Abstract
In Xenopus laevis egg cell cycle extracts that mimic early embryonic cell cycles, activation of MAP kinase and MAP kinase kinase occurs in M phase, slightly behind that of maturation promoting factor. To examine the possible role of MAP kinase in the in vitro cell cycle, we depleted the extracts of MAP kinase by using anti–Xenopus MAP kinase antibody. Like in the mock-treated extracts, the periodic activation and deactivation of MPF occurred normally in the MAP kinase–depleted extracts, suggesting that MAP kinase is dispensable for the normal M phase entry and exit in vitro. It has recently been reported that microtubule depolymerization by nocodazole treatment can block exit from mitosis in the extracts if enough sperm nuclei are present, and that the addition of MAP kinase– specific phosphatase MKP-1 overcomes this spindle assembly checkpoint, suggesting the involvement of MAP kinase in the checkpoint signal transduction. We show here that the spindle assembly checkpoint mechanism cannot operate in the MAP kinase–depleted extracts. But, adding recombinant Xenopus MAP kinase to the MAP kinase–depleted extracts restored the spindle assembly checkpoint. These results indicate unambiguously that classical MAP kinase is required for the spindle assembly checkpoint in the cell cycle extracts. In addition, we show that strong activation of MAP kinase by the addition of a constitutively active MAP kinase kinase kinase in the absence of sperm nuclei and nocodazole, induced mitotic arrest in the extracts. Therefore, activation of MAP kinase alone is sufficient for inducing the mitotic arrest in vitro.
The mitogen-activated protein kinase kinase (MAPKK)1/ MAP kinase cascade is thought to be important for a wide variety of signal transduction pathways (2, 4, 24, 25, 29, 30). In Xenopus laevis, for example, this classical MAP kinase cascade has been shown to play a crucial role in meiotic cell cycles during oocyte maturation, metaphase arrest of unfertilized eggs, and early embryonic development (5, 7, 9–13, 15–18, 26, 32). However, the role of the MAP kinase cascade in mitotic cell cycles has been poorly understood. In Xenopus unfertilized eggs which are arrested at the second meiotic metaphase, MAP kinase is fully activated, whereas in somatic or early embryonic cell cycles marked activation of MAP kinase at M phase has not been detected, although several reports observed the existence of an activated MAP kinase or an activated MAP kinase-like molecule in M phase (14, 31).
Recent work of Minshull et al. (20) has identified MAP kinase or a related molecule(s) as a component of the embryonic spindle assembly checkpoint in Xenopus egg cell cycle extracts. The Xenopus egg cell cycle extracts are cellfree extracts made from parthenogenetically activated Xenopus eggs (21, 23). They undergo multiple cell cycles, which are thought to mimic mitosis rather than meiosis, since Mos is degraded upon activation, and show many aspects of cell cycle in vitro. The cell cycle extracts have several advantages: well-synchronized cell cycles, availability of large quantities of materials, and easy biochemical operation. Minshull et al. (20) showed that the cell cycle extracts are arrested by spindle depolymerization if filled with high concentrations of sperm nuclei, and that addition of a MAP kinase phosphatase MKP-1 prevents these extracts from being arrested in M phase and drives the extracts previously arrested by the spindle assembly checkpoint into interphase. Cell cycle checkpoints are the systems that monitor the progress of the cell cycle and block further transitions in the cell cycle until certain events have been completed (22). The spindle assembly checkpoint is the mechanism that prevents metaphase cells from initiating anaphase until a bipolar spindle is formed and all chromosomes are attached to the spindle (33). From these results, Minshull et al. (20) suggested the involvement of MAP kinase in the spindle assembly checkpoint.
Although their elegant work clearly demonstrated the requirement of a substrate(s) for MKP-1 in the spindle assembly checkpoint, whether or not classical MAP kinase is required remains to be determined. This is because MKP-1 can dephosphorylate not only classical MAP kinase but also other substrates, including MAP kinase superfamily molecules such as SAPK/JNK and p38/HOG1 (19, 27, 28).
To examine directly the requirement of classical MAP kinase in the system, we produced a highly specific antiMAP kinase antibody and depleted the extracts of MAP kinase by using it. The results showed that the spindle assembly checkpoint did not work in the MAP kinase-depleted extracts, and that adding recombinant MAP kinase restored the checkpoint mechanism, indicating unambiguously the requirement of classical MAP kinase in the spindle assembly checkpoint in the Xenopus cell cycle extracts. Rather surprisingly, however, periodic activation and deactivation of maturation promoting factor (MPF) occurred normally in the MAP kinase-depleted extracts, suggesting that MAP kinase may not be required for normal cell cycle progress.
Materials and Methods
Preparation of Xenopus Egg Extracts
Xenopus egg cell cycle extracts and oocyte extracts were prepared essentially according to the method as described previously (21, 23). All cell cycle extracts were prepared from parthenogenetically activated eggs. Xenopus sperm nuclei were prepared as described (21, 23). Preparation of the extracts was done at 4°C. After adding sperm nuclei and/or nocodazole, we initiated the in vitro cell cycle by warming the extracts to room temperature (∼22°C). The time at which we shifted the temperature was t = 0.
Immunodepletion
The cell cycle extracts were centrifuged at 15,000 g for 15 min. As a result, they were divided into two fractions: one fifth portion is the cloudier lower layer and the rest is the clearer upper layer. The beads of AF-protein A–Toyopearl (Tosoh, Tokyo, Japan) resin were mixed and incubated with an equivalent volume of anti-MAP kinase antiserum which was raised against recombinant Xenopus MAP kinase or preimmune serum at 4°C for 1 h. The beads were washed three times with XB (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 10 mM K-Hepes, pH 7.7, 50 mM sucrose). The upper layer was then incubated with the beads on ice for 10 min, occasionally gently shaken by hand, and then centrifuged. The supernatant was gently mixed with the lower layer, which had been laid on ice. The mixture was centrifuged at 15,000 g for 10 min, the resulting lower layer was saved, and the resulting upper layer was again incubated with the beads for immunodepletion. Then, the beads-treated upper layer and the saved lower layer were mixed and used as MAP kinase–depleted extracts. We adopted this two-step immunodepletion procedure to avoid shaking of the lower layer with beads, because the lower layer is especially fragile to mechanical shock. All the steps should be done very gently not to perturb the cytoplasm; otherwise the immunodepletion procedure would have inhibited the ability of the extracts to produce cell cycles.
Preparation of Recombinant Proteins
Glutathione-S-transferase (GST)–conjugated kinase negative Xenopus MAP kinase (GST-KNMAPK) was expressed in Escherichia coli strain NM522 by incubating with 0.1 mM IPTG for 12 h at 37°C. Histidinetagged Xenopus MAPKK (His-MAPKK) was expressed in E. coli strain BL21 (DE3) pLysS by incubating with 0.4 mM IPTG for 12 h at 25°C. The region containing the kinase domain of Saccharomyces cerevisiae STE11 (residues 341–717) fused to GST (GST-Ste11ΔN) was expressed in E. coli strain XLIBlue by incubating with 0.3 mM IPTG for 4 h at 25°C. To purify GST fusion proteins, cells were pelleted and homogenized in STE (50 mM Tris-HCl, pH 8.5, 200 mM NaCl, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and 1% aprotinin). The homogenates were frozen, thawed, and sonicated. After clarification by centrifugation, the lysates were applied to GSH-agarose beads column. The proteins were eluted with 10 mM GSH in STE. His-MAPKK was purified as described (8). All the proteins were dialyzed against 20 mM Na-Hepes, pH 7.5.
Kinase Assays
Histone H1 kinase activity was measured essentially according to the method described (21), except that the reaction mixture contained 5 μg histone H1 and 0.5 μCi [γ-32P]ATP. To assay myelin basic protein (MBP) kinase activity, extracts were diluted 40 times with HB (20 mM Na-Hepes, pH 7.5, 2 mM MgCl2, and 2 mM EGTA). 5 μl of the diluted extract was incubated with 10 μg MBP in a final volume of 15 μl in the presence of 100 μM ATP, 0.5 μCi [γ-32P]ATP, and 15 mM MgCl2 for 10 min at 30°C. Activity to phosphorylate MAP kinase was measured by incubating samples with 5 μg GST-KNMAPK as described for MBP kinase assay. To measure MAPKK-K activity of GST-Ste11ΔN, immunoprecipitated samples were incubated with 2.5 μg His-MAPKK and 5 μg GST-KNMAPK in the presence of 100 μM ATP, 5 μCi [γ-32P]ATP, and 15 mM MgCl2 for 20 min at 30°C. All the reactions were terminated by adding the SDS-PAGE sample buffer. After boiling, samples were subjected to SDS-PAGE. Gels were analyzed by an image analyzer (FUJIX BAS2000; Fuji Photo Film, Tokyo, Japan). The kinase detection assay within MBP-containing gels was performed as described (6). To assay an activity to activate MAP kinase, samples were incubated with wild-type recombinant MAP kinase for 15 min at 25°C in the presence of 200 μM ATP and 15 mM MgCl2. The mixtures were then subjected to the kinase detection assay within MBP-containing gels, and activities of activated MAP kinase were measured. In some cases, MAPKK was immunoprecipitated with protein A–Sepharose (Pharmacia Fine Chemicals, Piscataway, NJ) and anti-MAPKK antibody as described (17).
Immunoblotting
Samples were run on a 12% SDS-PAGE gel. The electrophoresed proteins were transferred to a polyvinylidene difluoride membrane (Immobilon; Millipore Corp., Milford, MA). After blocking with 5% skim milk in TBS (20 mM Tris-HCl, pH 7.5, 150 mM NaCl), the membrane was incubated with primary antibody followed by horseradish peroxidase–conjugated secondary antibody. Reacted proteins were detected by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL).
Results
Activation of MAP Kinase and MAP Kinase Kinase at M Phase in Cell Cycle Extracts
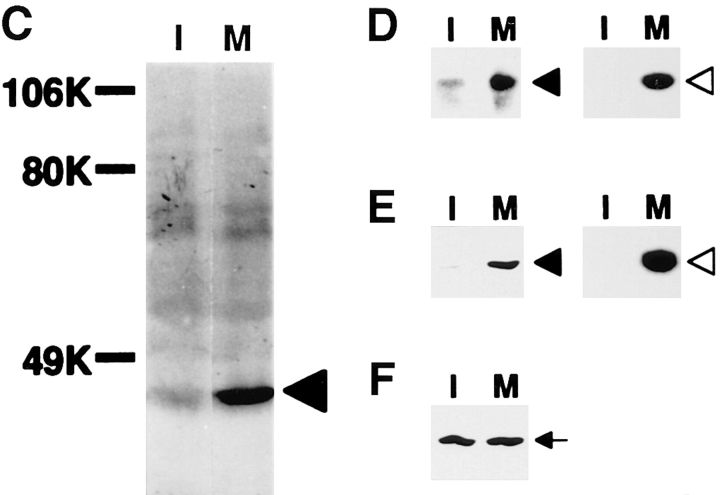
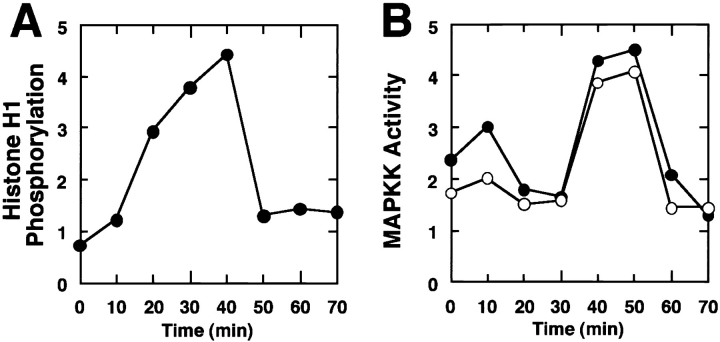
To see whether the activity of MAP kinase varies during cell cycle, we made several assays using Xenopus egg cell cycle extracts, taking advantage of their well-synchronized cell cycle. MPF activity, which is high at M phase, was assayed as histone H1 kinase activity (Fig. 1 A). It peaked at 40 min (M phase) and then suddenly decreased and returned to the basal level (interphase, I). MBP kinase activity was high in 40–50 min (data not shown; see Fig. 7 B, left). In-gel kinase assay for MAP kinase (data not shown and Fig. 7 C, left) and immunoblotting with anti-phosphotyrosine antibody (Fig. 1 C) revealed that 42-kD MAP kinase was tyrosine phosphorylated and activated during M phase. The level of the MAP kinase activity in M phase of this in vitro cell cycle was ∼30% of that seen in mature oocytes. Activities to phosphorylate MAP kinase (Fig. 1 B, closed circles) and to activate MAP kinase (Fig. 1 B, open circles) were also high at 40–50 min. These activities could be attributed to 45-kD MAP kinase kinase (MAPKK), as shown by the enhanced activity of immunoprecipitated MAPKK from M phase (Fig. 1, D and E, right). The amount of MAPKK did not vary during the cell cycle (Fig. 1 F). These data indicated that both MAPKK and MAP kinase are activated around M phase in the cell cycle extracts. It should be noted that the activation and deactivation of MAPKK and MAP kinase lagged slightly behind those of MPF (Fig. 1; see also Fig. 7, left). This is consistent with the data of Minshull et al. (20) that the transient activation of MAP kinase occurred just after the activation of MPF.
Figure 1.
Activation of MAP kinase and MAPKK in M phase of cell cycle extracts. Xenopus egg cell cycle extracts were incubated with 100 sperm nuclei/μl. Samples were taken and assayed for histone H1 kinase activity (A). MAPKK activities were assayed as the activity to phosphorylate kinase-negative recombinant MAP kinase (B, closed circle), and the activity to activate the ability of wild-type recombinant MAP kinase to phosphorylate MBP (B, open circle). Interphase (I) and mitotic (M) extracts were immunoblotted with anti-phosphotyrosine antibody PY20 (C). An arrow indicates MAP kinase. MAPKK activities of the same extracts were assayed as the activity to phosphorylate kinase-negative MAP kinase (KNMAPK; D) and the activity to activate MAP kinase (E). Assays were done in crude extracts (left) or with anti-MAPKK antibody immunoprecipitates (right). The same extracts were immunoblotted with anti-MAPKK antibody (F).
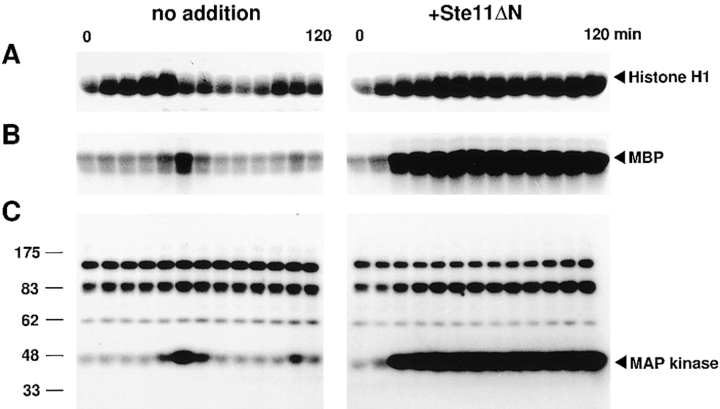
Figure 7.
Mitotic arrest by Ste11ΔN in cell cycle extracts. Cell cycle extracts were incubated with 5,000 sperm nuclei/μl with or without Ste11ΔN (100 μg/ml). Samples were withdrawn at 10 min intervals and assayed for histone H1 kinase activity (A), MBP kinase activity (B), and MAP kinase activity in MBP-containing gels (C).
Effect of MAP Kinase Depletion on the Normal Cell Cycle Progress In Vitro
To examine the role of MAP kinase in the in vitro cell cycle, we made MAP kinase–depleted extracts by immunodepletion. We first produced anti–Xenopus MAP kinase antiserum by immunizing rabbits with recombinant Xenopus MAP kinase. The obtained antiserum was highly specific for 42-kD Xenopus MAP kinase (Fig. 2 A). Immunodepletion procedures with this antiserum were done very gently and quickly, as described in Materials and Methods. Otherwise, the extracts lost viscosity and freshness and ceased to produce cell cycles.
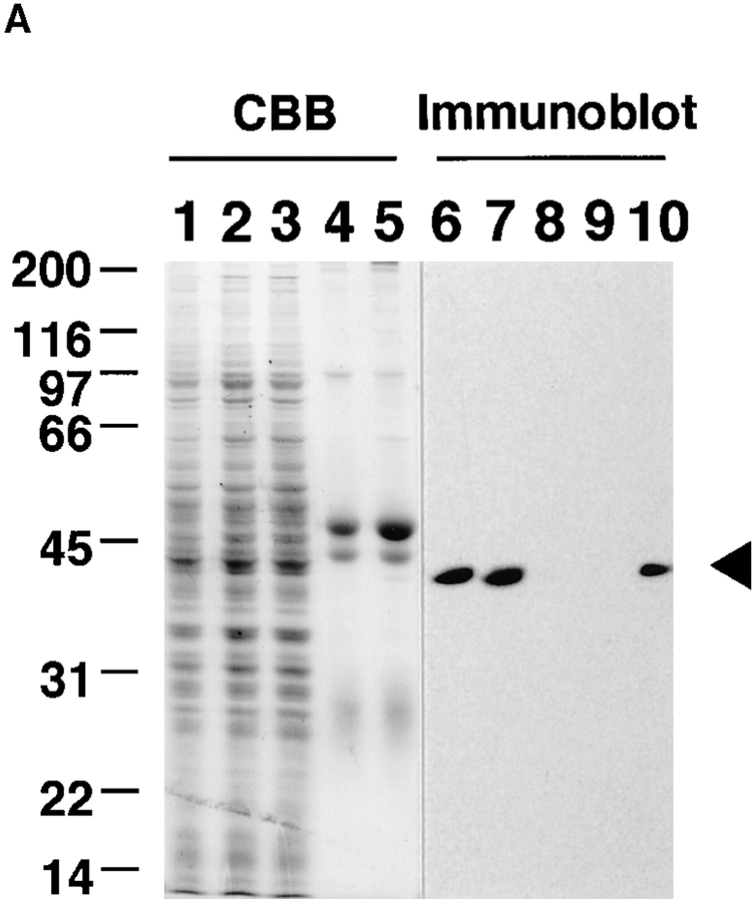
Figure 2.
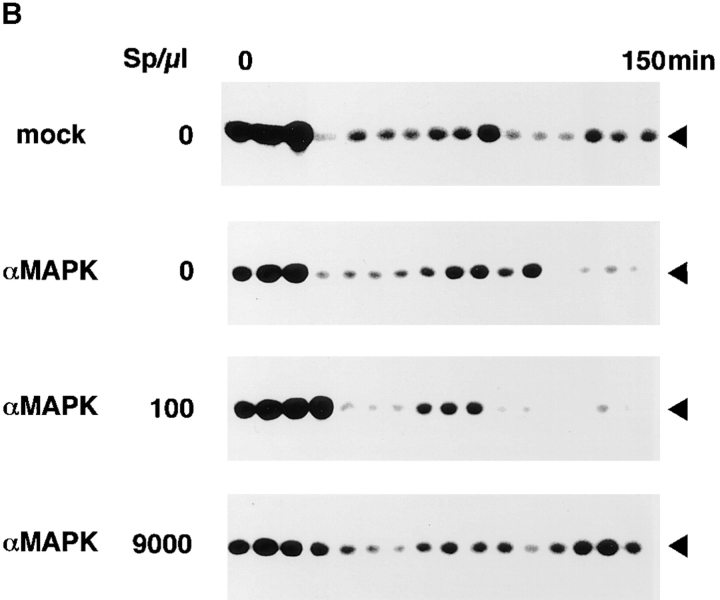
Effect of MAP kinase depletion on the normal cell cycle progress. Xenopus egg cell cycle extracts were subjected to immunodepletion with anti-MAP kinase antiserum or with preimmune serum (mock treatment), as described in Materials and Methods. (A) Total protein and MAP kinase were visualized by Coomassie brilliant blue staining and immunoblotting with anti-MAP kinase antibody, respectively. Extracts were untreated (lanes 1 and 6), mock treated (lanes 2 and 7), or treated with anti-MAP kinase antiserum (lanes 3 and 8), and the remaining extracts were analyzed. The precipitate with the protein A–Toyopearl beads in mock treatment (lanes 4 and 9) or in anti-MAP kinase antiserum treatment (lanes 5 and 10) was analyzed. An arrowhead indicates MAP kinase. (B) MPF activities were monitored as histone H1 kinase activities. Mock-treated extracts (mock) or MAP kinasedepleted extracts (αMAPK) were incubated with indicated concentrations of sperm nuclei (Sp). Samples were withdrawn at 10 min intervals. Assayed reactions were subjected to SDS-PAGE and autoradiographed.
MAP kinase–depleted extracts (anti-MAP kinase antiserum-treated extracts) and mock-treated extracts (preimmune serum–treated extracts) contained the majority of the total protein of untreated extracts as demonstrated by Coomassie brilliant blue staining (Fig. 2 A, lanes 1–3). In mock-treated extracts, the amount of MAP kinase was equivalent to the amount present in untreated extracts as judged by immunoblotting (Fig. 2 A, lanes 6 and 7). More than 95% of MAP kinase was removed from the extracts by the immunodepletion (Fig. 2 A, lane 8), with a recovery of MAP kinase in the immunoprecipitated pellet (Fig. 2 A, lane 10).
We monitored histone H1 kinase activity in these extracts. In both mock-treated and MAP kinase–depleted extracts, periodic activation of histone H1 kinase was observed (Fig. 2 B). All the extracts were in the first M phase at around 20–30 min in this series of experiments. They returned to interphase at 40 min followed by the second M phase at around 90–110 min. The concentration of sperm nuclei did not affect the kinetics of the cell cycle progress markedly (Fig. 2 B). In more than five series of these experiments, MAP kinase–depleted extracts were indistinguishable from mock-treated extracts in the kinetics of histone H1 kinase activity. Therefore, we can suggest that MAP kinase is not necessary for periodic activation of MPF in the cell cycle extracts.
Requirement of MAP Kinase for the Spindle Assembly Checkpoint
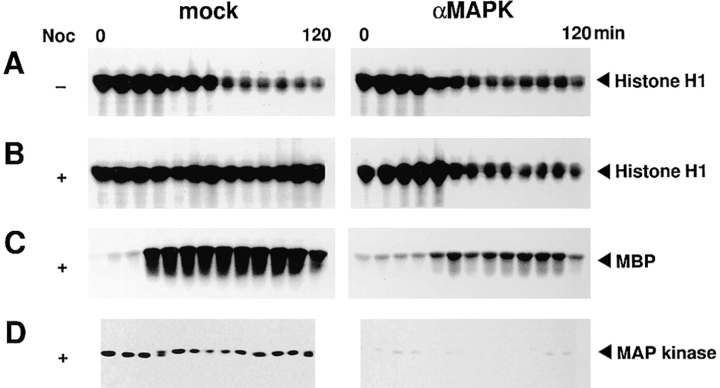
A previous report has shown that microtubule depolymerization by nocodazole treatment arrests cell cycle extracts in M phase if containing very high densities of sperm nuclei. The use of MKP-1 further suggested that MAP kinase, the most likely target of MKP-1, may have a role in the spindle assembly checkpoint (20). However, whether classical MAP kinase is involved in the mitotic arrest remains to be determined. To address this question, we used MAP kinase-depleted extracts. We monitored the level of MPF as histone H1 kinase activity in the extracts supplemented with 9,000 sperm nuclei/μl. In the absence of nocodazole, the extracts returned to interphase after the first M phase, irrespective of whether MAP kinase was depleted or not (Fig. 3 A). In the presence of nocodazole, mock-treated extracts were arrested at the first M phase and the high MPF activity was sustained (Fig. 3 B, mock), whereas MAP kinase–depleted extracts returned to interphase after the first M phase (Fig. 3 B, αMAPK). In the mock-treated extracts with nocodazole, MBP kinase activity became activated at 30 min, and the high activity was sustained (Fig. 3 C, mock). Consistent with this, immunoblotting with anti-MAP kinase antibody showed that about half of MAP kinase was activated at 30 min, and almost full activation of MAP kinase was achieved at 40 min and sustained thereafter (Fig. 3 D, mock). The MAP kinase–depleted extracts had markedly decreased levels of MBP kinase activity throughout (Fig. 3 C), which was consistent with almost complete loss of MAP kinase in the extracts (Fig. 3 D, αMAPK). The low levels of MBP kinase activity may be due to the trace amount of MAP kinase left and other kinases that can phosphorylate MBP. These results clearly indicated the requirement of MAP kinase in the spindle assembly checkpoint.
Figure 3.
Effect of MAP kinase depletion on microtubule depolymerization-induced mitotic arrest. Mock-treated extracts (mock) or MAP kinase–depleted extracts (αMAPK) were incubated with 9,000 sperm nuclei/μl in the presence or absence of 10 μg/ml nocodazole (Noc). Samples were withdrawn at 10 min intervals, assayed for histone H1 kinase activity (A and B), MBP kinase activity (C), or immunoblotted with anti-MAP kinase antibody (D).
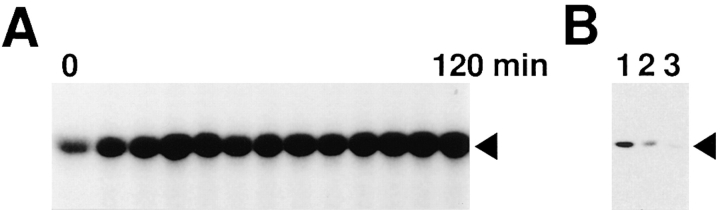
When about the half of MAP kinase was left due to insufficient immunodepletion (Fig. 4 B, lane 2), mitotic arrest was achieved by adding a high density of sperm nuclei and nocodazole to the extracts (Fig. 4 A). This supports the importance of MAP kinase in the spindle assembly checkpoint and may suggest the existence of the threshold of MAP kinase required for the mitotic arrest.
Figure 4.
Mitotic arrest by microtubule depolymerization in extracts insufficiently depleted of MAP kinase. (A) Cell cycle extracts which had been insufficiently immunodepleted of MAP kinase (B, lane 2) were incubated with 9,000 sperm nuclei/μl and nocodazole. Samples were withdrawn at 10 min intervals and assayed for histone H1 kinase activity. (B) Extracts which had been untreated (lane 1), insufficiently depleted of MAP kinase (lane 2), and depleted sufficiently of MAP kinase (lane 3) were immunoblotted with anti-MAP kinase antibody. The samples for lanes 2 and 3 were from the extracts assayed in A and Fig. 3 (αMAPK), respectively.
To confirm further that the observed defect in the establishment of mitotic arrest is specifically due to the removal of MAP kinase, we examined whether purified MAP kinase could rescue the defect in the MAP kinase-depleted extracts. We expressed histidine-tagged Xenopus MAP kinase in E. coli and purified it. Adding the purified recombinant Xenopus MAP kinase to the MAP kinase-depleted extracts restored the spindle assembly checkpoint; the extracts became arrested in M phase by the addition of a high concentration of sperm nuclei and nocodazole (Fig. 5 B). This result confirmed that MAP kinase is a component of the spindle assembly checkpoint.
Figure 5.
Rescue of mitotic arrest by microtubule depolymerization by addition of purified recombinant Xenopus MAP kinase in the MAP kinase-depleted extracts. MAP kinase-depleted extracts were incubated with 9,000 sperm nuclei/μl and 10 μg/ml nocodazole without (A) or with (B) histidine-tagged recombinant wild-type Xenopus MAP kinase. Samples were withdrawn at 10 min intervals and assayed for histone H1 kinase activity.
Mitotic Arrest by Ste11ΔN
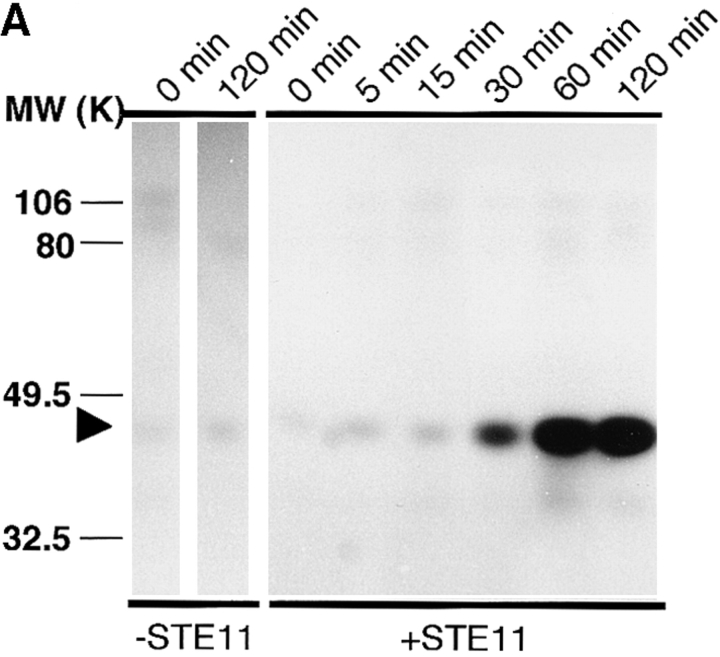
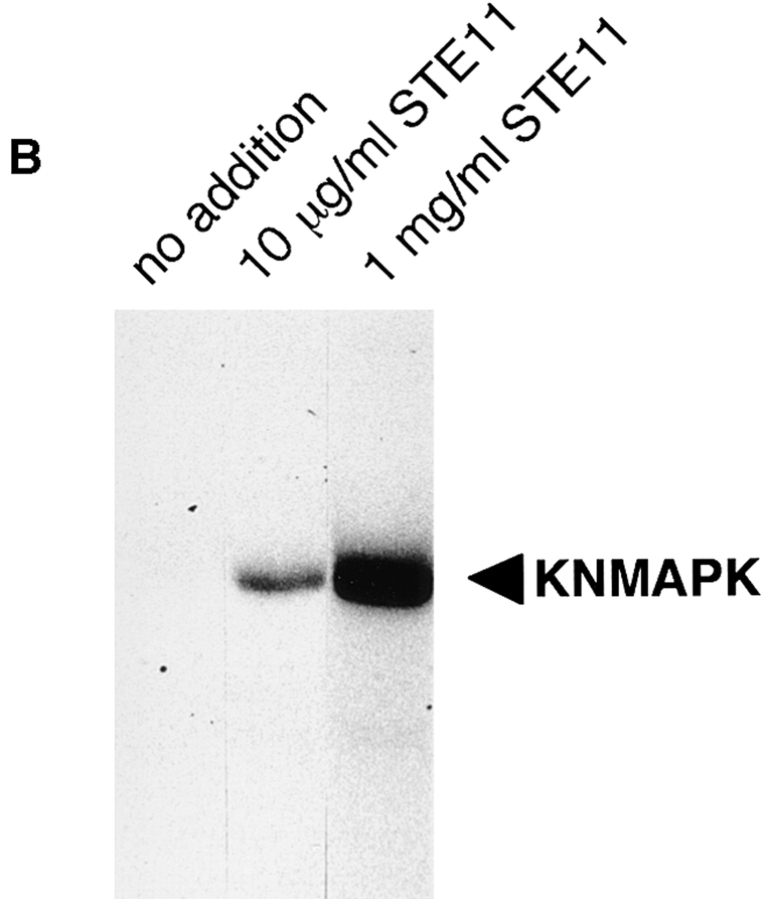
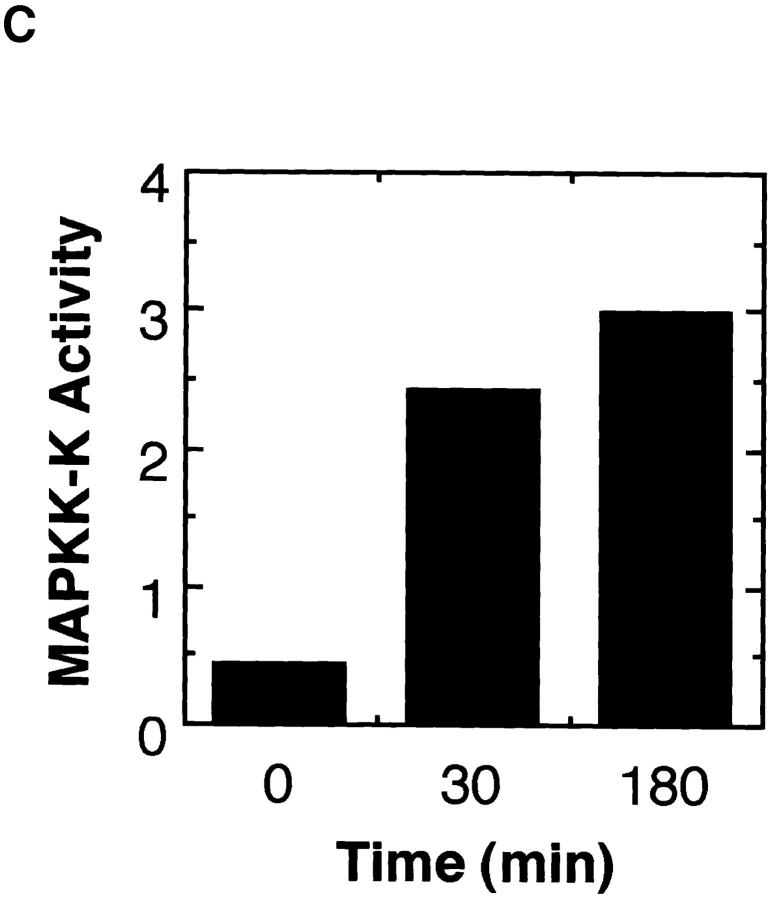
To see whether MAP kinase activity is sufficient to arrest cell cycle in M phase, we attempted to activate the MAP kinase cascade in cell cycle extracts. S. cerevisiae STE11 gene codes for an MAPKK-K family molecule, and the NH2-terminally truncated form is thought to be a constitutively active form (3). We expressed this form of recombinant Ste11 (Ste11ΔN) fused to GST in E. coli and purified it. As expected, addition of purified Ste11ΔN to Xenopus oocyte extracts resulted in activation of MAP kinase (Fig. 6 A) and MAPKK (Fig. 6 B). Immunoprecipitated Ste11ΔN showed high MAPKK-K activity (Fig. 6 C). This indicated that Ste11ΔN can act as an activator for MAP kinase in Xenopus egg cell-free systems.
Figure 6.
Ste11ΔN is a constitutively active MAPKK-K. (A) Xenopus oocyte extracts were incubated with or without 100 μg/ml recombinant Ste11ΔN. Samples were withdrawn at the indicated times and subjected to the kinase detection assay within MBP-containing gels. An arrowhead indicates MAP kinase. (B) Xenopus oocyte extracts were incubated with various concentrations of Ste11ΔN for 2 h. And then, MAPKK was immunoprecipitated and assayed for the activity to phosphorylate recombinant kinase negative MAP kinase. (C) Xenopus oocyte extracts were incubated with 100 μg/ml Ste11ΔN. Ste11ΔN was then immunoprecipitated from samples withdrawn at the indicated times and assayed for the activity to activate the ability of recombinant MAPKK to phosphorylate KNMAPK.
We monitored the MPF level in the cell cycle extracts with or without Ste11ΔN. As already shown, in the control extracts (without Ste11ΔN) periodic activation of MPF occurred; in this series of experiments the first peak was at 40 min and the second at 100 min (Fig. 7 A, no addition). In contrast, in the presence of Ste11ΔN the high level of MPF was sustained (Fig. 7 A, +Ste11ΔN). In this case, strong activation of MAP kinase occurred at 20–30 min, and this high activity was maintained (Fig. 7, B and C, +Ste11ΔN). Thus, Ste11ΔN induced strong activation of MAP kinase before the metaphase/anaphase transition of the first M phase and arrested the cell cycle extracts in M phase.
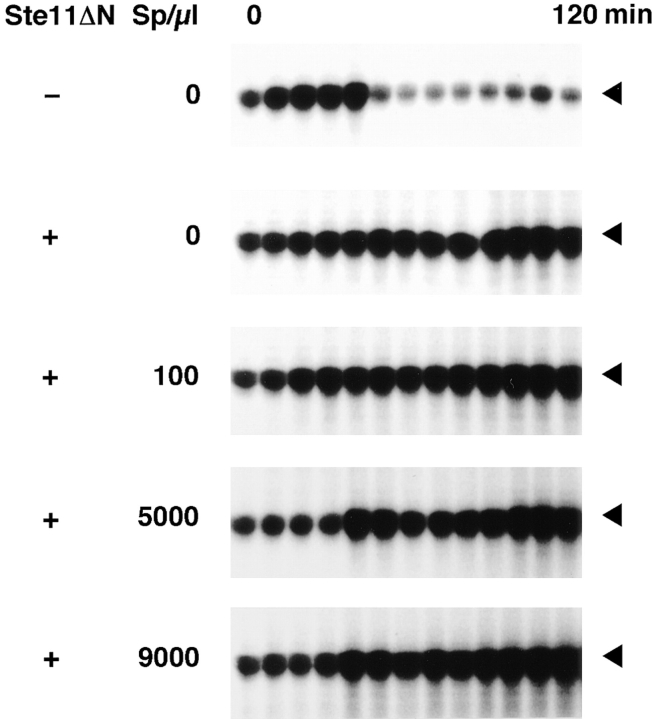
The mitotic arrest in cell cycle extracts by microtubule depolymerization requires the presence of a high density of sperm nuclei. To see whether the mitotic arrest by Ste11ΔN requires a certain density of sperm nuclei, we incubated extracts containing various concentrations of sperm nuclei with Ste11ΔN and assayed for histone H1 kinase. The extracts without Ste11ΔN returned to interphase after the first M phase and then entered the second M phase (Fig. 8, top). However, all the extracts with Ste11ΔN maintained high levels of histone H1 kinase activity and never returned to interphase from the first M phase, independent of the concentration of sperm nuclei (Fig. 8). This result indicated that the mitotic arrest induced by Ste11ΔN does not require sperm nuclei.
Figure 8.
Effect of sperm nuclei concentration on the Ste11ΔNinduced mitotic arrest. Cell cycle extracts were incubated without or with various concentrations of sperm nuclei (Sp) in the presence or absence of Ste11ΔN (100 μg/ml). Samples were withdrawn at 10 min intervals and assayed for histone H1 kinase activity.
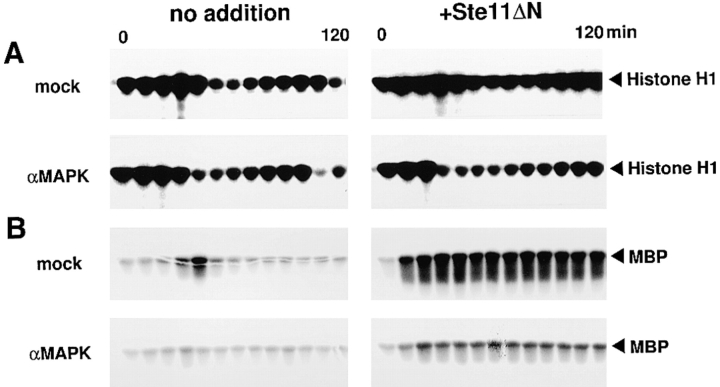
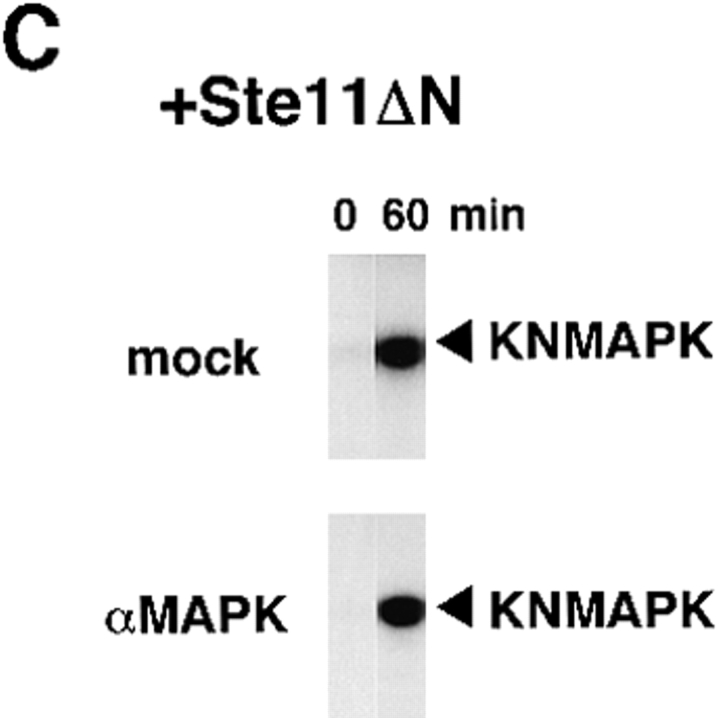
To examine whether Ste11ΔN induces the mitotic arrest through MAP kinase activation, we incubated the MAP kinase–depleted extracts and the mock-treated extracts with or without Ste11ΔN. In the absence of Ste11ΔN, both the mock-treated extracts and the MAP kinase–depleted extracts returned to interphase after the first M phase and entered the second M phase (Fig. 9 A, no addition). Almost complete absence of MBP kinase activity in the MAP kinase-depleted extracts confirmed the removal of MAP kinase (Fig. 9 B, αMAPK). These data not only represented another example of the data indicating that MAP kinase is unnecessary for normal cell cycle progress, as previously shown in Fig. 2 B, but also suggested that both the extracts were functionally intact. In the presence of Ste11ΔN, the mock-treated extracts were arrested in M phase (Fig. 9 A, +Ste11ΔN, mock), whereas the MAP kinase–depleted extracts were not arrested in M phase at all (Fig. 9 A, +Ste11ΔN, αMAPK). In the MAP kinase-depleted extracts, Ste11ΔN did not induce marked activation of MBP kinase activity (Fig. 9 B, +Ste11ΔN, αMAPK), as was consistent with the removal of MAP kinase, but did activate endogenous MAPKK (Fig. 9 C). These data clearly indicated that Ste11ΔN induces the mitotic arrest in the extracts through activation of MAP kinase, and suggested that activation of MAP kinase alone is sufficient for inducing the mitotic arrest in the cell cycle extracts.
Figure 9.
Ste11ΔN induces mitotic arrest through activation of MAP kinase. Mock-treated extracts (mock) or MAP kinase–depleted extracts (αMAPK) were incubated with 5,000 sperm nuclei/μl in the presence (+Ste11ΔN) or absence (no addition) of Ste11ΔN (100 μg/ml). Samples were withdrawn at 10 min intervals and assayed for histone H1 kinase activity (A) and MBP kinase activity (B). MAPKK was immunoprecipitated from the extracts in the presence of Ste11ΔN at 0 and 60 min, and the activity to phosphorylate KNMAPK was measured (C).
Discussion
In this study we used the technique of MAP kinase immunodepletion to examine the role of MAP kinase in the cell cycle progress and the spindle assembly checkpoint in the Xenopus cell cycle extracts. The result showed unambiguously that classical MAP kinase is required for the spindle assembly checkpoint. This confirms and extends the previous study of Minshull et al. (20). Furthermore, rather surprisingly, our present result showed that MAP kinase is unnecessary for periodic activation of MPF in the normal cell cycle progress. In addition, we have shown that MAP kinase activation alone is sufficient for inducing the mitotic arrest in vitro.
In the extracts, activation of MAPKK and MAP kinase during the cell cycle occurred slightly behind that of MPF (Figs. 1 and 7). In contrast, when the mitotic arrest was induced by microtubule depolymerization, activation of MAP kinase occurred before MPF began to be inactivated (Fig. 3). It can be speculated that activation of MAP kinase prior to the metaphase/anaphase transition is crucial for inducing the mitotic arrest. This is consistent with the recent report of Abrieu et al. (1) suggesting that MAP kinase does not inactivate but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. In fact, in the case of the Ste11ΔN-induced mitotic arrest, activation of MAP kinase occurred earlier than the peak of MPF activity (Fig. 7).
We depleted cell cycle extracts of MAP kinase by using anti-MAP kinase antibody. The immunodepletion procedure sometimes deteriorated the extracts; in such extracts periodic activation of MPF did not occur. Then, every experiment was done only when mock-treated extracts behaved as untreated extracts. Even when >95% of MAP kinase could be removed, periodic activation of MPF as assayed by histone H1 kinase activity occurred normally, and the kinetics of MPF activation in the MAP kinase– depleted extracts could be indistinguishable from that in the mock-treated extracts. Therefore, we may conclude that MAP kinase is not required for the activation and inactivation of MPF during the normal cell cycle progress. We cannot, however, rule out the possibility that only 5% of endogenous MAP kinase is sufficient to play a role in the cell cycle progress.
We have shown here that classical MAP kinase is essential for the spindle assembly checkpoint in the cell cycle extracts. This conclusion was derived from the following results. First, when almost all classical MAP kinase was removed by immunodepletion, microtubule depolymerization did not arrest the extracts in M phase. But, when immunodepletion was insufficient and about half of the MAP kinase was left, the mitotic arrest could be induced. Second, adding purified recombinant Xenopus MAP kinase to the MAP kinase–depleted extracts rescued the defect in mitotic arrest. As the previous pioneering work of Minshull et al. (20) demonstrated that a target of MKP-1 is required for the spindle assembly checkpoint, we can conclude that classical MAP kinase was the primary target of MKP-1 in these experiments.
We have shown further that an active form of MAPKK-K, Ste11ΔN, can induce mitotic arrest in the extracts through the activation of endogenous MAP kinase. The Ste11ΔNinduced mitotic arrest did not require the presence of exogenously added sperm nuclei, unlike microtubule depolymerization-induced mitotic arrest. Although there have been reports demonstrating that active MAP kinase is sufficient to induce mitotic arrest in cleaving embryos (11), the present study is the first demonstration of the mitotic arrest by activation of MAP kinase alone in vitro. Thus, we can suggest that once MAP kinase is activated by the spindle assembly defect, mitotic apparatus, chromosomes, or other cellular organelles are not required for inducing mitotic arrest, and the activated MAP kinase alone is sufficient for the mitotic arrest. We are now examining upstream pathways and downstream effectors of the MAP kinase cascade in the spindle assembly checkpoint mechanism.
Acknowledgments
We thank Dr. Andrew Murray for discussion, many helpful comments, and critical reading of the manuscript. We also thank F. Itoh for the production of anti-MAP kinase antiserum.
Abbreviations used in this paper
- GST
gluthathione-S-transferase
- MAP
mitogen activated protein
- MAPKK
MAP kinase kinase
- MAPKK-K
MAP kinase kinase kinase
- MBP
myelin basic protein
- MKP
Map kinase phosphatase
- MPF
maturation promoting factor
Footnotes
K. Takenaka is a Research Fellow of the Japan Society for the Promotion of Science. This work was supported by grants in aid from the Ministry of Education, Science, and Culture of Japan to E. Nishida.
Please address correspondence to Eisuke Nishida, Department of Biophysics, Graduate School of Science, Kyoto University, KitashirakawaOiwake, Sakyo-ku, Kyoto 606-01, Japan. Tel.: 81-75-753-4230; Fax: 81-75753-4235.
References
- 1.Abrieu A, Lorca T, Labbé JC, Morin N, Keyse S, Dorée M. MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopusegg extracts. J Cell Sci. 1996;109:239–246. doi: 10.1242/jcs.109.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Ammerer G. Sex, stress and integrity: the importance of MAP kinases in yeast. Curr Opin Genet Dev. 1994;4:90–95. doi: 10.1016/0959-437x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 3.Cairns BR, Ramer SW, Kornberg RD. Order of action of components in the yeast pheromone response pathway revealed with a dominant allele of the STE11 kinase and the multiple phosphorylation of the STE7 kinase. Genes Dev. 1992;6:1305–1318. doi: 10.1101/gad.6.7.1305. [DOI] [PubMed] [Google Scholar]
- 4.Errede B, Levin DE. A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol. 1993;5:254–260. doi: 10.1016/0955-0674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell JJ, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopusoocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh Y, Nishida E, Yamashita T, Hoshi M, Kawakami M, Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990;193:661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh Y, Nishida E, Matsuda S, Shiina N, Kosako H, Shiokawa K, Akiyama T, Ohta K, Sakai H. In vitro effects on microtubule dynamics of purified XenopusM phase-activated MAP kinase. Nature (Lond) 1991;349:251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh Y, Matsuda S, Takenaka K, Hattori S, Iwamatsu A, Ishikawa M, Kosako H, Nishida E. Characterization of recombinant XenopusMAP kinase kinases mutated at potential phosphorylation sites. Oncogene. 1994;9:1891–1898. [PubMed] [Google Scholar]
- 9.Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopusoocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh Y, Masuyama N, Suzuki A, Ueno N, Nishida E. Involvement of the MAP kinase cascade in Xenopusmesoderm induction. EMBO (Eur Mol Biol Organ) J. 1995;14:2491–2498. doi: 10.1002/j.1460-2075.1995.tb07246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopusembryos by MAP kinase. Science (Wash DC) 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- 12.Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopusoocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- 13.Hartley RS, Lewellyn AL, Maller JL. MAP kinase is activated during mesoderm induction in Xenopus laevis. . Dev Biol. 1994;163:521–524. doi: 10.1006/dbio.1994.1168. [DOI] [PubMed] [Google Scholar]
- 14.Heider H, Hug C, Lucocq JM. A 40-kDa myelin basic protein kinase, distinct from erk1 and erk2, is activated in mitotic HeLa cells. Eur J Biochem. 1994;219:513–520. doi: 10.1111/j.1432-1033.1994.tb19966.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosako H, Gotoh Y, Nishida E. Mitogen-activated protein kinase kinase is required for the mos-induced metaphase arrest. J Biol Chem. 1994;269:28354–28358. [PubMed] [Google Scholar]
- 17.Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopusoocyte maturation. EMBO (Eur Mol Biol Organ) J. 1994;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaBonne C, Burke B, Whitman M. Role of MAP kinase in mesoderm induction and axial patterning during Xenopusdevelopment. Development (Camb) 1995;121:1475–1486. doi: 10.1242/dev.121.5.1475. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogenactivated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 20.Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinasedependent spindle assembly checkpoint in Xenopusegg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 21.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:573–597. [PubMed] [Google Scholar]
- 22.Murray AW. Cell cycle checkpoints. Curr Opin Cell Biol. 1994;6:872–876. doi: 10.1016/0955-0674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 23.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature (Lond) 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 24.Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 25.Perrimon N. Signalling pathways initiated by receptor protein tyrosine kinases in Drosophila. . Curr Opin Cell Biol. 1994;6:260–266. doi: 10.1016/0955-0674(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 26.Posada J, Sanghera J, Pelech S, Aebersold R, Cooper JA. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991;11:2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 28.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 29.Ruderman JV. MAP kinase and the activation of quiescent cells. Curr Opin Cell Biol. 1993;5:207–213. doi: 10.1016/0955-0674(93)90104-x. [DOI] [PubMed] [Google Scholar]
- 30.Selfors LM, Stern MJ. MAP kinase function in C. elegans. . Bioessays. 1994;16:301–304. doi: 10.1002/bies.950160502. [DOI] [PubMed] [Google Scholar]
- 31.Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, Kohno M, Kasuga M, Yazaki Y, Akanuma Y. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- 32.Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopuscaused by activation of MAP kinase. Nature (Lond) 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- 33.Wells WAE. The spindle-assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]