, Cooper LJ, Ashton KM, Hindley AC, Nicholson CM, Fullwood NJ, Matanhelia SS, Martin FL. CYP1B1 expression in prostate is higher in the peripheral than in the transition zone. Cancer Lett. 2004;215:69–78. doi: 10.1016/j.canlet.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Sterling KM, Jr, Cutroneo KR. Constitutive and inducible expression of cytochromes P4501A (CYP1A1 and CYP1A2) in normal prostate and prostate cancer cells. J Cell Biochem. 2004;91:423–9. doi: 10.1002/jcb.10753. [DOI] [PubMed] [Google Scholar]
- 16.Bogen KT, Keating GA. U.S. dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol. 2001;11:155–68. doi: 10.1038/sj.jea.7500158. [DOI] [PubMed] [Google Scholar]
- 17.Kidd LC, Stillwell WG, Yu MC, Wishnok JS, Skipper PL, Ross RK, Henderson BE, Tannenbaum SR. Urinary excretion of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in White, African-American, and Asian-American men in Los Angeles County. Cancer Epidemiol Biomarkers Prev. 1999;8:439–45. [PubMed] [Google Scholar]
- 18.Ji H, Yu MC, Stillwell WG, Skipper PL, Ross RK, Henderson BE, Tannenbaum SR. Urinary excretion of 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline in white, black, and Asian men in Los Angeles County. Cancer Epidemiol Biomarkers Prev. 1994;3:407–11. [PubMed] [Google Scholar]
- 19.Nowell S, Ratnasinghe DL, Ambrosone CB, Williams S, Teague-Ross T, Trimble L, Runnels G, Carrol A, Green B, Stone A, Johnson D, Greene G, et al. Association of SULT1A1 phenotype and genotype with prostate cancer risk in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2004;13:270–6. doi: 10.1158/1055-9965.epi-03-0047. [DOI] [PubMed] [Google Scholar]
- 20.Rybicki BA, Neslund-Dudas C, Nock NL, Schultz LR, Eklund L, Rosbolt J, Bock CH, Monaghan KG. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev. 2006;30:412–22. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi S, Tamano S, Hirose M, Kimoto N, Ikeda Y, Sakakibara M, Tada M, Kadlubar FF, Ito N, Shirai T. Immunohistochemical demonstration of carcinogen-DNA adducts in tissues of rats given 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP): detection in paraffin-embedded sections and tissue distribution. Cancer Res. 1998;58:4307–13. [PubMed] [Google Scholar]
- 22.Zhu J, Chang P, Bondy ML, Sahin AA, Singletary SE, Takahashi S, Shirai T, Li D. Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine-DNA adducts in normal breast tissues and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:830–7. [PubMed] [Google Scholar]
- 23.Zhu J, Rashid A, Cleary K, Abbruzzese JL, Friess H, Takahashi S, Shirai T, Li D. Detection of 2-amino-1-methyl-6-phenylimidazo [4,5-b]-pyridine (PhIP)-DNA adducts in human pancreatic tissues. Biomarkers. 2006;11:319–28. doi: 10.1080/13547500600667911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybicki BA, Rundle A, Savera AT, Sankey SS, Tang D. Polycyclic aromatic hydrocarbon-DNA adducts in prostate cancer. Cancer Res. 2004;64:8854–9. doi: 10.1158/0008-5472.CAN-04-2323. [DOI] [PubMed] [Google Scholar]
- 25.Tang D, Liu JJ, Rundle A, Neslund-Dudas C, Savera AT, Bock CH, Nock NL, Yang JJ, Rybicki BA. Grilled meat consumption and PhIP-DNA adducts in prostate carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2007;16:803–8. doi: 10.1158/1055-9965.EPI-06-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Buheissi SZ, Cole KJ, Hewer A, Kumar V, Bryan RL, Hudson DL, Patel HR, Nathan S, Miller RA, Phillips DH. The expression of xenobiotic-metabolizing enzymes in human prostate and in prostate epithelial cells (PECs) derived from primary cultures. Prostate. 2006;66:876–85. doi: 10.1002/pros.20400. [DOI] [PubMed] [Google Scholar]
- 27.Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow RG. Defective DNA strand break repair after DNA damage in prostate cancer cells: implications for genetic instability and prostate cancer progression. Cancer Res. 2004;64:8526–33. doi: 10.1158/0008-5472.CAN-04-1601. [DOI] [PubMed] [Google Scholar]
- 28.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–8. [PubMed] [Google Scholar]
- 29.Pettaway CA, Troncoso P, Ramirez EI, Johnston DA, Steelhammer L, Babaian RJ. Prostate specific antigen and pathological features of prostate cancer in black and white patients: a comparative study based on radical prostatectomy specimens. J Urol. 1998;160:437–42. [PubMed] [Google Scholar]
- 30.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, Lloreta J, Johnston DA, Pettaway CA. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer. 2006;107:75–82. doi: 10.1002/cncr.21954. [DOI] [PubMed] [Google Scholar]
- 31.Kang JS, Maygarden SJ, Mohler JL, Pruthi RS. Comparison of clinical and pathological features in African-American and Caucasian patients with localized prostate cancer. BJU Int. 2004;93:1207–10. doi: 10.1111/j.1464-410X.2004.04846.x. [DOI] [PubMed] [Google Scholar]
- 32.Tewari A, Horninger W, Badani KK, Hasan M, Coon S, Crawford ED, Gamito EJ, Wei J, Taub D, Montie J, Porter C, Divine GW, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2005;96:29–33. doi: 10.1111/j.1464-410X.2005.05561.x. [DOI] [PubMed] [Google Scholar]
- 33.Moul JW, Sesterhenn IA, Connelly RR, Douglas T, Srivastava S, Mostofi FK, McLeod DG. Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. JAMA. 1995;274:1277–81. [PubMed] [Google Scholar]
- 34.Bogen KT, Keating GA, Chan JM, Paine LJ, Simms EL, Nelson DO, Holly EA. Highly elevated PSA and dietary PhIP intake in a prospective clinic-based study among African Americans. Prostate Cancer Prostatic Dis. doi: 10.1038/sj.pcan.4500941. in press. [DOI] [PubMed] [Google Scholar]
- 35.Relling MV, Lin JS, Ayers GD, Evans WE. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992;52:643–58. doi: 10.1038/clpt.1992.203. [DOI] [PubMed] [Google Scholar]
- 36.Lang NP, Butler MA, Massengill J, Lawson M, Stotts RC, Hauer-Jensen M, Kadlubar FF. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomarkers Rev. 1994;3:675–82. [PubMed] [Google Scholar]


