Abstract
Large, free polymannose oligosaccharides generated during glycoprotein biosynthesis rapidly appear in the cytosol of HepG2 cells where they undergo processing by a cytosolic endo H–like enzyme and a mannosidase to yield the linear isomer of Man5GlcNAc (Man[α1-2]Man[α1-2]Man[α1-3][Man α1-6]Man[β14]GlcNAc). Here we have examined the fate of these partially trimmed oligosaccharides in intact HepG2 cells. Subsequent to pulse–chase incubations with d-[2- 3H]mannose followed by permeabilization of cells with streptolysin O free oligosaccharides were isolated from the resulting cytosolic and membrane-bound compartments. Control pulse–chase experiments revealed that total cellular free oligosaccharides are lost from HepG2 cells with a half-life of 3–4 h. In contrast use of the vacuolar H+/ATPase inhibitor, concanamycin A, stabilized total cellular free oligosaccharides and enabled us to demonstrate a translocation of partially trimmed oligosaccharides from the cytosol into a membrane-bound compartment. This translocation process was unaffected by inhibitors of autophagy but inhibited if cells were treated with either 100 μM swainsonine, which provokes a cytosolic accumulation of large free oligosaccharides bearing 8-9 residues of mannose, or agents known to reduce cellular ATP levels which lead to the accumulation of the linear isomer of Man5GlcNAc in the cytosol. Subcellular fractionation studies on Percoll density gradients revealed that the cytosol-generated linear isomer of Man5GlcNAc is degraded in a membrane-bound compartment that cosediments with lysosomes.
The glycosylation of proteins with N-linked carbohydrate in the endoplasmic reticulum is a common and important posttranslational modification. Surprisingly, this process, accomplished by the transfer of a polymannose-type oligosaccharide from a lipid carrier (dolichol) onto polypeptide (Kornfeld and Kornfeld, 1985), is accompanied by the release of free polymannose-type oligosaccharides into the lumen of the ER (Anumula and Spiro, 1983; Cacan et al., 1987). As large amounts of free oligosaccharides are generated in this way an understanding of the fate of this material became important. It was initially thought that free oligosaccharides generated in the lumen of the ER might be exported from the cell by vesicular transport as a consequence of the effect of bulk flow (Wieland et al., 1987). In fact this was found not to be the case as free oligosaccharides were not recovered from the incubation media of cultured HepG2 cells (Moore and Spiro, 1990) but detected in the cytosol (Moore and Spiro, 1994). More recently free polymannose-type oligosaccharides bearing the terminal reducing di-N-acetylchitobiose moiety have been shown to be transported out of the ER into the cytosol in permeabilized HepG2 cells (Moore et al., 1995). In addition to the transfer of free oligosaccharides from the lumen of the ER into the cytosol, there is now evidence to suggest that some free oligosaccharides may be generated in the cytosol by either the release of cytosolically disposed oligosaccharides from dolichol (Kmiécik et al., 1995), or by the degradation of glycoproteins, initiated by a cytosolic N-glycanase (Suzuki et al., 1994), that have been translocated out of the ER into the cytosol (Wiertz et al., 1996). These reports highlight the crucial role that the cytosol plays in the processing and perhaps generation of free oligosaccharides which are generated during the biosynthesis and quality control of glycoproteins. What then is the fate of these free cytosolic oligosaccharides?
It has been known for some years that the cytosol contains both an endo H–like enzyme (Pierce et al., 1979) and an α-mannosidase (Shoup and Touster, 1976; Tulsiani and Touster, 1987). In vitro experiments with preparations of the cytosolic α-mannosidase have revealed it to possess two notable features, firstly it is inactive towards large polymannose-type oligosaccharides bearing the di-N-acetylchitobiose moiety at their reducing termini (Oku and Hase, 1991) and secondly its limit digest product is the linear isomer of Man5GlcNAc: Man(α1-2)Man(α1-2)Man(α1-3)(Man[α16])Man(β1-4)GlcNAc; Tulsiani and Touster, 1987; Oku and Hase, 1991). In intact cells cytosolic-free oligosaccharides generated during the biosynthesis of glycoproteins are apparently subjected to the actions of these two cytosolic enzymes to yield the linear isomer of Man5GlcNAc (Moore and Spiro, 1994).
Here we report on the fate of this cytosolic free oligosaccharide in intact HepG2 cells. It is shown that partially trimmed cytosolic oligosaccharides are translocated into a membrane bound compartment by a nonautophagic process that requires energy. Subcellular fractionation of HepG2 cell homogenates on Percoll density gradients revealed that cytosolic oligosaccharides are ultimately degraded in a compartment that cosediments with lysosomes. Along with a previous report describing the transport of free oligosaccharides from the lumen of the ER into the cytosol (Moore et al., 1995), this report describes a novel trafficking pathway for free oligosaccharides that links the endoplasmic reticulum to the lysosome via the cytosol.
Materials and Methods
Culture and Radiolabeling of Cells
HepG2 cells were cultivated in RPMI 1640 (GIBCO BRL, Paisley, UK) containing 10% FCS (GIBCO) as previously described (Moore and Spiro, 1990). Cells were pulse radiolabeled with 40 μCi d-[2-3H]mannose (20 Ci/ mmol; Amersham, Slough, UK) for 20 min in 0.5 ml glucose-free Dulbecco's modified Eagle Medium (GIBCO BRL) supplemented with 5% dialyzed FCS, 2 mM glutamine, 5 mM fucose, and 1 mM sodium pyruvate. Cells were chased in complete growth medium containing 5 mM fucose and 5 mM mannose. When pulse–chase studies were performed in the presence of swainsonine (Sigma Chemical Co., St. Louis, MO) and concanamycin A (Ciba-Geigy, Switzerland), the cells were preincubated with these inhibitors for 1 h before the onset of radiolabeling and were added to both the pulse and chase media at the appropriate concentrations. Before subcellular fractionation studies cells were pulse radiolabeled for 20 min with 200 μCi d-[2-3H]mannose and chased as described above. Other drugs including 3-methyladenine, asparagine, deoxyglucose, sodium azide, and oligomycin (all from Sigma Chemical Co.) were included in the chase media only and were added 45 min after the onset of the chase incubations, whereas leupeptin (Sigma Chemical Co.) was added at the beginning of each chase period.
Permeabilization of Cells
At the end of pulse–chase experiments, cells were released from tissue culture flasks with trypsin/EDTA and washed twice in 1.0 ml of permeabilization buffer: 250 mM mannitol, 5 mM Hepes (pH 7.3), 2 mM EGTA, 1 mM CaCl2, 2 mM MgCl2. Cells were then incubated at 4°C for 20 min in 0.5 ml of the permeabilization buffer containing 1 U/ml streptolysin O (Wellcome Diagnostics, Dartford, UK). Cells were recovered by centrifugation and the supernatant was kept. Subsequent to washing the cells twice with 0.5 ml permeabilization buffer at 4°C, they were incubated with 0.5 ml prewarmed permeabilization buffer (37°C) for 5 min and the permeabilized cells were then recovered by centrifugation to yield the membranebound compartment (MBC)1 fraction. The final supernatant was pooled with the SLO-containing supernatant and the two subsequent permeabilization buffer washes to yield 2 ml of the cytosolic compartment fraction (Cytosol).
Preparation of Free Oligosaccharides from Cytosolic and MBC Fractions of HepG2 Cells
Neutral free oligosaccharides were prepared from the cytosol and MBC fractions as previously described (Moore and Spiro, 1994). Briefly, the pellet of permeabilized cells (MBC) was extracted with chloroform/methanol/125 mM Hepes (pH 7.2) containing, 4 mM MgCl2, 3:2:1, and after vigorous shaking the upper methanolic phase was recovered, dried, and redissolved in water. This material and the cytosolic fractions were desalted on combined columns of AG 1-X2 (acetate form) and AG 50-X2 (H+ form), unbound neutral material was then loaded onto columns of charcoal, which were then washed with water before elution of oligosaccharide material from the charcoal with 30% ethanol. Free oligosaccharides were analyzed on plastic thin layer chromatography plates coated with silica (Merck, Darmstadt, Germany) which were developed in n-propanol/acetic acid/water (3:2:1) for 12 h. Resolved components were visualized by fluorography.
Structural Analysis of Man5GlcNAc Oligosaccharides
After resolution of free oligosaccharides by thin layer chromatography components of interest were eluted from the chromatography plates with water and passed over coupled columns of AG 50 (H+ form) and AG 1 (acetate form). Nonretained neutral components were dried and subjected to two mannosidase treatments. One aliquot was treated with 1 U Jack bean α-mannosidase (Sigma Chemical Co.) overnight at 37°C in 40 mM sodium acetate, pH 4.5. Another aliquot was digested overnight at 37°C with 5 μU α1-2 mannosidase (Oxford Glycosystems, Abingdon, UK) in 100 mM sodium acetate buffer, pH 5.0. The digestion products were then desalted as described above, concentrated, and resolved by thin layer chromatography on plastic sheets coated with cellulose (0.1 mm thickness; Merck). Chromatographs were developed in pyridine/ethyl acetate/water/ acetic acid 5:5:3:1 for 10 h, and after drying resolved components were visualized by fluorography. Quantitation of the resolved products was achieved by their elution from the cellulose plates and assaying radioactive components by scintillation counting.
Percoll Density Gradient Fractionation
HepG2 cells were washed three times with ice-cold PBS containing 1 mM CaCl2, 1 mM MgCl2 and once with ice-cold subcellular fractionation buffer (SFB), 250 mM sucrose, 20 mM Hepes, 1 mM EDTA, pH 7.2. The cells were scraped from tissue culture flasks in 5 ml SFB and cellular protein was assayed using a bicinchoninic acid protein assay kit (Sigma Chemical Co.). The cell pellet obtained after centrifugation at 600 g for 10 min was resuspended in SFB (1.5 mg/ml protein) and placed on ice for 15 min. Cell homogenization was carried out using a tight-fitting Dounce homogenizer (30 passages). After centrifuging the homogenate at 600 g for 10 min, the supernatant was removed and kept on ice, and the pellet was resuspended with SFB and rehomogenized and centrifuged as above. Pooled supernatants were adjusted to 5 ml with SFB and 3 ml of an 80% Percoll solution was added (Rijnboutt et al., 1992). The gradient was formed by centrifugation for 35 min at 92,570 gAv. (Rijnboutt et al., 1992). 400-μl fractions were collected from the top of the tube with a needle mounted on a syringe. To isolate free oligosaccharides from the Percoll gradient, fractions were pooled (see Fig. 9), diluted in SFB, and after centrifugation for 90 min at 100,000 gAv organelles were recovered separately from the Percoll pellet. Free oligosaccharides were prepared from organelle fractions as described above, except that before ion-exchange chromatography, sucrose was eliminated from the samples by Biogel P2 gel filtration.
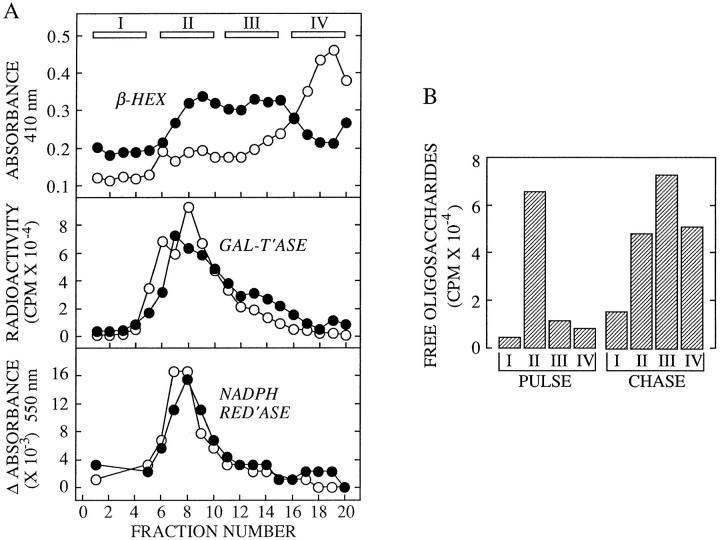
Figure 9.
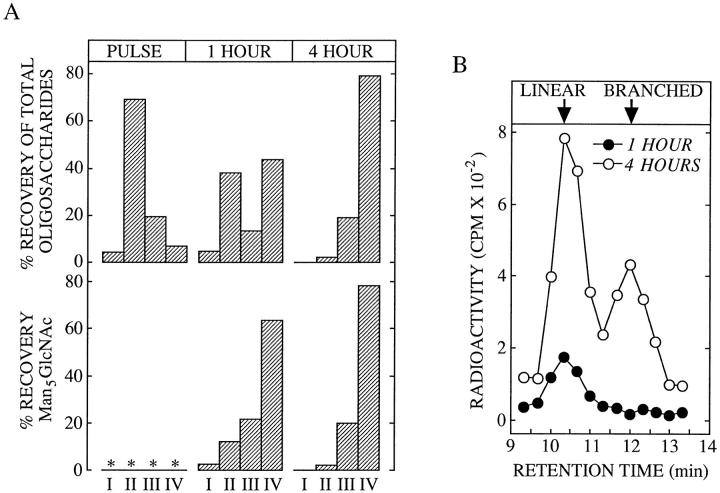
CCM A affects the distribution of a lysosomal marker enzyme on Percoll density gradients. (A) Control cells (open circles) or cells treated with CCM A for 4 h were homogenized and fractionated on Percoll density gradients as described in Materials and Methods. The sedimentation position of lysosomes, the Golgi apparatus, and ER were identified by performing assays for β-d-hexosaminidase (β- HEX), β-1, 4-galactosyltransferase (GAL T'ASE), and NADPH cytochrome C reductase (NADPH RED'ASE), respectively. (B) CCM A–treated cells were pulse radiolabeled (PULSE) and chased (CHASE) in the presence of CCM A for 4 h before subcellular fractionation as described above. After collecting the gradient in four fractions (I-IV) as shown in the upper panel of A, organelles were separated from Percoll and cytosol by centrifugation as described in Materials and Methods. Free oligosaccharides were isolated from the resulting organelles and assayed by scintillation counting.
HRP Uptake
HepG2 cells were washed with MEMH (MEM containing 1 mM sodium pyruvate, 1 mM l-glutamine and 20 mM Hepes/NaOH, pH 7.2) and HRP uptake was performed in MEMH containing 5 mg/ml HRP (Sigma Chemical Co.) for 5 or 15 min at 37°C (van Weert et al., 1995). Extracellular HRP was then removed by washing the cells with ice-cold MEMH over a period of 10 min, followed by either a 5-min or a 2-h chase period, corresponding to the 5 or 15 min pulse times, respectively (van Weert et al., 1995). HRP-loaded cells were then washed and homogenized as described above.
Enzymatic Assays
HRP activity was assayed in 50 mM phosphate buffer (pH 5.0) containing 0.1% Triton X-100, using 83 μg/ml O-dianisidine and 1% H2O2 as substrates (van Weert et al., 1995). The reaction was performed for 5 min at room temperature in the dark; absorbance was measured at 460 nm.
Lysosomal β-d-hexosaminidase activity was measured using p-nitrophenyl N-acetylglucosamine as described previously (Opheim and Touster, 1977). β1, 4 Galactosyltransferase was assayed by the method of Barker et al. (1972).
NADPH cytochrome c reductase activity was measured as previously described in a 50 mM phosphate, 0.1 mM EDTA buffer, pH 7.7, using 1 mg/ml NADPH and 25 μg/ml cytochrome c as substrates. Absorbance increases were measured at 550 nm over a 3-min period.
High pH Anion Exchange Chromatography
High pH anion exchange chromatography (HPAEC) was carried out on a Dionex apparatus as previously described (Townsend et al., 1991). Components were eluted at 1 ml/min with buffer A for 10 min followed by a linear gradient of 0–10% buffer B over 25 min (buffer A: 50 mM NaOH; buffer B: 500 mM sodium acetate in buffer A). Column effluent was monitored for radioactive components with a Flow Scintillation Analyzer Radiomatic Flow-one/β (Packard, Instrument Company, Meriden, CT) using a scintillation fluid (UltimaFlow one AP, Packard) flow rate of 2 ml/min. Standard oligosaccharides, prepared as previously described (Michalski et al., 1990; Haeuw et al., 1991) were monitored by pulsed electrochemical detection.
Results
Free Oligosaccharides Appear Rapidly in the Cytosol of HepG2 Cells during Glycoprotein Biosynthesis and Are Then Cleared from This Compartment
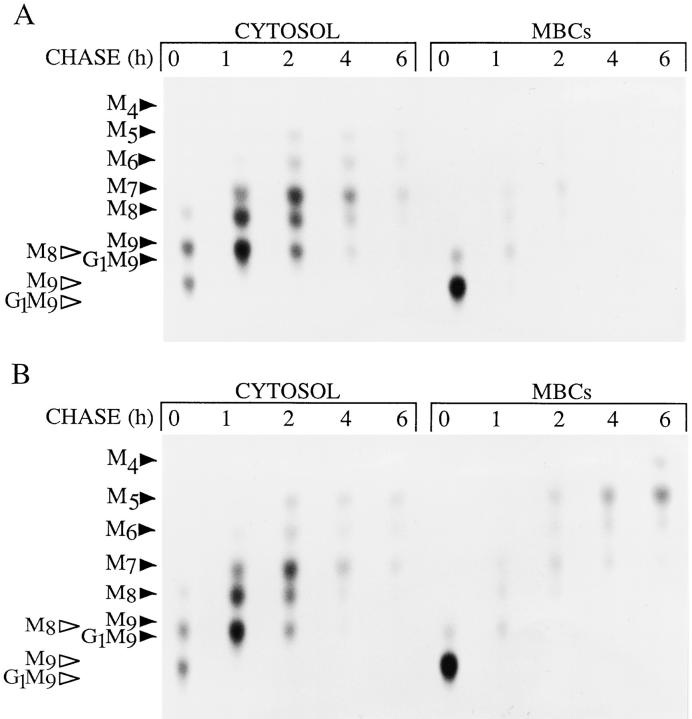
In a previous study we have shown that free oligosaccharides generated from oligosaccharide-lipid, bearing the di- N-acetylchitobiose moiety (OS-GN2), are rapidly transported out of the ER into the cytosol (Moore et al., 1995). This process was observed in vitro upon addition of ATP to permeabilized HepG2 cells. In the present report we have followed the subcellular trafficking of free polymannose oligosaccharides in vivo by permeabilizing the plasma membrane of [3H]mannose-radiolabeled HepG2 cells with SLO. After centrifugation of permeabilized cells, free oligosaccharides were isolated from the supernatant (cytosolic compartment) and the residual cell pellet, containing intact intracellular organelles (membrane bound compartments; MBCs). Fig. 1 A shows the results of such an experiment. During the pulse the MBC contains free oligosaccharides (OS-GN2; Fig. 1, open arrowheads) whereas the cytosol contains the same components and in addition their counterparts bearing a single residue of N-acetylglucosamine at their reducing termini (OS-GN1). Between the pulse and 1-h chase period there is a loss of OS-GN2 from the MBC and an increase in free oligosaccharides in the cytosolic compartment. These observations represent the previously described ER-to-cytosol transport process (Moore et al., 1995). After transport out of the ER, OSGN2 are subject to a cytosolic endo H–like activity to yield their OS-GN1 counterparts. OS-GN1 are now potential substrates for the cytosolic mannosidase which trims these components down to a limit digest product; the linear isomer of Man5GlcNAc (Moore and Spiro, 1994; see Fig. 2 B for representation of this structure). However, as can be seen in Fig. 1 A, free oligosaccharides neither accumulate in the cytosol nor reappear in an MBC. We reasoned that if cytosolic oligosaccharides are translocated into a degradative compartment they might be difficult to detect due to their rapid hydrolysis into free mannose and N-acetylglucosamine. Because the lysosome is the most likely compartment in which cytosolic oligosaccharides might be degraded we chose to perform pulse–chase experiments in the presence of a vacuolar H+/ATPase inhibitor.
Figure 1.
The effect of CCM A on the generation and fate of free oligosaccharides in HepG2 cells. Control (A) or CCM A–treated (B) HepG2 cells were pulse radiolabeled with d-[2-3H]mannose for 20 min, and then chased for the indicated times. Cells were then cooled, placed in suspension, and permeabilized with SLO as described in Materials and Methods to yield fractions corresponding to the cytosol (CYTOSOL) and membrane- bound compartments (MBCs). Free oligosaccharides were prepared from each of these fractions and then resolved by thin layer chromatography, on silica-coated plates. The abbreviations associated with the solid arrowheads are: G1M9, Glc1Man9GlcNAc; M9, Man9 GlcNAc; M8, Man8GlcNAc; M7, Man7GlcNAc; M6, Man6GlcNAc; M5, Man5GlcNAc; M4, Man4GlcNAc; M3, Man3GlcNAc. Those associated with the open arrowheads are: G1M9, Glc1Man9 GlcNAc2; M9, Man9GlcNAc2; M8, Man8GlcNAc2.
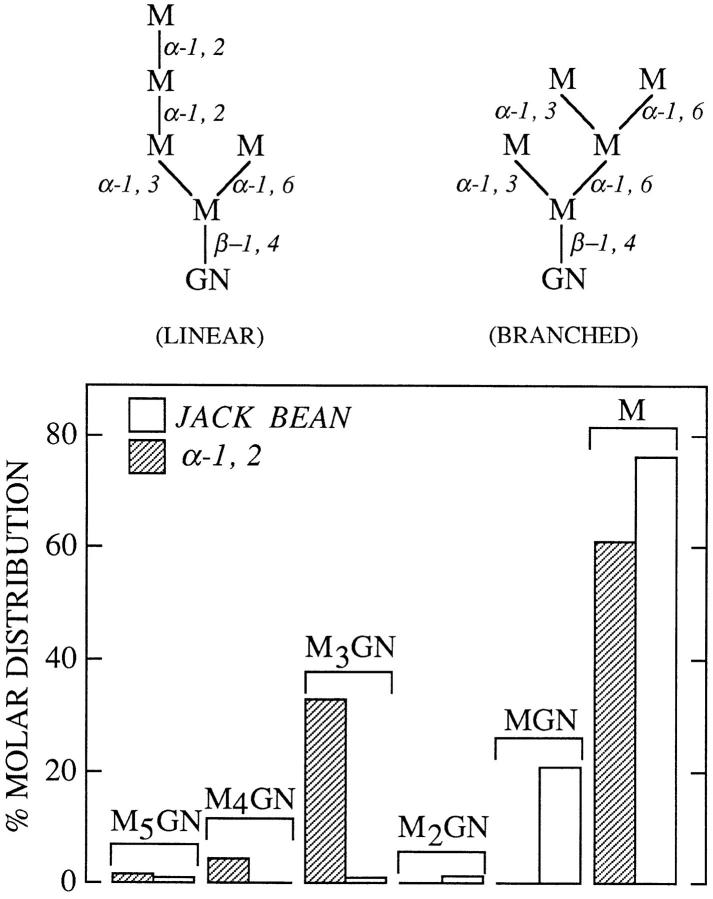
Figure 2.
The linear isomer of Man5GlcNAc accumulates in a membrane-bound compartment of CCM A–treated HepG2 cells. HepG2 cells were pulse radiolabeled and chased for 4 h in the presence of CCM A as described in Materials and Methods. The cells were then permeabilized with SLO and oligosaccharides were purified from the resulting MBC as previously described. The component migrating as Man5GlcNAc was recovered and aliquots of this oligosaccharide were then digested with either jack bean α-mannosidase (JACK BEAN) or an α-1, 2 mannosidase (α-1, 2). After desalting, the digestion products were resolved by thin layer chromatography on a cellulose-coated plate. Subsequent to visualization of resolved components by fluorography they were eluted from the chromatography plate and assayed by scintillation counting. The molar equivalents of the resolved components were calculated and, after summing, the percentage molar distribution of the digestion products was calculated. The cytosolic mannosidase generates an isomer (LINEAR) of Man5GlcNAc that contains two α-1, 2-linked mannose residues whereas Golgi mannosidase I leads to the formation of a Man5GlcNAc isomer (BRANCHED) devoid of α-1, 2-linked mannose residues. The abbreviations are as follows: M5GN, Man5GlcNAc; M4GN, Man4GlcNAc; M3GN, Man3GlcNAc; M2GN, Man2GlcNAc; MGN, ManGlcNAc; M, Mannose.
The Clearance of Free Oligosaccharides from the Cytosol Is Not Dependent upon Vacuolar H+/ATPase Activity
CCM A (Woo et al., 1992) is a vacuolar H+/ATPase which has been shown to abolish acidification of lysosomes without affecting cellular ATP levels (Woo et al., 1992: Kataoka et al., 1995), we reasoned that if free oligosaccharides are translocated into an acidic MBC from the cytosol this reagent may either inhibit their access to, or, failing that, block their degradation within an acidic compartment. Free oligosaccharides isolated from the cytosol and MBCs of cells from a pulse–chase experiment performed in the presence of CCM A are shown in Fig. 1 B, results show that when compared to the control experiment (Fig. 1 A), this reagent had very little effect on early events (0–1 h) during the pulse–chase experiment and we conclude from this that the generation of free oligosaccharides in the ER and their transport out of this compartment into the cytosol is unaffected by CCM A. In contrast Fig. 1 B shows that, after 1 h of chase, CCM A provokes a steady accumulation of free oligosaccharides bearing predominantly 7-4 residues of mannose, in an MBC. Although the effect of CCM A is most marked with respect to oligosaccharides recovered from the MBCs we noted systematically, after long chase periods, that this reagent also causes a small but significant accumulation of free oligosaccharide material in the cytosolic compartment. We also observed, after 8 h of chase in the presence of CCM A, that 7.9% of total free oligosaccharides produced by HepG2 cells could be recovered from the incubation medium, and after 20 h of chase this figure rose to 18.0%. As the quantity of free oligosaccharides recovered from the incubation media of CCM A–treated cells represented only a small fraction of total cellular free oligosaccharides observed during the time frame of our experiments the contribution made by these components to the quantitative aspects of our studies have not been taken into account.
These results show that CCM A has only small effects on the appearance and decay of radioactivity associated with free oligosaccharides in the cytosol of HepG2 cells but, in contrast, this reagent provokes a marked accumulation of free oligosaccharides associated with an MBC.
The Isomeric Structure of the Man5GlcNAc Isolated from MBCs of CCM A–treated HepG2 Cells Is Consistent with Its Cytosolic Origin
To evaluate the possibility that the CCM A–provoked accumulation of free oligosaccharides associated with an MBC is related to the loss of these components from the cytosol, we next investigated the isomeric configuration of the Man5GlcNAc associated with the MBCs. The cytosol is known to contain an endo H–like enzyme and an α-mannosidase which together process the large oligosaccharides that are transported out of the ER into the cytosol to yield the limit digest product Man5GlcNAc (Fig. 2, LINEAR) (Moore and Spiro, 1994). Although products closely related to CCM A are known to inhibit the degradation of proteins in the lysosomes of certain cell lines (Yoshimori et al., 1991), we could not rule out the possibility that in our hands CCM A caused the accumulation of partially degraded polymannose- or hybrid-type oligosaccharides derived from incomplete lysosomal glycoprotein degradation. If this were the case, then any resulting free Man5GlcNAc should have a structure consistent with its passage through the Golgi apparatus while N-linked to a protein. Accordingly, the Man5GlcNAc isolated from MBCs of CCM A–treated HepG2 cells would be expected to be the branched isomer of this oligosaccharide (Fig. 2, BRANCHED) (Kornfeld and Kornfeld, 1985). Thus transport of this latter component into an MBC of CCM A–treated HepG2 cells would lead to the accumulation of an isomer of Man5GlcNAc different from that expected from the limit digest product of the cytosolic mannosidase. To distinguish between the two possible origins of the Man5GlcNAc observed to accumulate in the presence of CCM A, we have isolated this component from the MBCs and subjected it to digestion with a nonspecific α-mannosidase (Jack bean) and an α-1, 2 mannosidase (Amano and Kobata, 1986) as shown in Fig. 2. The linear isomer of Man5GlcNAc has two α-1, 2 linked mannose residues whereas its branched counterpart contains no such linkages. Results show that the structure isolated from the MBCs of CCM A–treated HepG2 cells is sensitive to the α-1, 2 mannosidase yielding free mannose and the tetrasaccharide Man3GlcNAc. Jack bean α-mannosidase treatment of the Man5GlcNAc yielded free mannose and the disaccharide Manβ-1, 4 GlcNAc, in the ratio 4:1, indicating that this component possessed a single terminal reducing N-acetylglucosamine moiety and that all the α-linked mannose residues were accessible to an exomannosidase. Similar treatment of the Man4GlcNAc isolated from the MBCs of CCM A–treated HepG2 cells revealed it also to be sensitive to the α-1, 2 mannosidase yielding the digest products Man3GlcNAc and mannose (results not shown). These results clearly demonstrate that the isomeric configurations of the free oligosaccharides associated with the MBCs of CCM A–treated HepG2 cells are compatible with their having been generated by the cytosolic mannosidase and not as a consequence of incomplete degradation of free oligosaccharides derived from lysosomal glycoprotein catabolism.
The Loss of Free Oligosaccharides from the Cytosol Can Be Accounted for Both Quantitatively and Kinetically by Their Recovery within an MBC of CCM A–treated HepG2 Cells
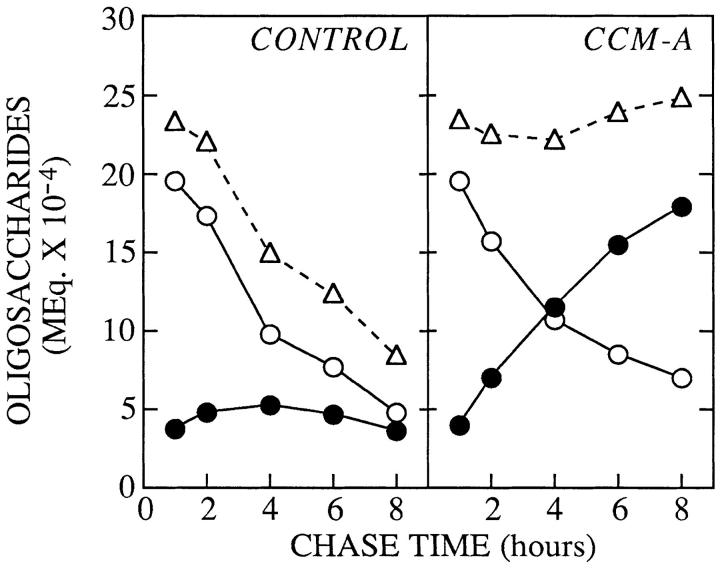
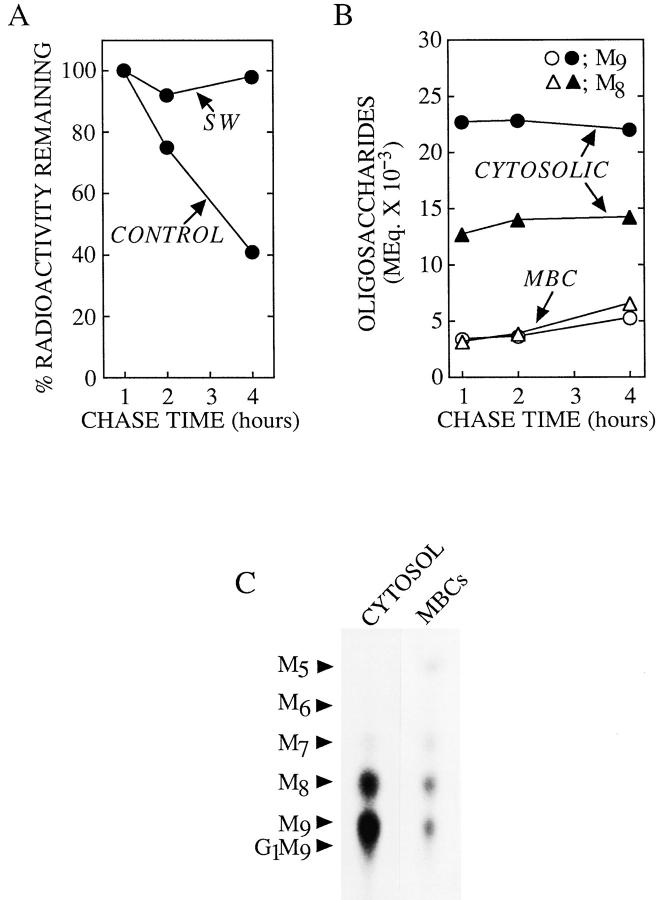
The ability of CCM A to block the degradation of MBCassociated free polymannose type oligosaccharides has enabled us to further establish the hypothesis that there is a cytosol-to-MBC translocation of free oligosaccharides. Accordingly, we have verified that the loss of free oligosaccharides from the cytosol could be quantitatively and kinetically accounted for by their reappearance in an MBC. Quantitation of the free oligosaccharides generated during the control and CCM A pulse–chase incubations is shown in Fig. 3. In the control incubations there is a loss of total free oligosaccharide from cells such that after 8 h of chase only 25% of the cytosolic free oligosaccharides remain. However in the presence of CCM A the total quantity of free oligosaccharide remains approximately constant between 1 and 8 h of chase and the loss of free oligosaccharides from the cytosol can be accounted for, both quantitatively and kinetically, by their recovery from the MBCs (Fig. 3).
Figure 3.
Quantitation of the transfer of free oligosaccharides from the cytosol into a membrane-bound compartment of CCM A–treated HepG2 cells. Cells were pulse–radiolabeled and chased in either the absence (CONTROL) or presence (CCM A) of CCM A. After isolation from both the cytosolic and membranebound compartments free oligosaccharides were resolved by thin layer chromatography as described in Fig. 1. Subsequent to fluorography each of the resolved components were quantitated by densitometry. The molar equivalent (MEq.) of any given oligosaccharide was then calculated by dividing its estimated quantity by the number of mannose residues the component contained. Molar equivalents of all cytosolic (open circles) oligosaccharides were summed and the same procedure was applied to free oligosaccharides derived from the MBCs (closed circles). The open triangles represent the sum of the molar equivalents of the cytosolic and MBC components. The values reported in this figure were calculated from data obtained from two pulse–chase experiments.
Next, we wanted to know whether the free oligosaccharides associated with the MBCs of CCM A–treated HepG2 cells are located within the MBC or are merely associated with the cytosolic face of its delineating membrane. This problem was addressed by performing a pulse–chase incubation in the presence of CCM A as described in Fig. 1. After 6 h of chase the cells were first permeabilized with SLO to release the remaining cytosolic oligosaccharides and then repermeabilized with saponin. Results indicated that >90% of the free oligosaccharides associated with the MBCs of SLO-permeabilized cells could be released upon their repermeabilization with saponin (results not shown). Identical results were obtained if the saponin treatment was substituted by freeze–thawing (not shown). These results confirm the hypothesis that in CCM A–treated HepG2 cells there is a translocation of free oligosaccharides from the cytosolic compartment into the lumen of an MBC.
Inhibition of Cytosolic Processing of Free Polymannose-type Oligosaccharides Affects Their Subcellular Trafficking
We then wanted to know if the structure of cytosolic-free oligosaccharides play a role in their transfer into an MBC. To achieve this the total cellular molar equivalent of each free oligosaccharide was calculated after 1, 2, and 4 h of chase in the presence of CCM A as shown in the upper part of Fig. 4. The percentage of each oligosaccharide occurring in the MBCs was then computed and displayed in the lower portion of Fig. 4. Irrespective of the total cellular quantity of Man9-8GlcNAc ∼17% of these two oligosaccharides are recovered from the MBC after 1, 2, and 4 h of chase. Because the permeabilization procedure used in these studies leads to the release of ∼80% of cellular lactate dehydrogenase (results not shown), the small percentage of Man9-8GlcNAc occurring in the MBCs during the chase incubations may represent free oligosaccharides in nonpermeabilized cells. However, as the chase progresses there is a steady increase in the proportion of free Man7-5- GlcNAc oligosaccharides occurring in the MBCs. These results suggest that oligosaccharides bearing 8 or 9 residues of mannose might be poorly transferred into a MBC. To gain more insight into the mechanism of the transfer of free oligosaccharides from the cytosol to a MBC, we examined the effect of inhibition of the cytosolic mannosidase on the transfer of cytosolic oligosaccharides into the MBC. If, as suggested above, large free oligosaccharides bearing 8 or 9 residues of mannose are poorly transferred from the cytosol into an MBC, then an inhibitor of the cytosolic mannosidase would be expected to slow down the transfer of the larger free polymannose-type oligosaccharides from the cytosol into an MBC of HepG2 cells. At high concentrations swainsonine (SW), a nonspecific mannosidase inhibitor (Elbein et al., 1981), inhibits the cytosolic α-mannosidase (Tulsiani and Touster, 1987) in addition to Golgi mannosidase II and lysosomal mannosidases. Accordingly, HepG2 cells were pulse radiolabeled and chased in the presence of 100 μM SW, and, subsequent to permeabilization with SLO, free oligosaccharides were prepared from both the MBCs and the cytosol as described for Fig. 1. Fig. 5 A demonstrates that after 4 h of chase SW causes an inhibition of the loss of radioactivity associated with free oligosaccharide material from the cytosolic compartment of HepG2 cells. Furthermore thin layer chromatography of the oligosaccharides recovered from the cytosolic compartment of HepG2 cells chased for 4 h in the presence of 100 μM SW revealed that Man9GlcNAc and Man8GlcNAc oligosaccharides are stabilized in the cytosol for up to 4 h and that there is little transfer of these components into the MBC. Although Fig. 5 B shows that free oligosaccharides bearing 9 and 8 residues of mannose are stabilized in the cytosolic compartment of SW-treated cells, we observed (Fig. 5 C) a small accumulation of Man5GlcNAc in the MBCs derived from these cells. As described above in addition to inhibiting the cytosolic mannosidase SW is also known to inhibit Golgi mannosidase II which leads to the production of glycoproteins bearing hybrid-type oligosaccharide chains (Elbein et al., 1981). Hybrid-type oligosaccharide chains possess a core structure which contains the branched Man5GlcNAc moiety (Fig. 2), now, if such glycoproteins, or free oligosaccharides, are transported from the Golgi complex to lysosomes, whose mannosidase activity has been compromised by SW, we would then expect to see an accumulation of a free oligosaccharide corresponding to the branched isomer of Man5GlcNAc in the MBCs of SWtreated cells. Results (not shown) demonstrated that 100 μM SW provokes the appearance of only the branched isomer of Man5GlcNAc in the MBCs of HepG2 cells. Thus, as expected from its ability to trap large free polymannose type oligosaccharides in the cytosol, 100 μM SW abolished the appearance of linear Man5GlcNAc in the MBCs. In summary, results show that incubation of HepG2 cells with 100 μM SW arrests the cytosolic trimming of free polymannose type oligosaccharides and slows down their egress from this compartment.
Figure 4.
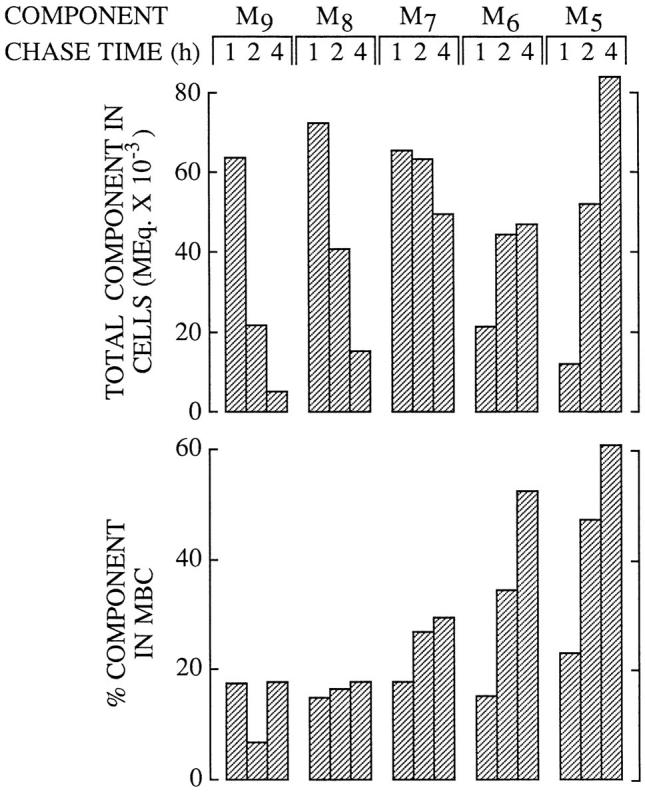
Different oligosaccharides appear in the membranebound compartment at different rates. Using the same data that was used for Fig. 3 the total cellular molar equivalents of the resolved oligosaccharides were displayed as shown at the 1, 2 and 4 h chase times (top), whereas the percent of each indicated component found to be present in the MBCs is plotted directly below. The abbreviations are; M9, Man9GlcNAc; M8, Man8GlcNAc; M7, Man7GlcNAc; M6, Man6GlcNAc; M5, Man5GlcNAc.
Figure 5.
The effects of swainsonine on the subcellular trafficking and processing of free polymannose oligosaccharides. HepG2 cells were pulse–radiolabeled and chased for the indicated times in the absence (CONTROL) or presence (SW) of 100 μM SW as described in the legend to Fig. 1. (A) After permeabilization of the cells free oligosaccharides were isolated from the cytosolic compartment and quantitated by scintillation counting. After 1 h of chase the quantity of free oligosaccharides in the cytosol are maximal (see Fig. 1) and this value has been set at 100%. During the chase the radioactivity associated with free oligosaccharides remaining in the cytosol has been expressed as a percent of the 1 h chase value. (B) After thin layer chromatographic resolution of free oligosaccharides isolated from the cytosolic compartment (CYTOSOLIC) and membrane-bound compartments (MBC) of cells radiolabeled and chased in the presence of 100 μM SW, components (M9, Man9GlcNAc; M8, Man8GlcNAc) were quantitated as described in the legend to Fig. 3. (C) After permeabilization of cells that had been chased for 4 h in the presence of 100 μM, SW-free oligosaccharides were isolated from the cytosolic (CYTOSOL) and membrane-bound compartments (MBCs) and resolved by thin layer chromatography on silica-coated plates. The abbreviations are as defined in the legend to Fig. 1.
The Golgi Apparatus Does Not Play a Major Role in the Trafficking of Free Oligosaccharides in HepG2 Cells
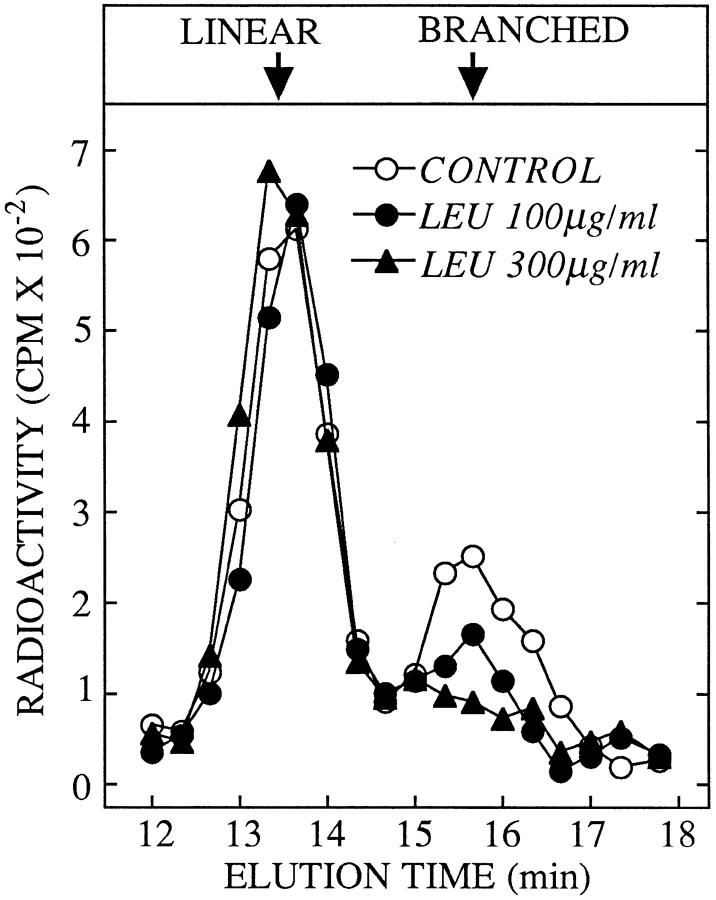
Taken together the results presented so far provide evidence to show that most free oligosaccharides follow a trafficking pathway from the ER into the cytosol and then into an MBC possessing a mannosidase with a low pH optimum. However, the appearance of the branched isomer of Man5GlcNAc in the MBCs of SW-treated cells suggested that there may also exist a vesicular trafficking pathway for free oligosaccharides involving the Golgi complex. Furthermore, as CCM A is known to affect the movement of glycoproteins through the Golgi complex (Yilla et al., 1993), it is possible that this agent inhibits the normal vesicular traffic of free oligosaccharides through this compartment and in so doing favors their traffic through the cytosol. To address this question we have performed pulse-chase experiments in the presence of 0.1 μM SW, a treatment which accurately reproduced the effects of CCM A on free oligosaccharide trafficking. Accordingly, at this low concentration, we found that SW neither inhibited the cytosolic processing of free oligosaccharides nor inhibited their loss from the cytosolic compartment. In addition, as observed with CCM A, 0.1 μM SW provoked an accumulation of Man5GlcNAc in the MBCs between the 1- and 8-h chase periods. At 4 h of chase we analyzed Man5GlcNAc isolated from the MBCs of cells treated with 0.1 μM SW as shown in Fig. 6 (CONTROL), and as can be seen there is now, in contrast to the situation observed in the presence of CCM A (Fig. 2), a mixture of the linear (cytosol-derived) and branched (Golgi-derived) isomers of Man5GlcNAc. The question now arises as to whether or not free oligosaccharides modified by Golgi enzymes, observed in the presence of SW, are transported through the Golgi as free oligosaccharides, or are in fact N-linked to glycoproteins while in transit through this compartment before their being released from the peptide backbone in an upstream degradative compartment. To resolve this problem we added leupeptin, an inhibitor of lysosomal protein degradation (Bond and Butler, 1987) which does not affect the vesicular import of material into lysosomes (Rohrer et al., 1995), to the SW-containing chase medium. As shown in Fig. 6 the quantity of the branched isomer of Man5GlcNAc is reduced in a dose-dependent manner without any affect being observed on the recovery of its linear counterpart. Therefore by using 0.1 μM SW to provoke an accumulation of Man5GlcNAc in the MBCs of HepG2 cells, we demonstrate that the two isomers of this free oligosaccharide have different origins. Results indicate that the bulk of free Man5GlcNAc produced by Golgi enzyme modifications (branched isomer) observed in the MBCs of SW-treated cells arises from its passage through the Golgi apparatus while N-linked to glycoprotein and not as a free oligosaccharide. Thus, although we cannot rule out the possibility that small amounts of free oligosaccharides are transported from the ER to the Golgi apparatus our results demonstrate that if this pathway does exist it represents only a minor trafficking route for free oligosaccharides in HepG2 cells.
Figure 6.
Leupeptin inhibits the formation of the branched isomer of Man5GlcNAc in swainsonine-treated HepG2 cells. HepG2 cells were pulse–radiolabeled and chased for 4 h in the presence of 0.1 μM SW (CONTROL). Where indicated the chase media were supplemented with leupeptin (LEU) at either 100 or 300 μg/ml. After permeabilization of the cells and isolation of free oligosaccharides from the MBCs the resulting radioactive components were resolved by HPAEC as described in Materials and Methods. The region of the chromatograph corresponding to the elution times of the linear (LINEAR) and branched (BRANCHED) isomers of Man5GlcNAc is shown.
Inhibitors of Macroautophagy Do Not Affect the Clearance of Free Oligosaccharides from the Cytosol of HepG2 Cells
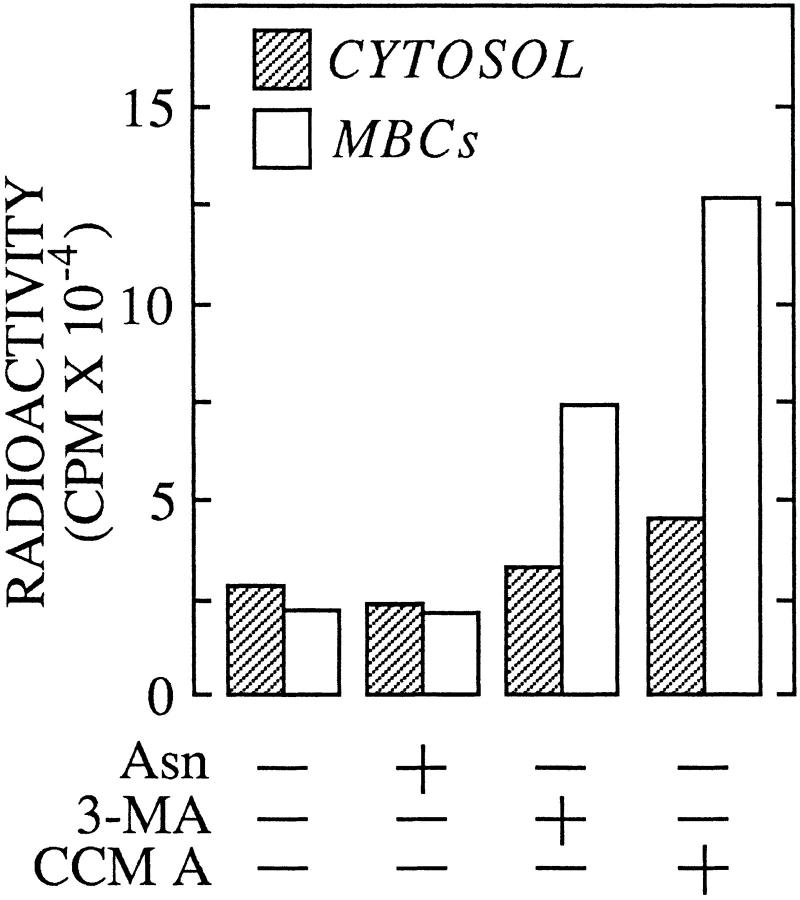
Having demonstrated that the bulk of free oligosaccharides are transferred from the cytosol into a degradative organelle, we went on to investigate the mechanism of this translocation process. Theoretically macroautophagy could be responsible for the clearance of oligosaccharides from the cytosol. To test this possibility we have performed chase incubations in the presence of well known inhibitors of macroautophagy. Pulse–chase experiments were performed as described in Fig. 1 except that the inhibitors were added to the chase incubations 45 min after the onset of the chase period. 3-MA inhibits the sequestration step of the autophagic process (Seglen and Gordon, 1982), therefore if autophagy were responsible for the clearance of free oligosaccharides from the cytosol, we would expect this reagent to cause an accumulation of fully trimmed free oligosaccharides in this compartment. Fig. 7 reveals this not to be the case and in fact after 8 h of chase, 3-MA had no effect on the clearance of free oligosaccharides from the cytosolic compartment of HepG2 cells. However, we did note that 3-MA caused an accumulation of free oligosaccharides in the MBCs, although this accumulation was not as large as that observed in the presence of CCM A, it is known that 3-MA interferes with the acidification of lysosomes (Caro et al., 1988). Asparagine has been shown to block the fusion of autophagosomes with the lysosomal compartment (Hoyvik et al., 1991) and would, if autophagy were responsible for the clearance of cytosolic oligosaccharides, lead to the accumulation of fully trimmed free oligosaccharides within MBCs. Again Fig. 7 demonstrates that this was not the case and shows that the chase incubation performed in the presence of asparagine is not significantly different from the control chase incubation.
Figure 7.
Inhibitors of macroautophagy do not affect the clearance of free oligosaccharides from the cytosol of HepG2 cells. Pulse–chase experiments were performed exactly as described for Fig. 1. Control or CCM A–treated cells were pulse radiolabeled and chased for 8 h in the presence of CCM A, 3-methyladenine (3-MA), or asparagine (Asn). After permeabilization of the cells as described in Materials and Methods, radioactivity associated with free oligosaccharides isolated from the cytosolic (CYTOSOL) and membrane-bound compartments (MBCs) was assayed by scintillation counting.
The Transfer of Free Oligosaccharides from the Cytosol into an MBC Requires Energy
To further investigate the nature of the cytosol-to-MBC translocation of free oligosaccharides in HepG2 cells, we have investigated the energy dependence of this process. For this purpose we have performed chase incubations in the presence of reagents known to deplete ATP levels in cultured cells (Krijnse-Locker et al., 1994). Fig. 8 A demonstrates that when HepG2 cells are chased in the presence of deoxyglucose and sodium azide the total molar equivalents of free oligosaccharides within the cytosol remains approximately constant when compared to that observed during control chase periods. Thin layer chromatographic examination of the cytosol- and MBC-derived oligosaccharides generated in cells treated with inhibitors of mitochondrial respiration for 8 h is shown in Fig. 8 B and shows that although cytosolic demannosylation of free oligosaccharides has been completed in drug-treated cells, the transfer of the cytosolic terminal digest product into an MBC is severely impaired leading to a marked cytosolic accumulation of Man5GlcNAc. We observed essentially identical results when we performed chase incubations in the presence of 10 μg/ml oligomycin, a specific inhibitor of the mitochondrial H+/ATPase (Ziegler and Penefsky, 1993) (results not shown).
Figure 8.
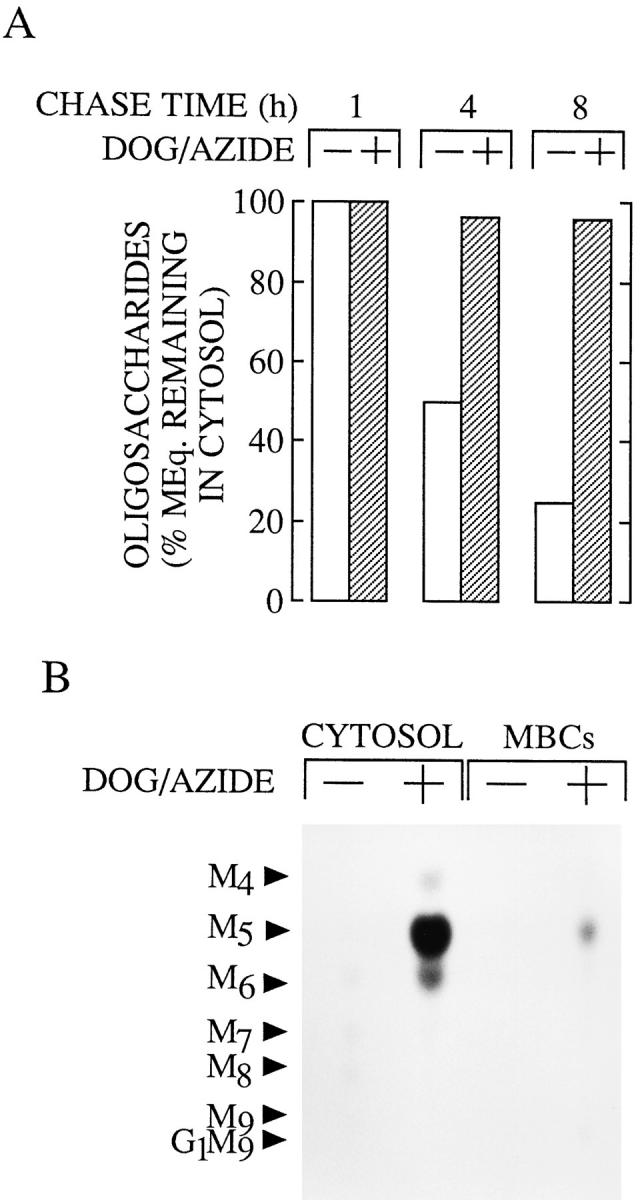
The effects of cellular ATP depleting agents on the clearance of free oligosaccharides from the cytosol of HepG2 cells. HepG2 cells were pulse–radiolabeled and chased for the indicated times as described in the legend to Fig. 1. Where indicated a mixture of deoxyglucose and sodium azide (DOG/AZIDE) was added to the chase medium 45 min after the onset of the chase period (to give a final concentration of 10 mM for each drug). (A) After permeabilization of the cells free oligosaccharides were prepared from the cytosolic fraction and the molar equivalents of these components were calculated as described in the legend of Fig. 3. As the quantity of free oligosaccharides reaches maximal levels after 1 h of chase this value was set at 100% for both the control and drug-treated cells. (B) Free oligosaccharides isolated from the cytosolic compartment (CYTOSOL) and membrane-bound compartments (MBCs) derived from cells chased for 8 h in the presence and absence of DOG/ AZIDE were separated by thin layer chromatography on silicacoated plates as described in the legend to Fig. 1. The abbreviations are as defined in the legend to Fig. 1. The oligosaccharide migrating as Man6GlcNAc is known to comprise a mixture of Glc1Man5GlcNAc and Man6GlcNAc (Moore and Spiro, 1994); the former is the likely degradation product of Glc1Man9GlcNAc by the cytosolic mannosidase.
Subcellular Fractionation Reveals That the Pseudolinear Isomer of Man5GlcNAc Is Degraded in Lysosomes
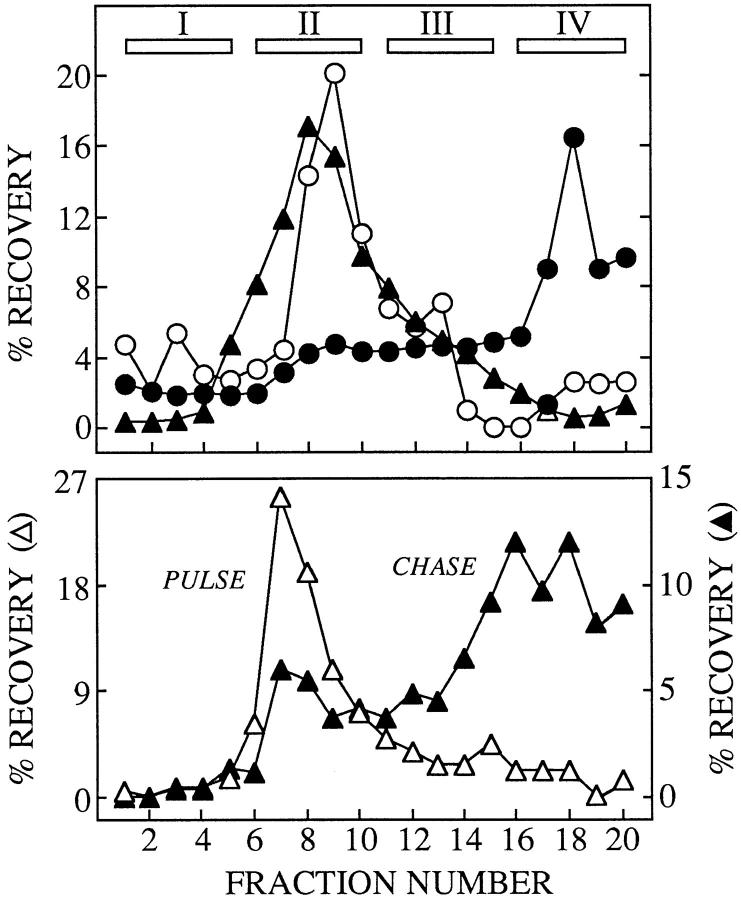
We have examined the identity of the MBC in which cytosolically generated free oligosaccharides are degraded by subcellular fractionation. As in the presence of CCM A, the linear isomer of Man5GlcNAc is stabilized in an MBC of permeabilized HepG2 cells we reasoned that in the absence of this drug free oligosaccharides are degraded within a subcellular compartment possessing a vacuolar H+/ATPase and an α-mannosidase with an acidic pH optimum. Although the lysosome best fits these criteria endosomes also possess a vacuolar H+/ATPase (Mellman et al., 1986), and in addition they are known to contain acidic hydrolases (Authier et al., 1994). To determine the nature of the MBC in which cytosolic-free oligosaccharides are degraded, we have fractionated HepG2 cell homogenates on a Percoll gradient known to be able to resolve endosomes from lysosomes (Rijnboutt et al., 1992). Initially we chose to fractionate HepG2 cells after treatment with CCM A, however results presented in Fig. 9 A demonstrate that when compared to subcellular fractionation of untreated cells this reagent caused a marked change in the distribution of the lysosomal marker enzyme, β-hexosaminidase, along the Percoll gradient while not disturbing the distributions of either the Golgi or endoplasmic reticulum marker enzymes. When CCM A–treated cells were pulse radiolabeled and then fractionated on a Percoll gradient we observed that free oligosaccharides (OS-GN2, not shown) are mainly localized to a region of the gradient containing endoplasmic reticulum and Golgi marker enzymes (Fig. 9 B). After 4 h of chase in the presence of CCM A, free oligosaccharides are now distributed throughout the gradient in similar but not identical fashion to that observed for the lysosomal enzyme β-hexosaminidase.
Due to difficulties in interpreting these results we have used 0.1 μM SW to provoke an accumulation of free oligosaccharides in the MBC of HepG2 cells before subcellular fractionation. As described above we have found that, at a concentration of 0.1 μM, SW mimics the effects of CCM A on the subcellular trafficking of free oligosaccharides in HepG2 cells. Treatment of HepG2 cells with 0.1 μM SW for 4 h did not dramatically affect the distributions of the lysosomal, Golgi, or endoplasmic reticulum marker enzymes along the Percoll gradient (compare the top panel of Fig. 10 with control fractionations in Fig. 9 A). To identify endosomal and lysosomal compartments, HepG2 cells, treated for 4 h with 0.1 μM SW, were allowed to endocytose a brief pulse of the fluid phase marker, HRP, and then chased for various times (van Weert et al., 1995). Cellular homogenates were then fractionated on Percoll density gradients as shown in Fig. 10 (bottom). Results show that, after the pulse, HRP is found uniquely in a region of the gradient (see top, fraction II), in which are found light membranes, including the endoplasmic reticulum and Golgi apparatus. After 4 h of chase the HRP is now mainly found at the bottom of the gradient, a region shown to contain the lysosomes (see top, fraction IV). These results are consistent with the transfer of the fluid phase marker from the endosomal compartment to the lysosomal compartment as previously described in HepG2 cells (van Weert et al., 1995). Cells treated with 0.1 μM SW were then pulse radiolabeled, chased for 1 and 4 h, homogenized, and then subjected to subcellular fractionation on Percoll density gradients. Fig. 11 A shows that after the pulse free oligosaccharides (OS-GN2, results not shown) are localized to a region of the gradient corresponding to the endoplasmic reticulum/Golgi region of the gradient, after 4 h of chase free oligosaccharides (mainly Man7-5GlcNAc, results not shown) now colocalize with the lysosomal marker enzyme β-hexosaminidase. Although only a small amount of free oligosaccharides were recovered from subcellular organelles after 1 h of chase, these components were distributed equally between regions of the gradient containing the lysosomal marker (fraction IV) and the endoplasmic reticulum/Golgi markers (fraction II). At this early chase time the gradient fraction II contained free oligosaccharides bearing 9 and 8 residues of mannose whereas those occurring in the gradient fraction IV comprised oligosaccharides bearing 7-5 residues of mannose (results not shown). After thin layer chromatography of free oligosaccharides recovered from the Percoll density gradients was performed, the Man5GlcNAc was recovered and quantitated as shown in the lower part of Fig. 11 A. We were unable to detect this oligosaccharide in the subcellular organelles of pulse-radiolabeled cells, but after 1 h of chase, small amounts of this component could be detected along the density gradient but were largely found in the lysosomal region of the gradient. After 4 h of chase the distribution of Man5GlcNAc along the Percoll gradient was indistinguishable from that of total free oligosaccharides and indicated that this component is uniquely localized in the lysosomal region of the gradient. HPAEC of the Man5GlcNAc recovered from the lysosomal region of the Percoll gradients (fraction IV) revealed that after 1 h of chase this fraction contained the linear isomer of Man5GlcNAc, indicative of its having been generated by the cytosolic mannosidase. After 4 h of chase both the linear and branched isomers of Man5GlcNAc could be detected in the lysosomal region of the Percoll gradient. Therefore we show that after 4 h of chase in either the presence of CCM A or 0.1 μM SW free oligosaccharides are closely associated with a lysosomal marker enzyme after subcellular fractionation on Percoll density gradients.
Figure 10.
Subcellular fractionation of SW-treated HepG2 cells on Percoll density gradients. (Top) Cells treated for 4 h with 0.1 μM SW were homogenized and fractionated on Percoll density gradients as described in the legend to Fig. 9. β-d-hexosaminidase (closed circles), β-1, 4-galactosyltransferase (triangles) and NADPH cytochrome C reductase (open circles) were assayed to identify the sedimentation positions of lysosomes, the Golgi apparatus and the ER, respectively. (Bottom) Cells were treated for 1 h with 0.1 μM SW, pulse labeled with HRP (open symbols), and then chased for 4 h (solid symbols) as described in Materials and Methods. HRP-labeled cells were homogenized and a postnuclear supernatant was fractionated on Percoll gradients. Material from the gradient was recovered in 20 fractions each of which was assayed for HRP activity.
Figure 11.
Characterization of free oligosaccharides isolated from organelles cosedimenting with lysosomal marker enzymes on Percoll gradients. (A) HepG2 cells pretreated with 0.1 μM SW for 1 h, were pulse radiolabeled with d-[2-3H]mannose and chased for 1 and 4 h in the presence of SW. After homogenization of the cells and fractionation of the postnuclear supernatants on Percoll gradients organelles were recovered from fractions I-IV (see Fig. 10). Free oligosaccharides (top) were recovered from the organelles of each fraction and assayed by scintillation counting. The results have been expressed as a percentage of the total free oligosaccharides recovered from the organelles. The quantities of radioactivity (cpm × 10−3) associated with free oligosaccharides isolated from the organelles were 74.7, 27.9, and 197.3, for the pulse, 1 and 4 h chase times, respectively. (Bottom) After thin layer chromatography of the oligosaccharides from each subcellular fraction the oligosaccharide migrating as Man5GlcNAc was quantitated by scintillation counting and recovery of this component as a percentage of the total Man5GlcNAc recovered from the subcellular fractions I–IV is represented for the pulse and the two chase times. The asterisk indicates that this component was not detected in the pulse incubation. (B) The Man5GlcNAc isolated from fraction IV of the Percoll density gradients as described above was analysed by HPAEC as described in Materials and Methods. The region of the chromatograph corresponding to the elution times of the linear (LINEAR) and branched (BRANCHED) isomers of Man5GlcNAc is shown.
Discussion
Permeabilization of the plasma membrane of HepG2 cells subsequent to pulse-chase incubations has enabled us to evaluate the hypothesis that cytosolic-free oligosaccharides are sequestered into and degraded by lysosomes. Short chase incubations revealed that free oligosaccharides rapidly appear in the cytosol at a time during which there is a loss of these components from the MBCs (Fig. 1, A and B). This observation can be accounted for by the previously observed rapid translocation of large, free polymannose-type oligosaccharides out of the ER into the cytosol (Moore et al., 1995). At present it is unclear whether this ER-to-cytosol transport of free oligosaccharides is the sole mechanism responsible for the appearance of free oligosaccharides in the cytosol. Recently, it has been proposed that newly synthesized glycoproteins may be translocated out of the ER and degraded in this compartment (Wiertz et al., 1996) by the actions of a cytosolic N-glycanase (Kitajima et al., 1995; Suzuki et al., 1994) and the proteasome (Ciechanover, 1994; Driscoll and Goldberg, 1990). Clearly such a phenomenon would also give rise to large, free polymannose-type oligosaccharides in the cytosol. Whatever the origin of cytosolic oligosaccharides in HepG2 cells radioactivity associated with these components reach maximal levels at ∼1 h of chase and declines thereafter (Fig. 1, A and B). We have tested the hypothesis that cytosolic-free oligosaccharides are translocated into lysosomes to be degraded. To do this we have performed pulse–chase experiments in the presence of a vacuolar H+/ATPase inhibitor. Concanamycins (Woo et al., 1992) are antibiotics which are closely related to bafilomycin A (Bowman et al., 1988), an agent which has been extensively used to investigate the acidification of intracellular organelles (Yoshimori et al., 1991; Yilla et al., 1993; Clague et al., 1994). In HepG2 cells the use of bafilomycin has demonstrated that vacuolar acidification is required for the transfer of fluid phase markers from endosomes to lysosomes (van Weert et al., 1995) and concanamycin B has been shown to perturb the trafficking and processing of glycoproteins in late Golgi compartments. Presumably then, vacuolar ATPase inhibitors block lysosomal degradation by inhibiting vesicular transport of certain substrates to the lysosome, and by increasing intralysosomal pH, thereby reducing acidic hydrolase activity. Accordingly, we reasoned that this reagent should allow us to examine the fate of cytosolic oligosaccharides without interference by those oligosaccharides generated during glycoprotein catabolism in the lysosome. We have shown that CCM A is able to substantially inhibit the SW-provoked appearance of the branched isomer of Man5GlcNAc in the MBC of HepG2 cells suggesting that glycoconjugates that have traversed the Golgi apparatus and which are destined for the lysosome are stabilized in the presence of this agent (data not shown). Here we show that despite its ability to block lysosomal glycoprotein degradation CCM A provoked an accumulation of free oligosaccharide in an MBC of HepG2 cells. This accumulation coincided precisely with the loss of free oligosaccharide from the cytosolic compartment. In addition we were able to show that the structures of the oligosaccharides that accumulated in an MBC of CCM A–treated cells were characteristic of their having been generated by the cytosolic mannosidase. These results clearly demonstrate the transfer of free oligosaccharides from the cytosol into an MBC. We went on to show that treating HepG2 cells with 100 μM SW blocked the trimming of cytosolic oligosaccharides by the cytosolic α-mannosidase and inhibited their loss from this compartment. As in the presence of 100 μM, SW oligosaccharides bearing 9 and 8 residues of mannose are stabilized with only a minor quantity of Man7GlcNAc being detected in the cytosolic compartment we conclude that most, if not all, cytosolic-free oligosaccharides must be derived from a preGolgi compartment (SW does not inhibit either the Golgi mannosidase I (Elbein et al., 1981), Fig. 5 C, or ER mannosidase I (Weng and Spiro, 1996). Our results show that Man7-5GlcNAc oligosaccharides can be cleared from the cytosol although our results strongly suggest that the smaller the oligosaccharide the more efficient its clearance from the cytosol. The small amounts of Man7GlcNAc that are transferred into the MBC are slowly trimmed during the chase suggesting that CCM A induced neutralization of degradative organelles is not complete (Fig. 1 B). Although lysosomal enzymes are known not to generate linear isomers of polymannose-type oligosaccharides (Michalski et al., 1990; Al Daher et al., 1991), it is not clear what isomer of Man5GlcNAc would be generated from Man7GlcNAc in the CCM A–sensitive MBC, but it is interesting to note that analysis of the MBC-derived Man5GlcNAc generated during CCM A chases always led to the detection of small amounts of an oligosaccharide that lost one mannose residue upon digestion with the α-1, 2 mannosidase (Fig. 2). In conclusion evidence demonstrates that cytosolic oligosaccharides are partially trimmed in the cytosol and transferred into a membrane bound compartment. Concerning the relationship between cytosolic trimming and clearance of free oligosaccharides from the cytosol two observations have to be discussed. First, Figs. 1 and 4 indicate that there is an apparent “hold up” of the trimming of cytosolic oligosaccharides at the Man7GlcNAc stage. Second, inspection of the rate of free oligosaccharide clearance from the cytosol indicates that this process is somewhat slower than the rate of appearance of these components in the cytosol (which is apparently complete after 1 h of chase; Fig. 3), a fact that would lead to a steady accumulation of oligosaccharide material in this compartment. These two observations may be related. We have preliminary data showing that the 5 mM mannose required to be added to the chase incubation media may both slow down the trimming of cytosolic-free oligosaccharides and slow down their clearance from this compartment (data not shown). Thus under physiological conditions it may be that cytosolic trimming is more efficient allowing a more rapid generation of the Man5GlcNAc, which may be cleared from the cytosol more efficiently than its more highly mannosylated counterparts.
After Trimming in the Cytosol-free Oligosaccharides Are Translocated into Lysosomes Where They Are Degraded
Subcellular fractionation of HepG2 cells chased in the presence of 0.1 μM SW for 1 and 4 h indicated that the distribution of free oligosaccharides along Percoll gradients was the same as that observed for the lysosomal marker enzyme β-hexosaminidase. Because we were interested in the final destination of cytosolic-free oligosaccharides, we have not directly addressed the question of whether free oligosaccharides are in fact transported from the cytosol directly into the lysosome. It is possible that the cytosol-toMBC oligosaccharide translocation machinery is located on a prelysosomal compartment which ultimately fuses with lysosomes. In this respect our results (Figs. 1–4) with the vacuolar ATPase inhibitor, CCM A, do not necessarily imply that the MBC into which free oligosaccharides are transported contains a pH-sensitive α-mannosidase. It is possible that free oligosaccharides may be stabilized in CCM A–treated HepG2 cells because the MBC into which they have been translocated cannot fuse with lysosomes whose membrane pH gradient has been perturbed. Indeed subcellular fractionation studies performed on CCM A–treated cells indicate that substantial amounts of free oligosaccharide are found in the endosomal region of the Percoll gradient suggesting that free oligosaccharides may not be transported directly into lysosomes. However, some of our observations suggest that free oligosaccharides may be transported directly into lysosomes. First, in the presence of CCM A, we were able to detect substantial amounts of free oligosaccharides bearing 3 and 4 residues of mannose in the vesicular compartment, suggesting that once translocated into this compartment, these components come into contact with and are slowly acted upon by an α-mannosidase with a low pH optimum. However, as endosomes may contain an acidic α-mannosidase (Authier et al., 1994), this result still leaves the possibility that free oligosaccharides are transported into endosomes and are then rapidly transferred to lysosomes by vesicular fusion. Although we cannot rule out this possibility, we noted that even when cells were chased in the presence of 0.1 μM SW for only 1 h, the majority of Man5GlcNAc recovered from the density gradient occurred in the lysosomal fraction and not in the endosomal fraction. The fact that substantial quantities of the endosomal marker HRP remain associated with endosomes 4 h after an HRP pulse suggests that fluid transfer between endosomes and lysosomes would not be rapid enough to account for the absence of free oligosaccharides in the endosomal compartment, if indeed these components had been translocated from the cytosol into endosomes, and then onto lysosomes by vesicular transport.
How Are Free Oligosaccharides Cleared from the Cytosol?
Examining the kinetics of loss of free oligosaccharides from cytosol yields useful information concerning the mechanism of this translocation process. First, as the cytoplasm of cells is continually sequestered by vesicles and delivered to lysosomes by macroautophagy, we wondered whether or not this bulk sequestration of the cytosol could account for the delivery of free cytosolic oligosaccharides to the lysosome. It has been shown that by starving hepatocytes of serum, autophagic sequestration can be stimulated 10-fold and under these conditions only 4% of the cytoplasm can be sequestered per hour (Kopitz et al., 1990). Here, despite the fact that HepG2 cells are chased in complete growth medium, a condition known to inhibit autophagy, we noted that oligosaccharides are cleared from the cytosol with a half-life of ∼3–4 h. Thus, it is apparent that autophagic sequestration cannot account for the transfer of free oligosaccharides into a vesicular compartment of HepG2 cells. In accordance with this we found that 3-MA, a well known inhibitor of autophagic sequestration (Seglen and Gordon, 1982), was without effect on the loss of oligosaccharide material from the cytoplasm. Furthermore as autophagic sequestration is a nonselective process, it should theoretically transfer all cytosolic oligosaccharides irrespective of structure into lysosomes. Our results with CCM A (Fig. 4) and 100 μM SW (Fig. 5) suggest that trimming of polymannose oligosaccharides to at least Man7GlcNAc is required before they are efficiently transferred into lysosomes, indicating that free oligosaccharides are not being sequestered into the lysosomal degradative compartment by bulk uptake of the cytosol. We demonstrate that the cytosol-to-lysosome translocation of free oligosaccharides is strongly impaired if the cells are chased under conditions known to deplete cellular ATP levels. This result is not surprising as in the presence of CCM A or 0.1 μM SW the cytosol-to-lysosome transfer of free oligosaccharides must occur against a substantial concentration gradient. It remains to be determined whether this transport process is accomplished by a transporter molecule or by an as yet unidentified mechanism such as a type of receptor mediated microautophagy. In conclusion our results suggest that free oligosaccharides are sequestred into lysosomes by an energy requiring process that displays oligosaccharide specificity.
What Are the Consequences of the Sequestration of Cytosolic-free Oligosaccharides in Lysosomes?
The results we have obtained with HepG2 cells throw light on a problem that has perplexed researchers investigating the genetic disorder, α-mannosidosis (see Discussion in Tulsiani and Touster, 1987; and conclusion in Daniel et al., 1992). Mannosidosis patients have a genetic deficiency in lysosomal α-mannosidase (Carroll et al., 1972) which leads to severe clinical symptoms. At a biochemical level the lesion is characterized by the presence of large quantities of free oligosaccharides in the tissues and urine from affected individuals. Surprisingly, however, a substantial proportion of the oligosaccharides isolated from the urine of mannosidosis patients possess structures not compatable with their having been formed by the incomplete degradation of complex oligosaccharides derived from lysosomal glycoprotein degradation. In fact the structures of these oligosaccharides were found to be linear in nature (Nordén et al., 1974; Strecker et al., 1976; Daniel et al., 1992), the largest of which being identical to the linear Man5GlcNAc shown in Fig. 2. The trafficking of free polymannose oligosaccharides from ER to cytosol and, after processing, into the lysosome may now explain the accumulation of the linear oligosaccharides observed in subjects deficient in lysosomal α-mannosidase, however, how these components gain access to the extracellular fluids still remains to be elucidated. In fact we have calculated that in CCM A–treated HepG2 cells 15% of all oligosaccharide structures (including N-linked polymannose-, complex-, and hybrid-type structures, and free oligosaccharides) occur as free polymannose species indicating that this recently outlined free oligosaccharide trafficking pathway must process large quantities of material in cells actively engaged in glycoprotein synthesis (data not shown). This observation is in line with the fact that the urine of mannosidosis patients may contain up to 250 mg/liter of small linear oligosaccharides (Strecker et al., 1976).
An intriguing question can now to be addressed: Why has the cell developed such an elaborate trafficking pathway for the degradation of free oligosaccharides generated in the lumen of the ER when two vesicular pathways already exist between the ER and lysosome? One explanation for this is that free oligosaccharides must be rapidly segregated from their N-linked counterparts in the ER in order to minimize their interference with the folding and trafficking of glycoproteins, a process now thought to involve lectins situated along the secretory pathway (Fiedler and Simmons, 1995). Alternatively free oligosaccharides maybe delivered to the cytosol for a purpose other than to be trimmed in order for their ultimate degradation in the lysosome. Interestingly, the cytosol contains an actin-binding protein, comitin (Weiner et al., 1993), which has recently been shown to be also a mannose-binding lectin (Jung et al., 1996). It was proposed that while the lectin moiety of this protein could bind to cytosolically disposed mannose-containing oligosaccharide lipids of the Golgi/ ER its actin-binding domain could tether it to microfilaments (Jung et al., 1996), thereby forming a bridge between the cytoskeleton and organelles of the secretory pathway. Could cytosolic-free oligosaccharides compete for binding sites on comitin thereby modulating this process?
In conclusion our results show that after their rapid appearance in the cytosol during glycoprotein biosynthesis free oligosaccharides are trimmed by the cytosolic mannosidase and transferred into lysosomes by an energy- dependent mechanism. Our results show that the lysosome is the site for the final degradation of these oligosaccharides and suggest that the lysosomal membrane is itself involved in the uptake of free polymannose-type oligosaccharides from the cytosol.
Acknowledgments
We thank Dr. J.R. Green (Ciba-Geigy Ltd.) for the gift of concanamycin A, Dr. J.-C. Michalski for supplying the two standard Man5GlcNAc oligosaccharides, and Dr. C. Rabouille for critical reading of the manuscript. HPAEC was performed by Dr. Thierry Fontaine (Laboratoire des Aspergillus, Institut Pasteur, Paris, Dir. Dr. J.-P. Latgé).
This work was supported by institutional funding from the Institut National de la Santé et de la Recherche Médicale (INSERM) and by grants from the Association Vaincre les Maladies Lysosomales and a European Community Human Mobility and Research Training Fellowship (to S.E.H. Moore).
Abbreviations used in this paper
- CCM A
concanamycin A
- DOG
deoxyglucose
- GlcNAc
N-acetylglucosamine
- Man
mannose
- MBC
membrane bound compartment
- 3-MA
3-methyladenine
- SLO
streptolysin O
- SW
swainsonine
Footnotes
Address all correspondence to S.E.H. Moore, INSERM U410, 16 rue Henri Huchard 75018 Paris, France. Tel.: 33 1 44856134. Fax: 33 1 42288765.
References
- Al Daher, S., R. De Gasperi, P. Daniel, N. Hall, C.D. Warren, and B. Winchester. Substrate specificity of human lysosomal α-d-mannosidase in relation to genetic α-mannosidosis. Biochem J. 1991;277:743–751. doi: 10.1042/bj2770743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano J, Kobata A. Purification and characterization of a novel α-mannosidase from Aspergillus saitoi. J Biochem. 1986;99:1645–1654. doi: 10.1093/oxfordjournals.jbchem.a135639. [DOI] [PubMed] [Google Scholar]
- Anumula KR, Spiro RG. Release of glucose-containing polymannose oligosaccharides during glycoprotein biosynthesis. J Biol Chem. 1983;258:15274–15282. [PubMed] [Google Scholar]
- Authier, F., B.I. Posner, and J.J.M. Bergeron. 1994. Hepatic endosomes are a major physiological locus of insulin and glucagon degradation in vivo. In Cellular Proteolytic Systems. A.J. Ciechanover and A.L. Schwartz, editors. 89–113.
- Barker R, Olsen KW, Shaper JH, Hill RL. Agarose derivatives of uridine diphosphate and N-acetyglucosamine for the purification of a galactosyltransferase . J Biol Chem. 1972;253:7135–7147. [PubMed] [Google Scholar]
- Bischoff J, Liscum L, Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various α-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986;261:4766–4774. [PubMed] [Google Scholar]
- Bond JS, Butler PE. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacan R, Cecchelli R, Verbert A. Catabolic pathway of oligosaccharide-diphospho-dolichol. Eur J Biochem. 1987;166:469–474. doi: 10.1111/j.1432-1033.1987.tb13539.x. [DOI] [PubMed] [Google Scholar]
- Caro LHP, Plomp PJAM, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-Methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur J Biochem. 1988;175:325–329. doi: 10.1111/j.1432-1033.1988.tb14200.x. [DOI] [PubMed] [Google Scholar]
- Carroll M, Dance N, Masson PK, Robinson D, Winchester BJ. Human mannosidosis—the enzyme defect. Biochem Biophys Res Commun. 1972;49:579–583. doi: 10.1016/0006-291x(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbé S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Daniel PF, Evans JE, De Gaspari R, Winchester B, Warren CD. A human lysosomal α(1-6)-mannosidase active on the branched trimannosyl core of complex glycans. Glycobiology. 1992;2:327–336. doi: 10.1093/glycob/2.4.327. [DOI] [PubMed] [Google Scholar]
- Driscoll J, Goldberg AL. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990;265:4789–4792. [PubMed] [Google Scholar]
- Elbein AD, Solf R, Dorling PR, Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci USA. 1981;78:7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Simmons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Haeuw J-F, Strecker G, Wieruszeski J-M, Montreuil J, Michalski J-C. Substrate specificity of rat liver cytosolic α-D-mannosidase. Eur J Biochem. 1991;202:1257–1268. doi: 10.1111/j.1432-1033.1991.tb16498.x. [DOI] [PubMed] [Google Scholar]
- Hoyvik H, Gorden PB, Berg TO, Stromhaug PE, Seglen PO. Inhibition of autophagic-lysosomal delivery and autophagic lactolysis by asparagine. J Cell Biol. 1991;113:1305–1312. doi: 10.1083/jcb.113.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E, Fucini P, Stewart M, Noegel AA, Schleicher M. Linking microfilaments to intracellular membranes: the actin-binding and vesicle- associated protein comitin exhibits a mannose-specific lectin activity. EMBO (Eur Mol Biol Organ) J. 1996;15:1238–1246. [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Muroi M, Ohkuma S, Waritani T, Magae J, Takatsuki A, Kondo S, Yamasaki M, Nagai K. Prodigiosin 25-C uncouples vacuolar type H+-ATPase, inhibits vacuolar acidification and affects glycoprotein processing. FEBS Lett. 1995;359:53–59. doi: 10.1016/0014-5793(94)01446-8. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Suzuki T, Kouchi Z, Inoue S, Inoue Y. Identification and distribution of peptide: N-glycanase (PNGase) in mouse organs. Arch Biochem Biophys. 1995;319:393–401. doi: 10.1006/abbi.1995.1309. [DOI] [PubMed] [Google Scholar]
- Kmiécik D, Herman V, Stroop CJM, Michalski J-C, Mir AM, Labiau O, Verbert A, Cacan R. Catabolism of glycan moieties of lipid intermediates leads to a single Man5GlcNAc oligosaccharide isomer: a study with permeabilized CHO cells. Glycobiology. 1995;5:483–494. doi: 10.1093/glycob/5.5.483. [DOI] [PubMed] [Google Scholar]
- Kopitz J, Kisen GO, Gorden PB, Bohley PO, Seglen PO. Nonselective autophagy of cytosolic enzymes by isolated rat hepatocytes. J Cell Biol. 1990;111:941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J, Ericsson M, Rottier PJM, Griffiths G. Characterisation of the budding compartment of mouse hepatitis virus: evidence that the transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski J-C, Haeuw J-F, Wieruszeski J-M, Montreuil J, Strecker G. In vitro hydrolysis of oligomannosyl oligosaccharides by the lysosomal α-D-mannosidases. Eur J Biochem. 1990;189:369–379. doi: 10.1111/j.1432-1033.1990.tb15498.x. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Ann Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Moore SEH, Bauvy C, Codogno P. Endoplasmic reticulum-to-cytosol transport of free polymannose oligosaccharides in permeabilised HepG2 cells. EMBO (Eur Mol Biol Organ) J. 1995;14:6034–6042. doi: 10.1002/j.1460-2075.1995.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SEH, Spiro RG. Demonstration that Golgi endo-α-Dmannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990;265:13104–13112. [PubMed] [Google Scholar]
- Moore SEH, Spiro RG. Intracellular compartmentalisation and degradation of free polymannose oligosaccharides released during glycoprotein biosynthesis. J Biol Chem. 1994;269:12715–12721. [PubMed] [Google Scholar]
- Nordén NE, Lundblad A, Svensson S, Autio S. Characterisation of two mannose-containing oligosaccharides isolated from the urine of patients with mannosidosis. Biochemistry. 1974;13:871–874. doi: 10.1021/bi00702a006. [DOI] [PubMed] [Google Scholar]
- Oku H, Hase S. Studies on the substrate specificity of neutral α-mannosidase purified from japanese quail oviduct by using sugar chains from glycoproteins. J Biochem. 1991;110:982–989. doi: 10.1093/oxfordjournals.jbchem.a123700. [DOI] [PubMed] [Google Scholar]
- Opheim DJ, Touster O. The purification and characterization of rat liver lysosomal α-L-fucosidase. J Biol Chem. 1977;252:739–743. [PubMed] [Google Scholar]
- Pierce RJ, Spik G, Montreuil J. Demonstration and cytosolic localisation of an endo-N-acetyl-β-D-glucosaminidase activity towards an asialoN-acetyl-lactosaminic-type substrate in rat liver. Biochem J. 1979;180:673–676. doi: 10.1042/bj1800673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnboutt S, Stoorvogel W, Geuze HJ, Strous GJ. Identification of subcellular compartments involved in biosynthetic processing of cathepsin-D. J Biol Chem. 1992;267:15665–15672. [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Johnson KF, Kornfeld S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J Cell Biol. 1995;130:1297–1306. doi: 10.1083/jcb.130.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup V, Touster O. Purification and characterisation of the α-dmannosidase of rat liver cytosol. J Biol Chem. 1976;251:3845–3852. [PubMed] [Google Scholar]
- Strecker G, Fournet B, Bouquelet S, Montreuil J, Dhondt JL, Farriaux J-P. Etude chimique des mannosides urinaires excrétées au cours de la mannosidose. Biochimie. 1976;58:579–586. doi: 10.1016/s0300-9084(76)80227-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kitajima K, Inoue S, Inoue Y. Does an animal peptide: N-glycanase have the dual role as an enzyme and a carbohydrate-binding protein? . Glycoconjugate J. 1994;11:469–476. doi: 10.1007/BF00731283. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Atkinson PH, Trimble RB. Separation of highmannose isomers from yeast and mammalian sources using high-pH anionexchange chromatography. Carbohydrate Res. 1991;215:211–217. doi: 10.1016/0008-6215(91)84021-6. [DOI] [PubMed] [Google Scholar]
- Tulsiani DRP, Touster O. Substrate specificities of rat kidney lysosomal and cytosolic α-D-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J Biol Chem. 1987;262:6506–6514. [PubMed] [Google Scholar]
- van Weert AWM, Dunn KW, Geuze HJ, Maxfield FR. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OH, Murphy J, Griffiths G, Schleicher M, Noegel AA. The actin-binding protein comitin (p24) is a component of the Golgi apparatus. J Cell Biol. 1993;123:23–34. doi: 10.1083/jcb.123.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Spiro RG. Endoplasmic reticulum kifunensine-resistant α-mannosidase is enzymatically and immunologically related to the cytosolic α-mannosidase. Arch Biochem Biophys. 1996;325:113–123. doi: 10.1006/abbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- Wieland FT, Gleason ML, Serafini TA, Rothman JE. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Wiertz EJHJ, Joner TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- Woo J-T, Shinohara C, Sakai K, Hasumi K, Endo A. Isolation, characterization and biological activities of concanamycins as inhibitors of lysosomal acidification. J Antibiotics. 1992;45:1108–1116. doi: 10.7164/antibiotics.45.1108. [DOI] [PubMed] [Google Scholar]
- Yilla M, Tan A, Ito K, Miwa K, Ploegh HL. Involvement of the vacuolar H+-ATPases in the secretory pathway of HepG2 cells. J Biol Chem. 1993;268:19092–19100. [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1: a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- Ziegler M, Penefsky HS. The adenine nucleotide translocase modulates oligomycin-induced quenching of pyranine fluorescence in submitochondrial particles. J Biol Chem. 1993;268:25320–25328. [PubMed] [Google Scholar]