Abstract
Transforming growth factor-β (TGFβ) is a dimeric peptide growth factor which regulates cellular differentiation and proliferation during development. Most cells secrete TGFβ as a large latent TGFβ complex containing mature TGFβ, latency associated peptide, and latent TGFβ-binding protein (LTBP)-1. The biological role of LTBP-1 in development remains unclear. Using a polyclonal antiserum specific for LTBP-1 (Ab39) and three-dimensional collagen gel culture assay of embryonic heart, we examined the tissue distribution of LTBP-1 and its functional role during the formation of endocardial cushion tissue in the mouse embryonic heart. Mature TGFβ protein was required at the onset of the endothelial-mesenchymal transformation to initiate endocardial cushion tissue formation. Double antibody staining showed that LTBP-1 colocalized with TGFβ1 as an extracellular fibrillar structure surrounding the endocardial cushion mesenchymal cells. Immunogold electronmicroscopy showed that LTBP-1 localized to 40–100 nm extracellular fibrillar structure and 5–10-nm microfibrils. The anti–LTBP-1 antiserum (Ab39) inhibited the endothelial-mesenchymal transformation in atrio-ventricular endocardial cells cocultured with associated myocardium on a three-dimensional collagen gel lattice. This inhibitory effect was reversed by administration of mature TGFβ proteins in culture. These results suggest that LTBP-1 exists as an extracellular fibrillar structure and plays a role in the storage of TGFβ as a large latent TGFβ complex.
Transforming growth factor-βs (TGFβs) are 25-kD dimeric peptide growth factors and are thought to regulate the signals by which primary and secondary inductions are initiated at different stages of embryogenesis in vertebrates. Several TGFβs and their receptors are expressed in developing organs, and their tissue distribution pattern has possible significance for signaling roles in epithelial-mesenchymal interaction during embryogenesis (Sporn et al., 1986; Barnard et al., 1990; MacLellan et al., 1993; Akhurst, 1994). Although it is clear that TGFβs are important molecules in the regulation of cellular differentiation and proliferation, it is still unknown how the activity of this growth factor is controlled under the physiological conditions. The biological activity of TGFβ is thought to be carefully controlled in a number of ways, via mRNA expression and protein synthesis, tissue or cellular distribution of receptors, presence of a latent form of TGFβ, activation of the latent form of TGFβ, and inactivation of the active form (Miyazono et al., 1993, 1994).
TGFβ is released from cells in a secretory form consisting of the mature growth factor associated with an NH2terminal peptide, a latency associated peptide (LAP)1, and a latent TGFβ-binding protein (LTBP)-1. This complex, the so-called large latent TGFβ complex, is unable to bind TGFβ receptors and is physiologically inactive (Pircher et al., 1986; Lawrence et al., 1985; Miyazono et al., 1991). The high molecular weight form of the large latent TGFβ complex contains LTBP-1, which is a glycoprotein of more than 190 kD and associated with the LAP via a disulfide bond (Miyazono et al., 1988; Wakefield et al., 1988; Saharinen et al., 1996). The mature TGFβ is noncovalently associated with the LAP; this complex is called the small latent TGFβ complex. The dissociation of the LAP renders the TGFβ biologically active. The large latent TGFβ complex is activated by proteolysis of the latent complex by plasmin (Lyons et al., 1988; Sato and Rifkin, 1989; Taipale et al., 1992). The latent TGFβ is also activated by thrombospondin in certain cell types (Schultz-Cherry and MurphyUllrich, 1993; Souchelnitskiy et al., 1995).
There are at least three forms of LTBP (LTBP-1, LTBP-2, and LTBP-3) which recently have been cloned and sequenced (Kanzaki et al., 1990; Tsuji et al., 1990; Moren et al., 1994; Gibson et al., 1995; Yin et al., 1995). LTBP-1 possesses 16–18 EGF-like domains, two of which contain hydroxyasparagine posttranscriptional modifications, and a novel motif containing eight cysteine residues (Kanzaki et al., 1990; Tsuji et al., 1990). These sequence characteristics of LTBP are also found in microfibrillar components, fibrillins, which suggests that LTBPs are involved in protein–protein interaction with cell surface molecules, as well as with the extracellular matrix (Apella et al., 1988; Rao et al., 1995). In the case of bovine endothelial cells cocultured with smooth muscle cells, targeting of the latent TGFβ complex to smooth muscle cells is required for the activation of latent TGFβ. Since LTBP-1 contains structural motifs involved in protein–protein interaction, LTBP-1 is thought to target the large latent TGFβ complex on the surface of certain cells in the triggering of the biological activity of TGFβ. Flaumenhaft et al. (1993) reported that LTBP-1 may play a role in the activation of the large latent TGFβ complex on the endothelial cell surface by concentrating the latent complex on the cell surface. In a model based on a human fibroblast culture, secreted TGFβ1 associates with the extracellular matrix via the targeting mechanism involving LTBP-1 of the large latent TGFβ1 complex. In this model, the association between LTBP-1 and the extracellular matrix is mainly covalent, and the release of latent TGFβ is due to a cleavage of LTBP-1 by plasmin (Taipale et al., 1992, 1994). It remains unclear what the biological functions and tissue distributions of LTBP-1 actually are in those regions where TGFβ-dependent cellular differentiation occurs during development.
Epithelial-mesenchymal interaction is one of the most important embryonic phenomena in the organization of the body axis, as well as in organogenesis. It is regulated via genes transcribed in a spatiotemporally restricted manner during development. Endothelial-mesenchymal transformation during the formation of endocardial cushion tissue is one of the most studied examples of epithelial-mesenchymal interaction in the embryogenesis (Markwald et al., 1975, 1977; Krug et al., 1985, 1987; Mjaatvedt and Markwald, 1989; Mjaatvedt et al., 1991; Nakajima et al., 1994, 1996). During its early development, the heart consists of two concentric epithelial layers, endocardium and myocardium, which are separated by an expanded acellular extracellular matrix (cardiac jelly). As development proceeds, some of the endothelial cells of both the outflow tract (OT) and atrioventricular (AV) regions change their phenotype to that of mesenchymal cells and migrate into the adjacent cardiac jelly. This is known as endothelial-mesenchymal transformation. It leads to the formation of endocardial cushion tissue of the primordia of both the valves and septa of the adult heart. This endothelial-mesenchymal transformation is regulated, at least in part, by TGFβ3 during endocardial cushion tissue formation in the chicken embryonic heart (Potts et al., 1989, 1991; Nakajima et al., 1994). During murine endocardial cushion tissue formation, three TGFβs (TGFβ1, TGFβ2, and TGFβ3) are preferentially distributed within the cushion tissueforming region in the heart, TGFβ1 being expressed in endothelial/mesenchymal cells and TGFβ2 and β3 in the outer myocardium (Akhurst et al., 1990; Dickson et al., 1993; Mahmood et al., 1992, 1995). Although the tissue distribution of TGFβs has been well examined at mRNA and protein levels, the functional significance of TGFβ in the formation of murine endocardial cushion tissue has yet to be determined. In addition, the tissue distribution and functional role of LTBP-1 during cushion tissue formation remains unknown.
Using an antiserum specific for LTBP-1 (Ab39), and an AV endothelial bioassay employing a three-dimensional collagen culture system (Bernanke and Markwald, 1982), we have examined the tissue distribution of LTBP-1 and its functional role during TGFβ-dependent endocardial cushion tissue formation in the mouse embryonic heart. Double immunohistochemical staining of antibodies specific for LTBP-1 (Ab39) and TGFβ1 revealed that an LTBP-1 molecule was codistributed with TGFβ1 as a fibrillar extracellular matrix in the cushion tissue at the onset of, or during cushion tissue formation. Immunoelectron microscopy showed that LTBP-1 was associated with 40– 100 nm fibrillar structures in the extracellular matrix surrounding mesenchymal cells. Ab39 inhibited mesenchymal formation in the AV endothelial bioassay and this inhibitory effect was reversed by administration of mature TGFβ proteins. These results suggest that LTBP-1 exists as an extracellular fibrillar structure and plays a role in the storage of the latent TGFβ complex during the formation of the endocardial cushion tissue.
Materials and Methods
Materials
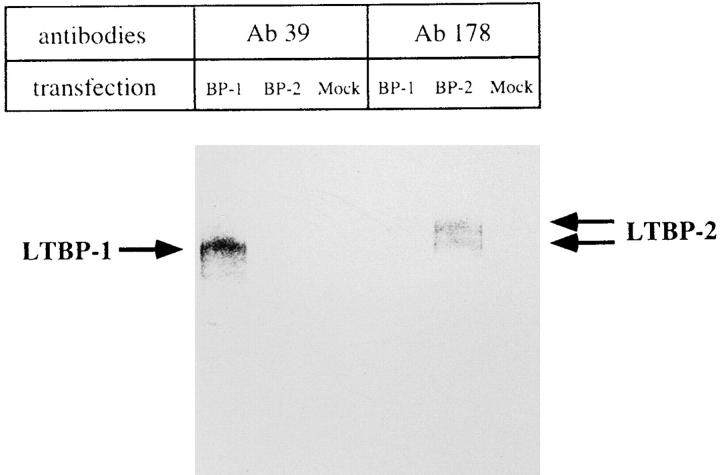
Rabbit antiserum (Ab39) was raised against native LTBP-1 purified from human platelets (Kanzaki et al., 1990; Miyazono et al., 1991); it does not cross-react with LTBP-2 (Moren et al., 1994; Fig. 1). This antiserum did not cross-react with mouse fibronectin in immunoblot (data not shown). Rabbit polyclonal antiserum (Ab178) was raised against a synthetic peptide for human LTBP-2 (Moren et al., 1994). A neutralizing antibody, anti–human TGF-β1 antibody (anti-TGFβ1, King Brewing, Kakogawashi, Japan), was raised against human recombinant TGFβ1 in rabbit, and affinity purified. This antibody reacts with TGFβ1 and TGFβ2 and inhibits the TGFβ1-induced production of collagen by NRK-49F to 75% of control at 50 μg/ml (manufacturer's description). Anti-TGFβ1-IgY (R & D systems, Minneapolis, MN) was raised against recombinant human TGFβ1 in chicken and this antibody shows<2% cross-reactivity with TGFβ2 and TGFβ3 in immunoblot (manufacturer's description). This antibody did not cross-react with LTBP-1 and mouse fibronectin in immunoblot (data not shown). A neutralizing antibody against chicken recombinant TGFβ3 (anti-TGFβ3) raised in goat was purchased from R & D Systems. This antibody completely neutralizes the TGFβ3-dependent inhibition of IL-4–dependent thymidine incorporation by HT-2 cells at 10 μg/ml, but does not inhibit the activity of TGFβ1, TGFβ2, or TGFβ5 (manufacturer's description). Recombinant human TGFβ1, purified human TGFβ2, and recombinant chicken TGFβ3 molecules were purchased from King Brewing, Nakarai tesque (Tokyo, Japan), and R & D Systems, respectively. Recombinant human LTBP-1 was generously donated by H. Ohashi (Kirin Brewery Co. Ltd., Maebashi, Japan).
Figure 1.
Specificity of anti–LTBP-1 antiserum (Ab39). COS-1 cells were transfected with human LTBP-1 (BP-1) or LTBP-2 (BP-2) cDNA. The cells were metabolically labeled, and resulting conditioned medium was immunoprecipitated with Ab39 or anti– LTBP-2 antiserum (Ab178) and analyzed by SDS-PAGE (5– 20% linear gradient gel) in the presence of dithiothreitol.
Animal Breeding
ICR (Jcl:ICR) mice were purchased from Japan Clea Co. (Tokyo, Japan). The mice were kept at 23°C with a 12/12-h light/dark cycle and were allowed free access to water and a standard pellet diet. Females were placed together with a male for 12 h, and mating was confirmed by the presence of a vaginal sperm plug (day 0 of gestation). On day 9.5, pregnant mice were sacrificed by cervical dislocation and a Caesarean section performed. The heart was removed from each embryo and subjected to the experiments described below.
Three-dimensional Collagen Gel Culture of the AV Region
Hydrated collagen gel (1 mg/ml type I rat-tail collagen; Collaborative Research, Waltham, MA) was prepared in 4-well dishes (Nunc, Roskilde, Denmark) as described by Bernanke and Markwald (1982). The hearts from the mouse embryos were collected and placed in Dulbecco's PBS. The AV region was dissected out and cut longitudinally to expose the lumen, and then placed on the drained collagen gel that had been saturated with CM199 (CM199; medium 199 containing 5% FBS, 5 μg/ml insulin, 5 μg/ ml transferrin, 5 ng/ml selenium, and streptomycin/penicillin, ITS; Collaborative Research, strept/pen; GIBCO BRL, Gaithersburg, MD, FBS; SANKO JYUNYAKU, Tokyo). After 12 h incubation, individual cultures were processed under the various test conditions described below. To examine the involvement of TGFβs in endothelial-mesenchymal transformation, AV endothelial cells cocultured with associated myocardium (AV explant) were incubated with antibodies against TGFβ (anti-TGFβ1, anti-TGFβ3, or both antibodies together) in CM199 and the results compared with that observed with CM199. To determine the biological activity of LTBP-1 during endothelial-mesenchymal transformation, AV explants were treated with various concentrations of Ab39 in CM199, with CM199 alone, or with CM199 containing Ab39 and TGFβ protein (TGFβ1, TGFβ2, or TGFβ3). Cultures were examined under a Hoffman modulation microscope for endothelial outgrowth and for the characteristics of epithelial-mesenchymal transformation (such as cell hypertrophy, loss of cell–cell contacts, formation of migratory processes, and mesenchymal invasion). The number of invading mesenchymal cells is shown as the mean ±SD. In these bioassays, an “inhibition of transformation” was considered to have occurred when the number of invading mesenchymal cells was more than two standard deviations below the number seen in a control explant cultured with CM199 alone. The determination of the endothelial outgrowth was measured with the end of an eyepiece equipped with a grid pattern and classified as follows: “−”, no endothelial outgrowth surrounding the myocardium; “+”, maximum diameter of endothelial monolayer was less than twice the maximum diameter of the myocardium; “++”, maximum diameter of endothelial monolayer was more than twice the maximum diameter of the myocardium.
Gel Electrophoresis and Immunoblotting
Whole embryos (9.5 or 14.5 d) were homogenized in ice-cooled RIPA buffer (50 mM Tris-HCl at pH 7.2, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate). The deoxycholate-insoluble pellet was collected at 8,000 g for 10 min. The pellet was washed in PBS, and then digested with plasmin (0.1 U/ml) in PBS for 1 h at 37°C. The resulting supernatant, containing ∼50 μg of protein, was concentrated by ultrafiltration (10-kD cutoff; Millipore, Tokyo), diluted into nonreducing SDS-PAGE solubilization buffer (63 mM Tris-HCl at pH 6.8, containing 2% SDS, and 6 M urea), boiled for 5 min, and subjected to 6% SDS-PAGE (Laemmli, 1970). Separated proteins were transferred to an Immobilon-P membrane (Millipore, Bedford, MA), nonspecific binding sites were blocked with 5% nonfat dried milk in Tris buffered saline, and the membrane was incubated with Ab39 (diluted 1:500) or Ab178 (diluted 1:200). After extensive washing, immunoreactivity was detected with the aid of an alkaline phosphatase–conjugated goat antibody against rabbit IgG (BioRad, Hercules, CA) and BCIP/NBT.
cDNA Transfection, Metabolic Labeling, Immunoprecipitation, and Gel Electrophoresis
COS-1 cells were transfected with pSV7d-LTBP-1 (Kanzaki et al., 1990), pcDNA3-LTBP-2, or pcDNA3 using LIPOFECTAMIN reagent (GIBCO BRL) following the manufacturer's recommendation. 2 d after transfection, cells were rinsed twice with PBS and the culture medium was exchanged to methionine- and cysteine-free DMEM supplemented with 0.1 mCi/ml of [35S]methionine and cysteine mixture (Pro-mix, Amersham, England). After 12 h of metabolic labeling, conditioned media were collected, concentrated, split equally into two tubes, subjected to immunoprecipitation by Ab39 or anti-LTBP-2 (Ab178) and analyzed by SDSPAGE (reducing condition) using 5–20% linear gradient gel as described previously (Moren et al., 1994). The gels were fixed, dried, and analyzed with a Fuji BAS 2000 Bio-Imaging Analyzer (FUJIFILM, Tokyo).
Indirect Immunofluorescence Microscopy
Embryos (9.5–10.5 d) were equilibrated in a graded series of sucrose solutions (10–20%, wt/vol) in PBS at 4°C for 12 h, embedded in OCT compound (Miles, IN), and frozen in 2-methylbutane cooled over liquid nitrogen. Frozen sections (6–8 μm) were cut on a cryostat, mounted on slides coated with 3-triethoxysilyl propylamine (Merck, Germany), and air dried. After rinsing in PBS for 15 min, sections were blocked with 1% bovine serum albumin (BSA) in PBS for 1 h, and incubated with primary antibody (Ab39 was diluted 1:500-1,000 in blocking solution) in a moist chamber for 2 h at room temperature. They were then rinsed in PBS, incubated with a tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC)- conjugated goat antibody against rabbit IgG (Cappel, Malvern, PA) for 1 h, rinsed with PBS and coverslipped with the mounting medium (0.2 M N-propylgallate in 10% PBS/90% glycerol). Similarly prepared fresh frozen sections were incubated with plasmin (0.1 U/ml in PBS) at 37°C for 1 h. The resulting sections were rinsed in PBS for 30 min and stained with Ab39, as described above.
The fresh frozen sections were processed for double antibody staining for anti-TGFβ1-IgY and Ab39. Sections were incubated with primary antibody mixture (Ab39, 1:500; anti-TGFβ1-IgY, 10 μg/ml in the blocking solution) for 2 h at room temperature, rinsed in PBS and incubated with TRITC-conjugated goat antibody against rabbit IgG for 1 h. They were then rinsed extensively in PBS and stained with fluorescein-5-isothiocyanate (FITC)–conjugated rabbit antibody against chicken IgY (Promega, Madison, WI), rinsed in PBS, and coverslipped with the mounting medium. The FITC-conjugated rabbit antibody against chicken IgY did not recognize Ab39-TRITC-conjugated goat antibody against rabbit IgG complex on tissue sections (data not shown).
AV explant cultures grown on collagen gels were processed for wholemount double immunostaining. Cultures were drained of medium, rinsed with PBS, fixed with 4% paraformaldehyde in PBS for 1 h, rinsed with PBS, blocked for 1 h with 1% BSA in PBS, incubated with primary antibody mixture (Ab39 and anti-TGFβ1-IgY) at 4°C overnight, rinsed with PBS, incubated with TRITC-conjugated goat antibody against rabbit IgG for 2 h, rinsed with PBS, incubated with FITC-conjugated rabbit antibody against chicken IgY, rinsed with PBS, transferred to slides, and coverslipped with the mounting medium. Specificity of immunostaining for LTBP-1 or TGFβ1 was confirmed by preincubating the antibody for 30 min with antigen (Ab39 vs purified human LTBP-1 or recombinant human LTBP-1; anti-TGFβ1-IgY vs recombinant human TGFβ1). Samples were observed under a conventional fluorescence microscope (BX60; OLYMPUS, Tokyo) and photographed using Tmax 400 (Kodak) or PROVIA 400 (FUJI FILM). The exposure times for FITC and TRITC images were 32 s and 40 s, respectively.
Immunogold Electron Microscopy
Post-embedding for indirect immunoelectron microscopy was performed using the method described by Tamaki and Yamashina (1994). In brief, the AV region was resected from the heart of each 9.5-d mouse embryo and fixed with 0.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M PBS with the aid of microwave irradiation (150 W; 120 s; maximum temperature, 37°C). Samples were then fixed for an additional 1 h at 4°C, rinsed in 7% sucrose in PBS for 12 h, and postfixed in 1% OsO4 containing 1.5% potassium ferrocyanide for 30 min at 4°C. Samples were dehydrated in a graded ethanol series (20 min at each concentration), embedded in LR White resin (London Resin, Berkshire, UK) after replacement, and the resin allowed to polymerize for 24 h at 58°C. Ultra-thin sections were cut, mounted on 200-mesh nickel grids, and pretreated for etching with a saturated aqueous solution of sodium metaperiodate for 30 s followed by five subsequent washes in distilled water. Nonspecific binding sites were blocked with 1% BSA in PBS for 30 min. The sections were incubated with Ab39 (diluted 1:500) for 12 h at 4°C, rinsed in PBS, and incubated with a 5-nm colloidal gold-conjugated secondary antibody (Amersham) for 1 h at room temperature. They were rinsed in PBS followed by distilled water, and stained with uranyl acetate and lead citrate.
The pre-embedding method for AV-regions was as follows. They were dissected out from 9.5-d embryos and incubated with Ab39 in 0.1% BSA/ PBS for 12 h at 4°C, rinsed in PBS, and incubated in colloidal gold-conjugated secondary antibody in 0.1% BSA/PBS at 4°C for 12 h. After the extensive washing in PBS, they were fixed in 2.5% glutaraldehyde/4% paraformaldehyde in PBS for 2 h at 4°C and postfixed in 1% OsO4/PBS for 40 min at 4°C. Samples were dehydrated in a graded ethanol series and embedded in Epon. Ultra-thin sections were cut, mounted on 150-mesh grids and stained with uranyl acetate and lead citrate. Specificity for immunostaining was confirmed by preincubating the antiserum with purified human LTBP-1 or recombinant human LTBP-1. Samples were observed using a transmission electron microscope (JEM-100C; JEOL, Tokyo) operated at 80 kV.
Results
Ab39 Recognizes Mouse LTBP-1
In this study, we used a polyclonal antiserum (Ab39) raised against a purified human LTBP-1 (Kanzaki et al., 1990; Miyazono et al., 1991). It was important to confirm that Ab39 does not cross-react with LTBP-2 (Moren et al., 1994) and recognizes mouse LTBP-1 (Nunes et al., 1995). Radiolabeled conditioned medium obtained from COS-1 cells transfected with human LTBP-1 or LTBP-2 cDNA was subjected to immunoprecipitation with Ab39 or antiLTBP-2 antiserum (Ab178). Resulting immunoprecipitates were analyzed by gel electrophoresis/BAS 2000. As shown in Fig. 1, Ab39 immunoprecipitated 205-kD protein (LTBP-1) in the conditioned medium from COS-1 cells transfected with human LTBP-1 cDNA, whereas it did not precipitate any detectable protein in that transfected with human LTBP-2 cDNA. Ab178 recognized LTBP-2 protein and did not cross-react with LTBP-1 (Fig. 1). Using these antisera, we also performed an immunoblot analysis of the plasmin digest of a deoxycholate insoluble fraction obtained from homogenizing embryos (9.5 or 14.5 d). Both the 9.5- and 14.5-d embryos contained an Ab39 immunoreactive 190kD protein (Fig. 2). Our preliminary immunoblot showed that Ab178 reacted with a 180-kD band in the extracts of 14.5-d embryos; there was no detectable immunoreactivity in the extracts of 9.5-d embryos (data not shown). Thus, Ab39 appears to recognize mouse LTBP-1 and does not react with mouse LTBP-2.
Figure 2.
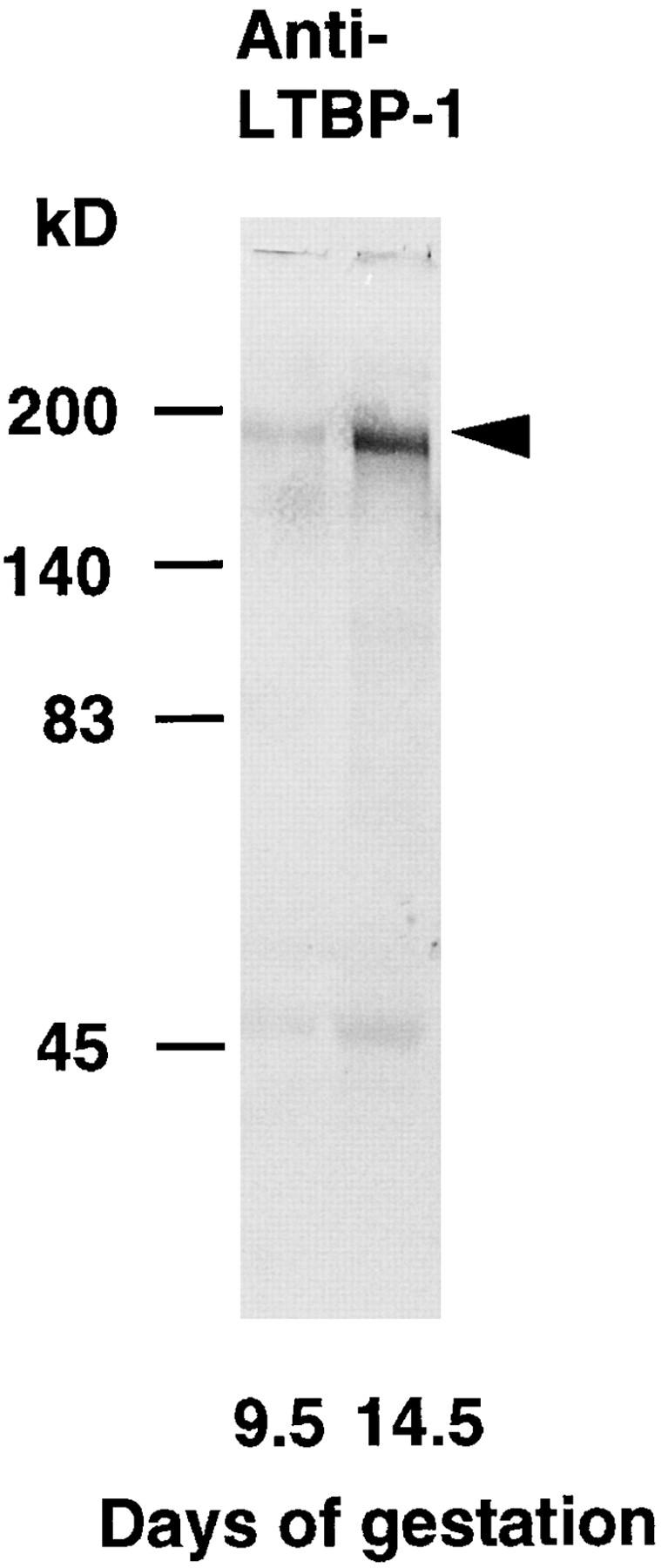
Immunoblot of plasmin digest of a deoxycholate insoluble fraction obtained from homogenizing 9.5- or 14.5-d whole embryo probed with anti–LTBP-1 antiserum (Ab39). Deoxycholate insoluble fraction of whole homogenate of the embryos was digested with plasmin, and the resulting supernatant containing 50 μg of protein was electrophoresed (6% SDS-PAGE) under nonreducing conditions. Separated proteins were transferred to Immobilon-P and stained with Ab39. Ab39 recognizes a 190-kD band both in 9.5- and in 14.5-d embryo (arrowhead).
TGFβ Is Required to Initiate the Formation of Endocardial Cushion Tissue
Before examining the function of LTBP-1 during endocardial cushion tissue formation, we examined whether TGFβs were required for the initiation of this embryonic phenomenon in an AV endothelial coculture with associated myocardium (AV explant) on a three-dimensional collagen gel lattice. Although there have been studies of the tissue distribution of TGFβs during mouse endocardial cushion formation, there is no evidence of a regulatory effect of TGFβs on endothelial-mesenchymal transformation in mouse embryonic heart. AV explants, containing endocardium and myocardium, were prepared from 9.5-d mouse embryonic hearts and cultured with or without neutralizing antibodies against TGFβs in CM199 (Table I). We administrated either anti-TGFβ1, anti-TGFβ3, or both antibodies to the explant culture at an effective concentration of antibody according to manufacturer's description. From each AV explant cultured in CM199 alone, there was an invasion of mesenchymal cells into the gel lattice and many mesenchymal cells were observed after 60 h in culture. An anti-TGFβ1 inhibited this mesenchymal invasion: the number of mesenchymal cells was significantly lower and the percentage of the explant invaded was reduced to 75% of control. Culture with both anti-TGFβ1 and antiTGFβ3 antibodies completely inhibited mesenchymal formation. However, an anti-TGFβ3 antibody alone did not inhibit mesenchymal formation in the gel lattice. These results indicate that TGFβs are required for the generation of the endothelial-mesenchymal transformation. However, it is still uncertain which TGFβs are involved in endothelial-mesenchymal transformation in vivo during endocardial cushion tissue formation.
Table I.
Anti-TGFβ Antibody Blocking Experiment in AtrioVentricular Explant Culture
| Culture condition | Mesenchymal formation (60 h) | |||
|---|---|---|---|---|
| No. of invaded cells (mean ± SD) | Transformation (explants) | |||
| % | ||||
| Control | ||||
| CM199 (n = 8) | 134 ± 30 | 100 | ||
| NR + NGIg/CM199 | 136 ± 33 | 100 | ||
| (n = 12) | ||||
| Experimental | ||||
| Anti-TGFβ1 (n = 8) | 97 ± 34* | 75 | ||
| Anti-TGFβ3 (n = 8) | 141 ± 15 | 100 | ||
| Anti-TGFβ1 + 3 (n = 8) | 66 ± 37* | 27 | ||
Anti-TGFβ1 antibody (50 μg/ml) alone, and the combined administration of the antibodies, anti-TGFβ1 (50 μg/ml) and anti-TGFβ3 (10 μg/ml), both significantly inhibited mesenchymal cell invasion in comparison with control culture (*, P < 0.05. Mann-Whitney U test). Anti-TGFβ3 antibody (10 μg/ml) alone did not. An inhibition of transformation was defined when the number of invading mesenchymal cells was less than two standard deviations below the numbers seen in a control culture. CM199, medium 199 containing 5% FBS; NR + NGIg, 50 μg/ml of normal rabbit IgG and 10 μg/ml of normal goat IgG; n, number of explants tested; No., number.
Large Latent TGFβ Complex Is Distributed as an Extracellular Material in Mouse Endocardial Cushion Tissue
At 9.0–9.5 d of gestation, the heart consists of two concentric epithelial layers, endocardium and myocardium, which are separated by an expanded cardiac jelly. At 9.5–10 d of gestation, endothelial cells in the OT and AV regions change their phenotype to that of mesenchymal cells. They invade into the adjacent cardiac jelly leading to the formation of endocardial cushion tissue (Markwald et al., 1975, 1977; Kaufman, 1992).
At the onset of this endothelial-mesenchymal transformation, a fibrous structure containing LTBP-1–like molecules was observed beneath hypertrophied endothelial cells, transforming cells, of OT and AV (Fig. 3, a and b). As development proceeds, endothelially derived mesenchymal cells accumulate within the AV and OT cardiac jelly, forming endocardial cushion tissue. A fibrillar arrangement of LTBP-1–like molecule was observed surrounding the migrating mesenchymal cells within the cushion tissue (Fig. 3, a–d). This Ab39-immunoreactive molecule could be removed by plasmin treatment in nonfixed fresh frozen tissue-sections before incubation with the primary antibody (Fig. 3, e and f). These results suggested that the fibrillar arrangement of LTBP-1–like molecule was associated with the extracellular matrix via the plasmin-sensitive extracellular matrix binding sequence (Taipale et al., 1994). The extracellular matrix of mesenchymal tissue surrounding the dorsal aorta, gut epithelium, and neural tube also contained LTBP-1–like molecule (our unpublished observations). Immunogold electronmicroscopy revealed that a 40–100-nm low electron dense extracellular fibrillar network surrounding the mesenchymal cells was decorated by Ab39-gold particles (Fig. 4 a). In addition to 40–100-nm extracellular fibrillar structure, microfibrils having a diameter of 5–10 nm were stained with Ab39 by the pre-embedding method (Fig. 4 b). The immunostaining was blocked by a preincubation of Ab39 with purified human LTBP-1 (5 μg/ ml; Fig. 3, g and h and 4 c) or recombinant human LTBP-1 (50 μg/ml; data not shown).
Figure 3.
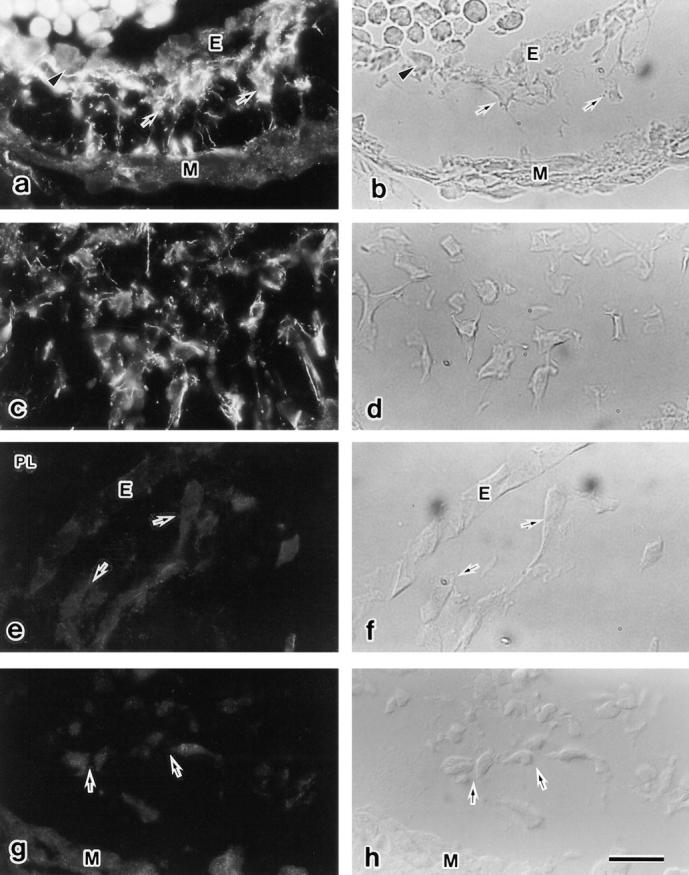
Immunohistochemical staining for LTBP-1 in fresh frozen sections of endocardial cushion tissue. (a and b) At the onset of endothelial-mesenchymal transformation (9.5 d), fibrillar LTBP-1–like molecules are distributed beneath the hypertrophied transforming cell (arrowhead) and surrounding mesenchymal cells (arrows). There is no LTBP-1 beneath the stationary endothelial cells (E). (c and d) Fibrillar LTBP-1–like molecules are observed surrounding the invaded mesenchymal cells in 10.5 d AV cushion tissue. (e and f) Fresh frozen section treated with plasmin (PL) and stained with Ab39. Ab39-immunoreactivity disappeared after plasmin digestion. (g and h) The Ab39-immunostaining was blocked by a pre-incubation with purified human LTBP-1. E, endocardium; M, myocardium; arrows, mesenchymal cells. b, d, f, and h are light microscopic images of a, c, e, and g. Bar, 200 μm.
Figure 4.

Immunogold electron microscopic detection of LTBP-1 in endocardial cushion tissue. (a) A 40–100 nm extracellular fibrillar network surrounding the invading mesenchymal cell (Me) is decorated with an Ab39-gold-particles complex (arrowheads). (b) Extracellular microfibril is stained with Ab39 (pre-embedding method). (c) The immunoreactivity of Ab39 was blocked by preincubation of an antibody with purified human LTBP-1. Bar, 0.5 μm.
To determine whether extracellular TGFβ1 (Akhurst et al., 1990) colocalizes with LTBP-1 during the endocardial cushion tissue formation, we examined immunohistochemical colocalization of TGFβ1 and LTBP-1 in tissue sections (9.75 d embryonic heart) and cultured AV explants on collagen gel lattice. There are several anti-TGFβ1 antibodies, some antibodies are able to detect a cellular TGFβ1, while the others react with extracellular TGFβ1 (Thompson et al., 1989). We used anti-TGFβ1 IgY, purchased from R & D systems, because this antibody stained extracellular TGFβ1. Fresh frozen sections from 9.75-d embryos were processed for double immunostaining for TGFβ1 and LTBP-1. Results showed that extracellular anti-TGFβ1 immunoreactive fibers were observed surrounding the mesenchymal cells and the TGFβ1-immunoreactive materials coincided with Ab39 immunoreactivity (Fig. 5, a and b). Double antibody staining was also performed against cultured AV explant on collagen gel lattice. AV explants were prepared from 9.5-d embryonic heart and cultured on the gel lattice. After 48 h in culture, where endothelial-mesenchymal transformation progressively occurred, cultures were fixed and double-stained with anti-TGFβ1 IgY and Ab39. The extracellular fibrillar codistribution of TGFβ1 and LTBP-1 surrounding the invaded mesenchymal cells was observed (Fig. 5, e and f). The double antibody staining was blocked by preincubation of an antibody-mixture containing purified human LTBP-1 (5 μg/ml) and recombinant human TGFβ1 (1 μg/ml; Fig. 5, c and d and g and h). Our present observations indicate that the large latent TGFβ complex is distributed as an extracellular fibrillar matrix in the developing endocardial cushion tissue.
Figure 5.

Immunohistochemical colocalization of TGFβ1 and LTBP-1 in 9.75-d embryonic heart and cultured atrioventricular (AV) explant. Double antibody staining for anti-TGFβ1 IgY (a, c, e, and g) and anti–LTBP-1 antiserum (Ab39; b, d, f, and h) was performed. Extracellular fibrillar structures containing TGFβ1 (arrowheads in a and e) coincides with LTBP-1 (arrowheads in b and f) surrounding the AV cushion tissue mesenchymal cells. The immunoreactivities of anti-TGFβ1 IgY and AB39 were blocked by preincubation of antibody mixture with recombinant human TGFβ1 and purified human LTBP-1 (c and d and g and h). Note that an explant (EX in e and f) containing myocardium, mesenchyme, and endocardium shows immunoreactivity. Bar, 20 μm.
Ab39 Inhibits Mesenchymal Formation in Culture
To determine whether LTBP-1 was required for the generation of the endothelial-mesenchymal transformation, we cultured AV explants from 9.5-d embryonic hearts with or without the presence of Ab39 in CM199 (Table II). Antiserum against LTBP-1 (AB39) inhibited the mesenchymal cell invasion into the gel lattice in a dose-dependent manner. At a concentration of 1 μl/ml of Ab39 in CM199, not only the endothelial outgrowth on the gel lattice, but also mesenchymal invasion into the gel lattice was inhibited, by comparison with culture in CM199 alone (Fig. 6, a–d) or normal rabbit serum in CM199. At lower concentrations of Ab39, only the mesenchymal invasion (number of invading mesenchymal cells and percentage of explant transformed into mesenchyme) was inhibited. These results indicate that LTBP-1 was required for the generation of the endothelial outgrowth and for endothelial-mesenchymal transformation. They also suggest that a smaller amount of LTBP-1 was required for the formation of the endothelial outgrowth.
Table II.
Anti–LTBP-1 Antiserum (Ab39) Blocking Experiment in AV Explant Culture
| Culture condition | Mesenchyme formation (60 h) | Endothelial outgrowth | ||||
|---|---|---|---|---|---|---|
| No. of invaded cells (mean ± SD) | Transformation (explants) | |||||
| % | ||||||
| Control | ||||||
| CM199 (n = 8) | 134 ± 30 | 100 | ++ | |||
| NRS/CM199 (n = 5) | 121 ± 21 | 100 | ++ | |||
| Ab39/CM199 | ||||||
| 0.01 μl/ml (n = 10) | 92 ± 24* | 80 | ++ | |||
| 0.01 μl/ml (n = 10) | 76 ± 19* | 50 | ++ | |||
| 1 μl/ml (n = 11) | 15 ± 4* | 0 | + | |||
| Ab39 (1 μl/ml) | ||||||
| +TGFβ (2 ng/ml) | ||||||
| β1 (n = 6) | 123 ± 28 | 100 | ++ | |||
| β2 (n = 6) | 111 ± 15 | 100 | ++ | |||
| β3 (n = 6) | 70 ± 26* | 33 | ++ | |||
Ab39 significantly inhibited mesenchymal cell invasion into the gel lattice (*, P < 0.05. Mann-Whitney U test). The inhibitory effect was reversed by administration of TGFβ proteins. Explant cultures in CM199 containing Ab39 and mature TGFβ1 or TGFβ2 generated mesenchymal cell invasion to a similar extent as to control cultures. Addition of TGFβ3 was less effective than TGFβ1 or TGFβ2 in rescuing the inhibitory effect of Ab39. CM199, medium 199 containing 5% FBS; NRS, normal rabbit serum; n, number of explants tested; No., number.
Figure 6.

Three-dimensional collagen gel culture of the AV region. AV explants were prepared from 9.5-d mouse embryonic heart and cultured on the gel lattice. After 12 h incubation, explants were subjected to various culture conditions as described in Materials and Methods. (a and b) After an additional 48 h in culture with CM199, an endothelial outgrowth (E in a) and mesenchymal cell invasion into the gel lattice (arrows in b) were both present. (c and d) When explants were cultured with Ab39 (1 μl/ml), endothelial outgrowth (E in c) and mesenchymal cell invasion (d) into the gel lattice were both inhibited. (e and f) Rescue experiment: AV explant cultured in the presence of both Ab39 (1 μl/ml) and mature TGFβ1 (2 ng/ml) in CM199 showed definite endothelial outgrowth (E in e) and mesenchymal cell invasion (arrows in f) in the gel lattice. E, endothelial outgrowth; EX, explant; arrows, mesenchymal cell; a, c, and e, gel surface; b, d, and f, under the gel surface observed by optical sectioning, Bar, 200 μm.
To resolve the question as to whether LTBP-1 was required for activation of the latent TGFβ complex or as an extracellular scaffold for mesenchymal cell migration, we performed an explant culture rescue experiment. In this, we added TGFβ proteins to the above-mentioned neutralizing culture experiment with Ab39. The results of this rescue experiment are summarized in Table II. The previous Ab39 neutralizing experiment had indicated that 1 μl/ml of Ab39 in CM199 completely blocked the mesenchymal cell invasion into the gel lattice. In the rescue experiment, the concentration of Ab39 was maintained at 1 μl/ml in CM199. TGFβ1 or TGFβ2 each restored the inhibitory effect of Ab39 at a concentration of 2 ng/ml (Fig. 6, e and f). The rescue effect of TGFβ3 was less than that of TGFβ1 or TGFβ2. A high concentration of exogenously applied TGFβs (20 ng/ml) did not exert any greater rescue effect than that observed with 2 ng/ml on the endothelial outgrowth and mesenchymal cell invasion (data not shown). The results of our culture experiments suggested that LTBP-1 was required to induce the biological activity of the mature TGFβ, rather than for the erection of an extracellular scaffold during the TGFβ-dependent endothelialmesenchymal transformation. They also suggested that an excess amount of TGFβ appeared to inhibit both cellular differentiation and proliferation in this culture model.
Discussion
Functional Importance of TGFβ during the Endothelial-Mesenchymal Transformation
Antibodies against TGFβs, which were exogenously administered to the AV endocardium cocultured with associated myocardium, effectively blocked the invasion of mesenchymal cells into the collagen gel lattice. Therefore, TGFβs are presumably required in the endocardium-to-mesenchymal cell transformation that occurs during cushion tissue formation. Three different types of TGFβ are preferentially expressed in the endocardial cushion tissue-forming region of the mouse embryonic heart at mRNA and protein levels. TGFβ1 is expressed in both the premigratory endocardium and invading mesenchymal cells, whereas TGFβ2 and TGFβ3 are expressed in the external myocardium (Akhurst et al., 1990; Dickson et al., 1993; Mahmood et al., 1993 and 1995). In the chicken embryo, TGFβ3 is thought to be involved in endocardial cushion tissue formation because administration of either antisense oligonucleotides or an antibody specific to TGFβ3 effectively blocked mesenchymal formation in a three-dimensional collagen gel culture model (Potts et al., 1991; Runyan et al., 1993). The tissue distribution pattern of TGFβ3 in the chicken endocardial cushion tissue-forming region is similar to that of TGFβ1 in the embryonic heart of the mouse (Akhurst et al., 1990; Choy et al., 1991; Nakajima et al., 1994). In the development of the mouse heart, it appears that TGFβs do play a role in eliciting endothelial-mesenchymal transformation during the formation of endocardial cushion tissue. However, it is still unclear which TGFβ isoform is predominantly involved in this embryonic phenomenon.
A combined administration of antibodies (anti-TGFβ1 and anti-TGFβ3) to an AV explant culture inhibited mesenchymal formation more effectively than did a single antibody. Furthermore, TGFβ1, TGFβ2, or TGFβ3 all led to some recovery of the inhibitory effect of Ab39 against endothelial-mesenchymal transformation in an AV explant culture. It has been reported that there is no obvious cardiovascular abnormality in the early development of TGFβ1 null (TGFβ1−/−) embryos (Letterio et al., 1994; Diebold et al., 1995; Dickson et al., 1995), despite the fact that TGFβ1 is expressed within the developing heart (Akhurst et al. 1990; Akhurst, 1994; Dickson et al., 1993; Mahmood et al., 1992, 1995). Furthermore, fetuses from a TGFβ1 null mother are affected with secondary cardiac muscle hypertrophy caused by severe vascular defects and defective hematopoiesis within the extra-embryonic mesoderm of the yolk sac (Letterio et al., 1994; Dickson et al., 1995). These results, together with our observations, strongly suggest that there is a functional redundancy of TGFβs in the formation of endocardial cushion tissue, although TGFβs are required there during mouse cardiogenesis.
Extracellular Distribution of LTBP-1
Immunohistochemical observation of LTBP-1–like proteins within the developing endocardial cushion tissue showed that LTBP-1–like molecule was distributed as a fibrillar structure surrounding mesenchymal cells. Immunogold electronmicroscopy revealed the LTBP-1–like molecule localized to a 40–100-nm fibrillar network structure. The tissue distribution pattern of this 40–100-nm fibrillar structure is similar to that of LTBP-2, a component of elastin-associated microfibrils (Gibson et al., 1995). In addition, LTBP-1 was associated with 5–10-nm microfibrils. Sequence analysis of LTBPs has revealed a characteristic of matrix or adhesion molecules (Kanzaki et al., 1990; Tsuji et al., 1990; Moren et al. 1994; Gibson et al., 1995; Yin et al., 1995) and they are classified as the fibrillin family of extracellular matrix proteins (Sakai et al., 1986, 1991; Maddox et al., 1989; Rosenbloom et al., 1993; Zhang et al., 1994). Immunohistochemical examination of mature TGFβ1 in developing endocardial cushion tissue revealed not only a cellular distribution, but also an extracellular fibrillar deposition of TGFβ1 (Fig. 5, a and e; Akhurst et al., 1990; Mahmood et al., 1993, 1995). We showed that the extracellular distribution of mature TGFβ1 coincided with that of LTBP-1 (Figs. 5 b and 6 b). The tissue distribution pattern of LTBP-1 is similar to that of both tenascin (Akhurst et al., 1990) and fibronectin (Mjaatvedt et al., 1987). Recently, LTBP-2 was shown to associate with the microfibrillar component of elastic fibers in the bovine fetal nuchal ligament and aorta (Gibson et al., 1995). During fetal rat calvarial cell culture formation of extracellular fibrillar, LTBP-1 is observed and this structure precedes the presence of type I collagen fibers (Dallas et al., 1995). In human fibroblast culture, LTBP-1 is associated with cellular fibronectin as a component of extracellular microfibrils (Taipale et al., 1996). In COS-1 transfected with the LTBP-1 cDNA, interaction of LTBP-1 with collagen type I could also be seen (Olofsson et al., 1995). Immunohistochemical colocalization of the extracellular LTBP-1 and TGFβ1, as well as immunoelectron microscopic observation for LTBP-1 suggest that the large latent TGFβ complex is presumably a component of extracellular microfibrils that are associated with 40-100 nm extracellular fibrillar network. The tissue distribution pattern of LTBP-1 and mature TGFβ1 is compatible with the hypothetical role of LTBP-1, i.e., that LTBP-1 concentrates/stores TGFβ within those tissues in which TGFβ is a prerequisite for the progression of endothelial-mesenchymal transformation.
Role for LTBP-1 during Cushion Tissue Formation
The antibody specific for LTBP-1 (Ab39) inhibited the endothelially derived mesenchymal-cell invasion into the collagen gel lattice in culture and the inhibition was reversed by the exogenous administration of a mature form of TGFβ proteins. Therefore, these results suggested that LTBP-1 may be required for the activation or concentration of TGFβ within certain regions. Most cells secrete TGFβ as a high molecular mass of latent TGFβ which contains the mature growth factor associated with LAP and LTBP-1 (Kanzaki et al., 1990; Tsuji et al., 1990). Neither the large latent TGFβ complex nor the small latent TGFβ complex has any biological activity as a growth factor. Active TGFβs are highly hydrophobic and basic proteins which are rapidly lost from solution (Brown et al., 1990); they therefore bind to many proteins with a relatively high affinity. Matrix components binding active TGFβ could have a role in rendering nascently activated TGFβ more soluble and in delivering it to the cell surface receptors (Lopez-Casillas et al., 1993; Taipale et al., 1994; Dallas et al., 1995). On the basis of results obtained using heterotypic culture of bovine endothelial and smooth muscle cells, in which targeting of latent TGFβ to smooth muscle cells is required for the activation of latent TGFβ, it has been proposed that delivery of the latent TGFβ complex to the cell surface occurs via LAP or LTBP-1 (Sato et al., 1993; Flaumenhaft et al., 1993). The mechanism underlying the targeting process such as LAP-mediated activation remains unknown but mannose 6-phosphate in LAP appears to play an important role in certain cell types (Dennis and Rifkin, 1991). Flaumenhaft et al. (1993) have reported that either the antibody specific to LTBP-1 (Ab39) or an excess of exogenously applied free LTBP-1 can inhibit the activation of latent TGFβ. This suggests that LTBP-1 may participate in the activation of TGFβ by concentrating the latent TGFβ on the cell surface, where activation occurs. The large latent form of TGFβ associates with the extracellular matrix covalently via LTBP-1 and the release of the small latent complex from the extracellular matrix is a consequence of the proteolytic cleavage of LTBP-1 (Taipale et al., 1993). To date, it remains unknown which sequences confer this putative targeting mechanism on LTBP-1. Recently, LTBP-1 with a longer NH2-terminal portion (LTBP-1L) has been identified, and this LTBP-1L binds more effectively to the extracellular matrix than does LTBP-1 (Oloffson et al., 1995). Together with previously reported results, our observation may suggest a two-step process for the activation for latent TGFβ. In the first step, the secreted large latent TGFβ complex would be targeted to the extracellular matrix via LTBP-1 for the storage/concentration of TGFβ. In the second, proteinase-released latent TGFβ complex from the matrix may possibly retarget the TGFβ to cell surface binding sites by some mechanism such as mannose 6-phosphate receptor.
In conclusion, during the formation of endocardial cushion tissue formation, the large latent TGFβ complex, containing LTBP-1, LAP, and the mature form of TGFβ1, is distributed as an extracellular fibrillar structure surrounding cells where TGFβ-dependent endothelial-mesenchymal transformation is regulated. LTBP-1 may play a role in either the storage or concentration of TGFβ for the activation of TGFβ.
Abbreviations used in this paper
- AV
atrioventricular
- EX
explant
- LAP
latency associated peptide
- LTBP
latent transforming growth factor-β
- binding protein; M
myocardium
- Me
mesenchymal cell
- OT
outflow tract
- PL
plasmin
Footnotes
K. Miyazono is supported by Japan Heart Foundation & IBM Japan Research Grant Foundation for 1995.
Please address all correspondence to Y. Nakajima, Department of Anatomy, Saitama Medical School, 38 Morophongo, Moroyama-cho, Irumagun, Saitama 350-04 Japan. Tel: 81 492 76 1148. Fax: 81 492 95 8026.
References
- Akhurst, R.J. 1994. The transforming growth factor β family in vertebrate embryogenesis. In Growth Factors and Signal Transduction in Development. M. Nilsen-Hamilton, editor. Wiley-Liss, Inc. New York. pp. 97–122.
- Akhurst RJ, Lehnert SA, Faissner A, Duffie E. TGF β in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990;108:645–656. doi: 10.1242/dev.108.4.645. [DOI] [PubMed] [Google Scholar]
- Appella E, Weber IT, Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988;231:1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor β. Biochem Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physiochemical activation of recombinant latent transforming growth factor-βs 1, 2, and 3. Growth Factors. 1990;3:35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- Choy M, Armstrong MT, Armstrong PB. Transforming growth factor-β1 localized within the heart of the chick embryo. Anat Embryol. 1991;183:345–352. doi: 10.1007/BF00196835. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-β binding protein in storage of latent TGF-β in the extracellular matrix and as structural matrix protein. J Cell Biol. 1995;131:539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor β requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci USA. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ. RNA and protein localization of TGF β 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993;117:625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor β1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin C-H, Rifkin DB. Role of the latent TGF-β binding protein in the activation of latent TGF-β cocultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, Hatzinikolas G, Davis E C, Baker E, Sutherland GR, Mecham RP. Bovine latent transforming growth factor β1-binding protein 2: immunolocalization to elastin-associated microfibrils. Mol Cell Biol. 1995;15:6932–6942. doi: 10.1128/mcb.15.12.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, Claesson-Welsh L, Heldin C-H. TGF-β1 binding protein: a component of the large latent complex of TGF-β1 with multiple repeat sequences. Cell. 1990;61:1051–1061. doi: 10.1016/0092-8674(90)90069-q. [DOI] [PubMed] [Google Scholar]
- Kaufman, M.H. 1992. The Atlas of Mouse Development. Academic Press, London.
- Krug EL, Runyan RB, Markwald RR. Protein extracts from early embryonic hearts initiate cardiac endothelial cytodifferentiation. Dev Biol. 1985;112:414–426. doi: 10.1016/0012-1606(85)90414-2. [DOI] [PubMed] [Google Scholar]
- Krug EL, Mjaatvedt CH, Markwald RR. Extracellular matrix from embryonic myocardium elicits an early morphogenetic event in cardiac endothelial differentiation. Dev Biol. 1987;120:348–355. doi: 10.1016/0012-1606(87)90237-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Pircher R, Jullien P. Conversion of a high molecular weight latent TGF-β under acidic conditions. Biochem Biophys Res Commun. 1985;113:1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Gieser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor β knockout. Science (Wash DC) 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-β from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF-β signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- MacLellan WR, Brand T, Schneider MD. Transforming growth factor-β in cardiac ontogeny and adaptation. Circ Res. 1993;73:783–791. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- Maddox BK, Sakai LY, Keene DR, Glanville RW. Connective tissue microfibrils. Isolation and characterization of three large pepsin-resistant domains of fibrillin. J Biol Chem. 1989;264:21381–21385. [PubMed] [Google Scholar]
- Mahmood R, Flanders KC, Morriss-Kay GM. Interactions between retinoids and TGFβs in mouse morphogenesis. Development. 1992;115:67–74. doi: 10.1242/dev.115.1.67. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Flanders KC, Morriss-Kay GM. The effects of retinoid status on TGF β expression during mouse embryogenesis. Anat Embryol. 1995;192:21–33. doi: 10.1007/BF00186988. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Adams-Smith WN. Structural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42:160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Amer J Anat. 1977;148:85–120. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Hellman U, Wernstedt C, Heldin C-H. Latent high molecular complex of transforming growth factor β1. J Biol Chem. 1988;263:6407–6415. [PubMed] [Google Scholar]
- Miyazono K, Ichijo H, Heldin C-H. Transforming growth factor-β: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Ichijo H, Heldin C-H. Receptors for transforming growth factor-β. Adv Immunol. 1994;55:181–220. [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin C-H. A role of the latent TGF-β1-binding protein in the assembly and secretion of TGF-β1. EMBO (Eur Mol Biol Organ) J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Markwald RR. Induction of an epithelial mesenchymal transition by an in vivo adheron-like complex. Dev Biol. 1989;136:118–128. doi: 10.1016/0012-1606(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Krug EL, Markwald RR. An antiserum (ES1) against a particulate form of extracellular matrix blocks the transition of cardiac endothelium into mesenchyme in culture. Dev Biol. 1991;145:219–230. doi: 10.1016/0012-1606(91)90121-i. [DOI] [PubMed] [Google Scholar]
- Moren A, Olofsson A, Stenman G, Sahlin P, Kanzaki T, Claesson-Welsh L, ten Dijke P, Miyazono K, Heldin C-H. Identification and characterization of LTBP-2, a novel latent transforming growth factor-β binding protein. J Biol Chem. 1994;269:32469–32478. [PubMed] [Google Scholar]
- Nakajima Y, Krug EL, Markwald RR. Myocardial regulation of transforming growth factor-β expression by outflow tract endothelium in the early embryonic chick heart. Dev Biol. 1994;165:615–625. doi: 10.1006/dbio.1994.1280. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Morishima M, Nakazawa M, Momma K. Inhibition of outflow cushion mesenchyme formation in retinoic acid-induced complete transposition of the great arteries. Cardiovasc Res. 1996;31:E77–85. [PubMed] [Google Scholar]
- Nunes I, Shapiro RH, Rifkin DB. Characterization of latent TGF-β activation by murine peritoneal macrophages. J Immunol. 1995;155:1450–1459. [PubMed] [Google Scholar]
- Olofsson A, Ichijo H, Moren A, ten Dijke P, Miyazono K, Heldin C-H. Efficient association of an amino-terminally extended form of human latent transforming growth factor-β binding protein with the extracellular matrix. J Biol Chem. 1995;270:31294–31297. doi: 10.1074/jbc.270.52.31294. [DOI] [PubMed] [Google Scholar]
- Pircher R, Jullien P, Lawrence DA. β-Transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986;136:30–37. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor β. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor β. Proc Natl Acad Sci USA. 1991;88:1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart D. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein–protein interactions. Cell. 1995;82:131–141. doi: 10.1016/0092-8674(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB (Fed Am Soc Exp Biochem) J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- Runyan RB, Potts JD, Weeks DL. TGF-β3-mediated tissue interaction during embryonic heart development. Mol Repro Dev. 1992;32:152–159. doi: 10.1002/mrd.1080320211. [DOI] [PubMed] [Google Scholar]
- Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-β with an eight cysteine repeat of its binding protein LTBP-1. EMBO (Eur Mol Biol Organ) J. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Glanville RW, Bachinger HP. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991;266:14763–14770. [PubMed] [Google Scholar]
- Sato Y, Rifkin D B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Okada F, Abe M, Seguchi T, Kuwano M, Sato S, Furuya A, Hanai N, Tamaoki T. The mechanism for the activation of latent TGF-β during co-culture of endothelial cells and smooth muscle cells: cell-type specific targeting of latent TGF–β to smooth muscle cells. J Cell Biol. 1993;123:1249–1254. doi: 10.1083/jcb.123.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchelnitskiy S, Chambaz EM, Feige J-J. Thrombospondins selectively activate one of the two latent forms of transforming growth factor-β present in adrenocortical cell-conditioned medium. Endocrinology. 1995;136:5118–5126. doi: 10.1210/endo.136.11.7588249. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-β: biological function and chemical structure. Science (Wash DC) 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Taipale J, Koli K, Keski-Oja J. Release of transforming growth factor-β1 from the pericellular matrix of cultured human fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem. 1992;267:25378–25385. [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin C-H, Keski-Oja J. Latent transforming growth factor-β1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Hadman K, Keski-Oja J. Latent transforming growth factor-β1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- Tamaki H, Yamashina S. Improved method for post-embedding cytochemistry using reduced osmium and LR white resin. J Histochem Cytochem. 1994;42:1285–1293. doi: 10.1177/42.9.8064136. [DOI] [PubMed] [Google Scholar]
- Thompson NL, Flanders KC, Smith JM, Ellingsworth LR, Roberts AB, Sporn MS. Expression of transforming growth facto-β1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1986;108:661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Okada F, Yamaguchi K, Nakamura T. Molecular cloning of the large subunit of transforming growth factor type β masking protein and expression of the mRNA in various rat tissues. Proc Natl Acad Sci USA. 1990;87:8835–8839. doi: 10.1073/pnas.87.22.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-β from human platelets: a high molecular weight complex containing precursor sequences. J Biol Chem. 1988;263:7646–7654. [PubMed] [Google Scholar]
- Yin, W., E. Smiley, J. Germiller, R.P. Mecham, J.B. Florer, R.J. Wenstrup, and J. Bonadio. 1995. Isolation of a novel latent transforming growth factor-β binding protein gene (LTBP-3). J. Biol. Chem. 270:10147–10160. [DOI] [PubMed]
- Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]