Abstract
We have recently cloned and characterized ankyrin-3 (also called ankyrinG), a new ankyrin that is widely distributed, especially in epithelial tissues, muscle, and neuronal axons (Peters, L.L., K.M. John, F.M. Lu, E.M. Eicher, A. Higgins, M. Yialamas, L.C. Turtzo, A.J. Otsuka, and S.E. Lux. 1995. J. Cell Biol. 130: 313–330). Here we show that in mouse macrophages, ankyrin-3 is expressed exclusively as two small isoforms (120 and 100 kD) that lack the NH2-terminal repeats. Sequence analysis of isolated Ank3 cDNA clones, obtained by reverse transcription and amplification of mouse macrophage RNA (GenBank Nos. U89274 and U89275), reveals spectrin-binding and regulatory domains identical to those in kidney ankyrin-3 (GenBank No. L40631) preceded by a 29–amino acid segment of the membrane (“repeat”) domain, beginning near the end of the last repeat. Antibodies specific for the regulatory and spectrin-binding domains of ankyrin-3 localize the protein to the surface of intracellular vesicles throughout the macrophage cytoplasm. It is not found on the plasma membrane. Also, epitope-tagged mouse macrophage ankyrin-3, transiently expressed in COS cells, associates with intracellular, not plasma, membranes. In contrast, ankyrin-1 (erythrocyte ankyrin, ankyrinR), which is also expressed in mouse macrophages, is located exclusively on the plasma membrane. The ankyrin-3–positive vesicles appear dark on phasecontrast microscopy. Two observations suggest that they are lysosomes. First, they are a late compartment in the endocytic pathway. They are only accessible to a fluorescent endocytic tracer (FITC-dextran) after a 24-h incubation, at which time all of the FITC-dextran– containing vesicles contain ankyrin-3 and vice versa. Second, the ankyrin-3–positive vesicles contain lysosomal-associated membrane glycoprotein (LAMP-1), a recognized lysosomal marker. This is the first evidence for the association of an ankyrin with lysosomes and is an example of two ankyrins present in the same cell that segregate to different locations.
The ankyrins are a family of plasma membrane–associated proteins that link integral membrane proteins to the underlying membrane skeleton. There are now three family members: erythrocyte ankyrin (ankyrin-1, Ank1, AnkR) (42, 48), brain ankyrin (ankyrin-2, Ank2, AnkB) (58), and epithelial or general ankyrin (ankyrin-3, Ank3, AnkG) (37, 60).
In the RBC, ankyrin-1 links the transmembrane anion exchanger, band 3, to β-spectrin and the plasma membrane skeleton (3, 4, 13, 33). This linkage is critical for the stability of the lipid bilayer (61). Defects in ankyrin-1 or its attached proteins result in a spherocytic hemolytic anemia (11, 21, 49, 75) due to excessive vesiculation of RBC membrane lipids. Ankyrin-1 is also expressed in muscle (6), endothelial cells (Lux, S.E., unpublished data), cerebellar Purkinje cells and granule cells, and a subset of spinal cord and hippocampal neurons (34, 59).
Ankyrin-2 is the major form of ankyrin in the nervous system. The 220-kD protein is found in most neuron cell bodies and dendrites, as well as in glia (58). A 440-kD isoform is expressed in the fetal brain, targeted specifically to unmyelinated axons and dendrites (8, 40).
Ankyrin-3 is much more widely distributed than its sister genes and is the major ankyrin in epithelia, myocytes, hepatocytes, melanocytes, megakaryocytes, Leydig cells, and neuronal axons (60). In the brain, ankyrin-3 is expressed as isoforms of 270- and 480-kD and localizes to the nodes of Ranvier and to the initial segments of axons (34, 37, 60).
All three ankyrins have a similar, three-domain structure. The NH2-terminal, plasma membrane–binding or “repeat” domain contains 24 tandem 33–amino acid repeats and binds many of the integral membrane protein ligands of ankyrins (12, 50–52). The central, spectrin-binding domain binds spectrin and fodrin (nonerythroid spectrin) (2, 3, 14, 33, 62) and is critical for attaching the underlying membrane skeleton to the lipid bilayer. The COOHterminal, regulatory domain influences the affinity of ankyrin for its ligands via an acidic, alternately spliced segment in the middle of the domain (14).
The kidney contains multiple isoforms of ankyrin-3: 215, 200, 170, 120, and 105 kD (60). The two smallest isoforms lack the repeat domain and differ only by the presence or absence of the acidic regulatory domain insert (60). These two small ankyrin-3 isoforms are the only forms observed in intestine, testis, and liver. Immunohistochemical staining suggests they reside in the cytoplasm instead of in the plasma membrane (60).
We find that two similar, small, NH2-terminal truncated proteins are also the only isoforms of ankyrin-3 in bone marrow–derived macrophages, where they associate with intracellular membranes of phase-dense lysosomes (i.e., lysosomes that appear dense in the phase-contrast microscope). Judging from when they are labeled by soluble, fluid-phase tracer molecules (25, 68, 71), these vesicles occur very late in the endocytic pathway. Specifically, 24 h after an initial 15-min pulse, fluorescence from endocytically incorporated dextrans completely coincides with staining from ankyrin-3 antibodies. Taken together with immunofluorescent experiments that show both ankyrin-3 and lysosomal-associated membrane glycoprotein 1 (LAMP-1)1 on the same late endocytic vesicles, we identify these vesicles as lysosomes.
Materials and Methods
Macrophages
Murine bone marrow–derived macrophages were obtained as previously described (70). Briefly, femurs from C3H-HeJ +/+ mice (The Jackson Laboratory, Bar Harbor, ME) were removed and their marrow was extruded and cultured in bone marrow macrophage medium (BMM) for 6–7 d. BMM comprises DME (GIBCO BRL, Gaithersburg, MD) containing 30% L cell–conditioned medium, a source of macrophage colony stimulating factor, and 20% heat-inactivated FBS (Biocell Laboratories, Rancho Dominguez, CA). Cells were harvested and either plated onto glass coverslips and incubated overnight to allow cells to adhere for immunofluorescence or replated at one-third density and allowed to grow for an additional 2 d before harvesting protein or RNA.
Antibodies
Ankyrin-3 antibodies were generated and characterized previously in our laboratory (60). Briefly, a unique portion of the regulatory domain of mouse ankyrin-3 was fused to glutathione-S-transferase (GST) and used as an antigen (Ank3-R1 antibody). Also, antibodies were prepared to the “B” insert (DKCTWFKIPKVQEVL), which lies between the repeat and spectrin-binding domains, and to the NH2-terminal end of the kidney isoform lacking the repeat domain (MALPHSEDAITGDTD). These are referred to as Ank3-B and 5′Ank3, respectively (60). Anti–human ankyrin-1 antibodies were prepared against a mixture of native and SDS-denatured ankyrin as previously described (66). Anti–hemagglutinin (HA) mAb (12CA5) was obtained from Boehringer Mannheim Biochemicals (Indianapolis, IN). Anti–LAMP-1 sera were obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences (Johns Hopkins University School of Medicine, Baltimore, MD), and from the Department of Biological Sciences (University of Iowa, Iowa City, IA) (under contract N01-HD-6-2915 from the National Institute of Child Health and Human Development). The anti-rab9 antiserum was provided by Dr. Angela Wandinger-Ness (Northwestern University, Evanston, IL). Anti–α-spectrin, anti-adducin, and anti– protein 4.1 antibodies were provided by Dr. Shih-Chun Liu (St. Elizabeth's Hospital, Brighton, MA) (17,46). The adducin antiserum was raised against both α- and β-adducin. Anti–canine erythrocyte β-spectrin and ankyrin antibodies, provided by Dr. Kenneth Beck (Stanford University, Stanford, CA) (1), detect Golgi spectrin and ankyrin, respectively, in MDCK cells (1).
Anti–human erythrocyte protein 4.1 and eight different anti–human spectrin antisera were provided by Dr. Orah Platt (Children's Hospital, Boston, MA). Affinity-purified antibodies against β-actin were provided by Dr. Ira Herman (Tufts University School of Medicine, Boston, MA) (28). Anti–GP-260 antibodies, which were raised against an Acanthamoeba protein immunologically related to β-spectrin, were provided by Dr. Tom Pollard (Johns Hopkins University School of Medicine, Baltimore, MD) (63). Anti–human brain fodrin antibodies were provided by Dr. Jon Morrow (Yale University School of Medicine, New Haven, CT) (27). Anti–bovine brain fodrin antibodies were provided by Dr. Shin Lin (Johns Hopkins University) (45). Affinity-purified anti–guinea pig brain fodrin antibodies were provided by Dr. Mark Willard (Washington University School of Medicine, St. Louis, MO) (43). All of the fodrin antisera were raised against both α- and β-fodrin.
Preparation of Proteins
Kidney membrane proteins were prepared as previously described (60). Macrophage protein extracts were prepared by lysing dense but not confluent macrophages from one well of a six-well dish into 1 ml of extraction buffer (16) containing 0.5% SDS, 0.1% Triton X-100, 40 mM Hepes, pH 7.15, 50 mM Pipes, pH 6.9, 75 mM NaCl, 1 mM MgCl2, 0.5 mM EGTA, 0.1 mg/ml leupeptin, 0.1 mg/ml pepstatinA, and 0.1 mg/ml PMSF (first dissolved in 100% ethanol). Extracts were immediately added to one-third volume of 4× Laemmli sample buffer (41), boiled for 5 min, and applied to SDS polyacrylamide gels.
Immunoblotting
SDS-PAGE was performed using the Laemmli buffer system (41) with 5% stacking and 10% running gels. 30 μl (50–100 μg) of macrophage extracts or kidney membrane proteins and 30 μl (∼200 μg) of prestained molecular weight markers (Bio Rad Laboratories, Hercules, CA) were run overnight at a constant 30 V. The proteins were transferred to ImmobilonP membranes (Millipore Corp., Bedford, MA) in 48 mM Tris-HCl, 39 mM glycine, 20% methanol, and 0.0375% SDS, pH 8.3, using a BioRad semidry transfer apparatus. Filters were blocked for a minimum of 1 h at room temperature in TTBS (10 mM Tris HCl, pH 7.0, 0.05% Tween-20, and 150 mM NaCl) containing 5% BSA. Filters were washed three times for 5 min each in TTBS, and then incubated for 1 h at room temperature in primary antibody diluted 1:500 in TTBS. Washing was repeated as above, and then filters were incubated at room temperature with goat anti–rabbit IgG conjugated with alkaline phosphatase (Bio Rad Laboratories) according to the manufacturer's instructions. Filters were washed again as above, and bound antibody was visualized with nitroblue tetrazolium and 5-bromo-4chloro-3-indolyl phosphate (BioRad kit).
Preparation of RNA
Medium was removed from macrophage cultures by aspiration, and each monolayer was washed twice with ice-cold PBS lacking calcium and magnesium. For 150-mm dishes, 4.5 ml of 10 mM EDTA, pH 8.0, containing 0.5% SDS was added, and the lysate was scraped off the dish and collected into a glass centrifuge tube. Then 4.5 ml of 0.1 M sodium acetate, pH 5.2, containing 10 mM EDTA, pH 8.0, was used to rinse the plate; the eluate was collected and added to the lysate. Water saturated phenol (9 ml) was added to the lysate and the mixture was shaken vigorously at room temperature for 2 min. Aqueous and organic phases were separated by a 10min centrifuge spin at 4°C at 3,000 g. The aqueous phase was reextracted twice more, once in a 24:24:1 mixture of phenol/chloroform/isoamyl alcohol and once in a 24:1 mixture of chloroform/isoamyl alcohol. Ice-cold 1 M Tris-HCl (pH 8.0, 990 μl) and 5 M NaCl (405 μl) were added to the aqueous phase and mixed. 2 vol of ice-cold ethanol were then added, and the precipitated RNA was centrifuged for 15 min at 4°C at 12,000 g. The RNA was redissolved and reprecipitated as above, and then dissolved in icecold 10 mM Tris and 1 mM EDTA, pH 8.0.
Reverse Transcription–PCR
Macrophage RNA (5 μg) was heated to 65°C for 5 min and transferred to ice for an additional 5 min. Denatured RNA was then added to a buffer containing 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 0.5 mM each of dGTP, dATP, dCTP, and dTTP, 10–50 μg/ml oligo(dT), 1 μl (40 U) of RNAsin (Promega, Madison, WI), and 4 μl (800 U) of Moloney murine leukemia virus reverse transcriptase (GIBCO BRL) and incubated at 42°C for 60 min. RNA was then hydrolyzed by adding NaOH to a final concentration of 0.375 N at 65°C for 30 min. The base was neutralized with an equal molar amount of acetic acid. cDNA was amplified using AmpliTAQ (Boehringer Mannheim Biochemicals) with isoform-specific ankyrin-3 primers (see Fig. 1) (15 ng/μl) in a buffer containing 100 mM Tris-HCl, pH 8.3, 15 mM MgCl2, 500 mM KCl, and 0.2 mM dNTP. The PCR program was 94°C (30 s), 60°C (30 s), and 72°C (5 min) for 30 cycles followed by extension at 72°C (7 min).
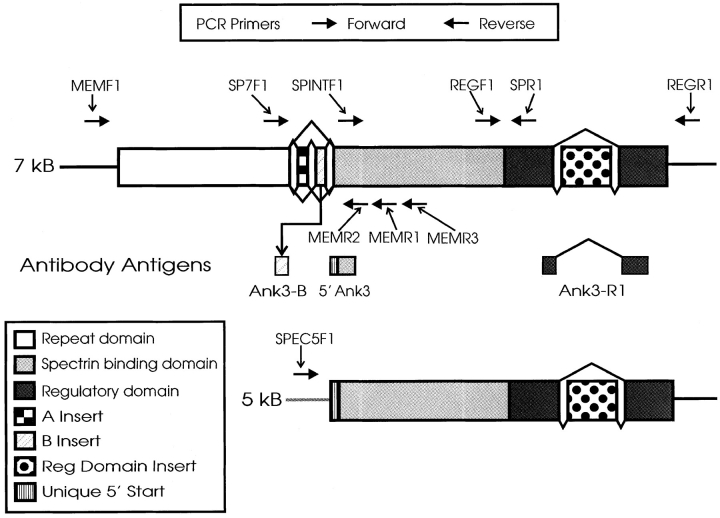
Figure 1.
Schematic diagram of ankyrin-3 protein isoforms and location of antibody epitopes and PCR primers. Ank3-B is a peptide antibody against the B insert, 5′Ank3 is a peptide antibody directed against the NH2-terminal region of the spectrin-binding domain and the short 5′ exon from the 120- and 105-kD isoforms present in kidney, and Ank3-R1 is a polyclonal antibody against the regulatory domain. The positions of the antigens used for those antibodies are displayed as well as the positions of the forward and reverse primers used in RT-PCR experiments.
Primer sequences were: REGR1; 5′-ACTGGCAGTATGACCAAGTGGTCCTGGACTGAC-3′ (Tm = 70.7°C); REGF1; 5′-CACATAAAAAGGCTGAGAAGGCAGACAGACGCC-3′ (Tm = 70.7°C); SPR1; 5′-GGACTCATGCTGGGTTCAGTCAAGTAGCTGTAG-3′ (Tm = 70.0°C); SPINTF1; 5′-GAAGATGCCATCACAGGGGACACTGACAAG-3′ (Tm = 69.5°C); SP7F1; 5′-ACAAAATGAATGTCCCAGAAACGATGAATGAAG-3′ (Tm = 63.3°C); SPEC5F1; 5′-GGACGGCTTACTCTAAACCCCTGCTTAAGGAAT-3′ (Tm = 69.4°C); MEMF1; 5′-GATCTCCTGCCTCGTCTCAACTCCCCTGATCTC-3′ (Tm = 73.2°C).
5′-RACE PCR
Amplification of the 5′ end of macrophage ankyrin-3 was carried out as described by the manufacturer using the 5′/3′ RACE kit (Boehringer Mannheim Biochemicals). Briefly, RACE products were generated by MEMR3 priming the reverse transcription, and MEMR1 and MEMR2 priming the primary and nested PCR reactions. Additional reactions used the MEMR2 primer for the reverse transcription, and 5PRR1 and 5PRR2 (which are located at the extreme 3′ end of the repeat domain) for priming the primary and nested PCR reactions.
Primer sequences were: MEMR1; 5′-ACCTTCTGCTGGCAGGGAGTCATCACCTAGCTC-3′ (Tm = 72.9°C); MEMR2; 5′-AAGGTCCTGTGGCCCGAGATACTTGTCAGTGTC-3′ (Tm = 71.9°C); MEMR3; 5′-GTAGGACCTATCCGAACTGAAGGAGCGGAGGC-3′ (Tm = 71.3°C); 5PRR1; 5′-AGGCGTTGTTCTGAAGCAAGACATTGATGATAT-3′ (Tm = 65.7°C); 5PRR2; 5′-CCTGCTGAGCAGCCTGGTGCAGTGCTGTGTATC-3′ (Tm = 74.4°C).
DNA Sequencing
PCR products were isolated and subcloned into the pCR II vector using the TA cloning kit (Invitrogen, San Diego, CA). Plasmid DNA was prepared by the cetyltrimethylammonium bromide method (15) and sequenced on both strands by the dideoxynucleotide chain termination method (65) using Sequenase version 2.0 (United States Biochemical Corp., Cleveland, OH) and synthetic oligonucleotide primers.
Immunofluorescence Microscopy
For ankyrin-3 localization, cells plated onto glass coverslips were fixed in 4% paraformaldehyde at room temperature for 5 min, and then lysed in a 0.1% Triton X-100 buffer containing 40 mM Hepes, pH 7.15, 50 mM Pipes, pH 6.9, 75 mM NaCl, 1 mM MgCl2, and 0.5 mM EGTA as described previously (16). For LAMP-1 localization, cells plated onto glass coverslips were fixed in 50 mM phosphate buffer, pH 7.8, containing 2% paraformaldehyde, 9 mg/ml lysine, and 2 mg/ml NaIO4 for 1–2 h. The fixed cells were permeabilized in 0.01% saponin.
For double immunofluorescence experiments, cells were fixed as described for LAMP-1 but were lysed in 0.25% saponin. Both LAMP-1 and ankyrin-3 were detectable under these conditions, although staining of each protein was less intense than it was under the optimal conditions of cell lysis described above (LAMP-1 = 0.01% saponin; ankyrin-3 = 0.1% Triton X-100). This was particularly true for ankyrin-3, which was barely detectable in cells lysed with saponin. In a survey experiment, 0.25% saponin was the only concentration where both ankyrin-3 and LAMP-1 could be detected. The number of LAMP-1–positive or dextran-filled vesicles observed was reduced when higher concentrations of saponin were used for lysis or completely ablated when Triton X-100 was used instead of saponin.
All cells were then washed three times (5 min each) in 2 ml of PBS containing 0.02% NaN3 at room temperature, and then incubated at room temperature for 1 h with primary antibodies diluted in PBS. Cells were washed in PBS again and incubated for 1 h at room temperature with a 1:800 dilution of a goat anti–rabbit or goat anti–mouse secondary antibody conjugated with the fluorochrome CY-3 (Jackson ImmunoResearch Laboratories, West Grove, PA). Stained cells were washed a final time, mounted in 9:1 glycerol/PBS, and visualized on an Axiochrom microscope (Carl Zeiss Inc., Thornwood, NY). Images were captured on T-Max 400 film (Eastman Kodak Co., Rochester, NY).
Labeling of Endocytic Organelles
Dextran was used as an endocytic tracer. Macrophages were incubated at 37°C for 15 min in BMM containing 10 mg/ml of an anionic, fluoresceinconjugated dextran (M r 10,000) that could be fixed with paraformaldehyde (Molecular Probes, Eugene, OR). The cells were then washed and chased with BMM (not containing dextran) for periods up to 24 h. The dextran-treated cells were processed at specific time points for immunofluorescence microscopy as described above.
Expression of Epitope-tagged Ankyrin-3 in COS Cells
The HA epitope was fused to the COOH terminus of mouse macrophage ankyrin-3 and ligated into the pcDNA3 vector (Invitrogen). The vector used in transfection experiments was purified by alkaline lysis and cesium chloride density gradient centrifugation as described previously (64). Purified vector was transfected into COS cells using the ProFection CaPO4 transfection kit (Promega, Madison, WI) according to manufacturer's recommendations. Cells were allowed to take up the DNA for 16 h, washed, and incubated for an additional 2 d before HA-tagged ankyrin-3 was visualized via immunofluorescence using the monoclonal 12CA5 anti-HA antibody (Boehringer Mannheim Biochemicals) as described above.
Results
Macrophage Ankyrin-3 Lacks the NH2-terminal Repeats
In the mouse kidney, ankyrin-3 is expressed as multiple isoforms (see Fig. 2) that are detected by Western blotting with the Ank3-R1 antibody (60) (Fig. 1). Antibody staining is specific since it is successfully competed by the immunizing antigen (Fig. 2, lane C). The calculated molecular masses, based on mobility in SDS polyacrylamide gels, are 215, 200, 170, 120, and 105 kD (60). We have previously shown that the two smallest isoforms (105 and 120 kD) lack the NH2-terminal repeat domain (60). Bone marrow– derived macrophages also express small (120 and 100 kD) isoforms of ankyrin-3 (Fig. 2, lane D, arrows). These isoforms are specific (Fig. 2, lane E) and are the only forms of ankyrin-3 consistently observed in macrophages. Some gels contain other, lower molecular mass bands (<80 kD) that stain specifically with Ank3-R1 (Fig. 2, lanes D and E), but these are probably proteolytic fragments of the larger bands because they vary in size and intensity from experiment to experiment.
Figure 2.

Western blot analysis. (A) Mouse kidney membranes (50 μg) stained with a 1:500 dilution of the Ank3-R1 antibody. (B) Mouse kidney membranes stained with ANK3-R1 plus a 200-fold molar excess of GST. (C) Mouse kidney membranes stained with ANK3-R1 plus a 200-fold molar excess of the GST– ankyrin-3 regulatory domain fusion peptide used to generate Ank3-R1. Note that there are five major isoforms of ankyrin-3 expressed in the kidney, all of which are detected by and specific to the Ank3-R1 antibody. (D) Mouse bone marrow macrophage extract (50 μg) stained with Ank3-R1. (E) Mouse bone marrow macrophage extract stained with ANK3-R1 plus a 200fold molar excess of the Ank3R1 epitope. Note that only the 100- and 120-kD isoforms are expressed in bone marrow macrophages.
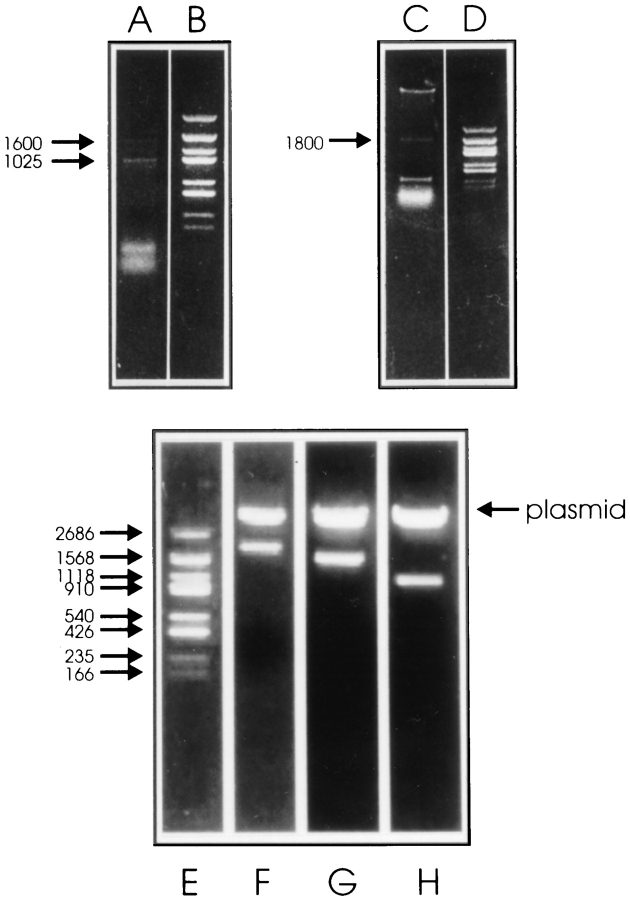
To further characterize the macrophage forms of ankyrin-3 and the analogous kidney isoforms, we recloned ankyrin-3 from mouse bone marrow–derived macrophage RNA by reverse transcription (RT)–PCR. Primers that flank the regulatory domain (REGF1 and REGR1; Fig. 1) produce two PCR products of ∼1,025 and 1,600 bp (Fig. 3, lane A, arrows). Subcloning (Fig. 3, lanes G and H) and sequencing show these represent the regulatory domain with and without the acidic, alternatively spliced exon that is located in the center of the domain (for review see Fig. 1). The spectrin-binding domain is also present in macrophage RNA as shown by the 1,800-bp product (Fig. 3, lane C, arrow) obtained using primers SPINTF1 and SPR1 (Fig. 1). The identity of this domain was also confirmed by subcloning (Fig. 3, lane F) and sequencing. Interestingly, primers MEMF1 and MEMR1 (Fig. 1) did not yield a product. This result supports the protein analysis and confirms that the NH2-terminal repeats of ankyrin-3 are not present in macrophage RNA. All macrophage ankyrin-3 cDNAs were completely sequenced and compared with ankyrin-3 cDNA clones from kidney. This analysis revealed that macrophage ankyrin-3 contains all of the sequence of the spectrin-binding (amino acids [aa] 874–1,455) and regulatory (aa 1,456–1,960) domains previously reported from mouse kidney (60) (GenBank No. L40631). The two isoforms of ankyrin-3 expressed in macrophages differ from each other only by the presence or absence of an insert in the regulatory domain (aa 1,588–1,783) that is also found in kidney ankyrin-3 (60) and is analogous in size and charge to the “2.1 insert” in the regulatory domain of ankyrin-1 (42, 48).
Figure 3.
RT-PCR analysis of bone marrow macrophage mRNA. (A) Regulatory domain RT-PCR using REGF1 and REGR1 primers (See Materials and Methods and Fig. 1). Note two bands (arrows) of ∼1,025 and 1,600 bp. These were subcloned (lanes H and G, respectively) and sequenced. (C) Spectrin-binding domain RT-PCR using SPINTF1 and SPR1 primers. Note one band of ∼1,800 bp (arrow), which was subcloned (lane F) and sequenced. Other bands shown in lane C were nonspecific products. (Lanes B, D, and E) DNA size standards.
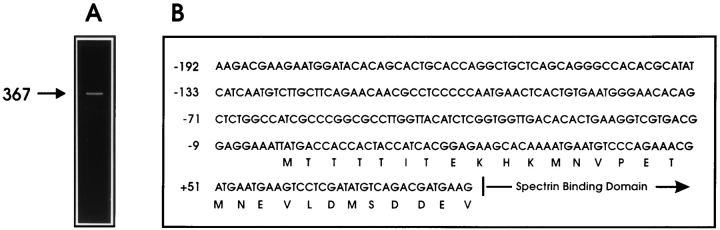
It is important to note that the primer SP5F1, which hybridizes to the 5′ untranslated region of the short kidney ankyrin-3 transcripts that lack the repeat domain (Fig. 1), fails to yield any PCR product from macrophage cDNA. This experiment suggests that the 5′ end of the macrophage cDNA is distinctive from the kidney clones. Analysis of this region of macrophage ankyrin-3 via 5′–rapid amplification of cDNA ends (RACE) PCR confirms the presence of upstream coding sequence (Fig. 4 B). Four separate RACE reactions were performed using two different RNA samples and two sets of primers (see Materials and Methods). Products generated from these experiments (Fig. 4 A) end at base 2,280 (60) located at the 3′ end of the 22nd repeat. These data suggest the start site for macrophage ankyrin-3 is 29 amino acids upstream of the spectrin-binding domain (Fig. 4 B). There are three other possible start sites downstream to the first methionine encoded by the sequence revealed in our 5′-RACE experiment. All of these methionine residues are in adequate Kozak contexts (for review see 39) for efficient translation initialization. We have assigned methionine806 (60) as the start site because it is the first in-frame methionine encoded in our 5′RACE product. The cDNA sequence of full-length macrophage ankyrin-3 has been deposited in (GenBank No. U89275). The calculated molecular mass of this protein is 128,323 daltons, which is close to its estimated size of 120 kD based on mobility in SDS polyacrylamide gels. This NH2 sequence corresponds to the extreme COOH-terminal end of the membrane (“repeat”) domain (60), beginning at the end of the last repeat. The 100-kD isoform corresponds to the full-length protein minus the acidic regulatory domain insert (GenBank No. U89274; calculated molecular mass 106,827 daltons).
Figure 4.
RACE analysis of the 5′ end of macrophage ankyrin-3. (A) 378-bp 5′ RACE product using A3MEMR3 for the reverse transcription, and A3MEMR1 and A3MEMR2 for primary and nested PCR reactions, respectively. (B) Schematic diagram of sequence encoded by the 5′ end of macrophage ankyrin-3.
Ankyrin-3 Associates with Intracellular Vesicles in the Macrophage
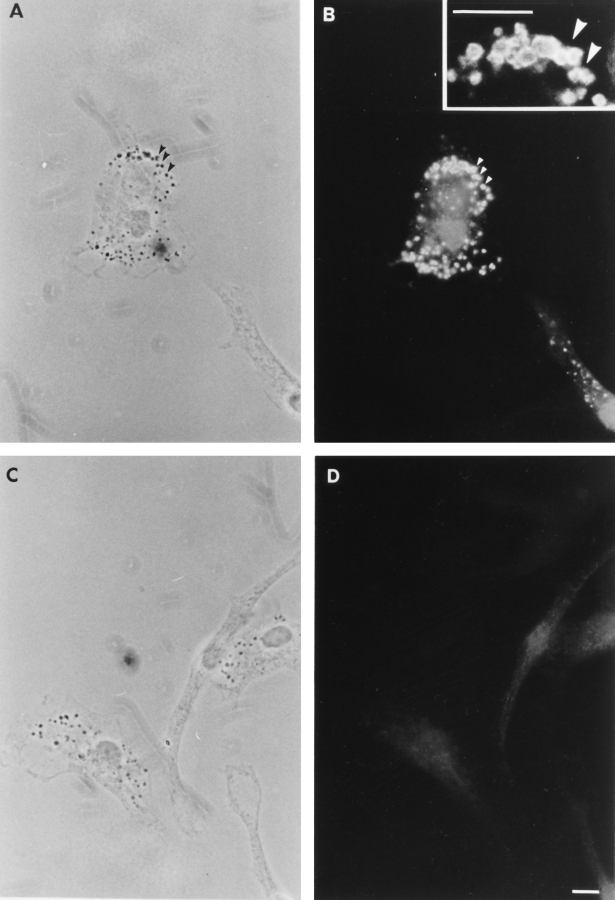
Immunofluorescence microscopy using the Ank3-R1 antibody, which recognizes both isoforms of ankyrin-3 expressed in macrophages, reveals that ankyrin-3 surrounds intracellular vesicles throughout the macrophage cytoplasm (Fig. 5, A and B) and is not associated with the plasma membrane. This surprising result was confirmed with a second antibody, 5′Ank3, which also recognizes both isoforms of macrophage ankyrin-3 (data not shown). 5′Ank3 was raised against a synthetic peptide representing the six unique NH2-terminal residues of the small (105 and 120 kD) isoforms of kidney ankyrin-3, plus the first nine residues of the spectrin-binding domain (60) (Fig. 1). In contrast, an antibody directed against an alternatively spliced exon of ankyrin-3 that is only present in the full-length ankyrin-3 isoforms (Ank3-B; Fig. 1) (60) does not stain macrophage membranes at all (data not shown). This observation supports the RT-PCR and immunoblotting experiments, identifying vesicular ankyrin-3 as truncated isoforms.
Figure 5.
Localization of ankyrin-3 in mouse bone marrow macrophages. (A and C) Phase-contrast photomicrographs. (B) Macrophage stained with Ank3-R1 antibody (inset, high power view). (D) Preimmune serum. Note the intense staining surrounding phase-dense vesicles throughout the macrophage cytoplasm (A and B, arrowheads). Bar, 10 μm.
Macrophage Ankyrin-3 cDNA Expression in COS Cells
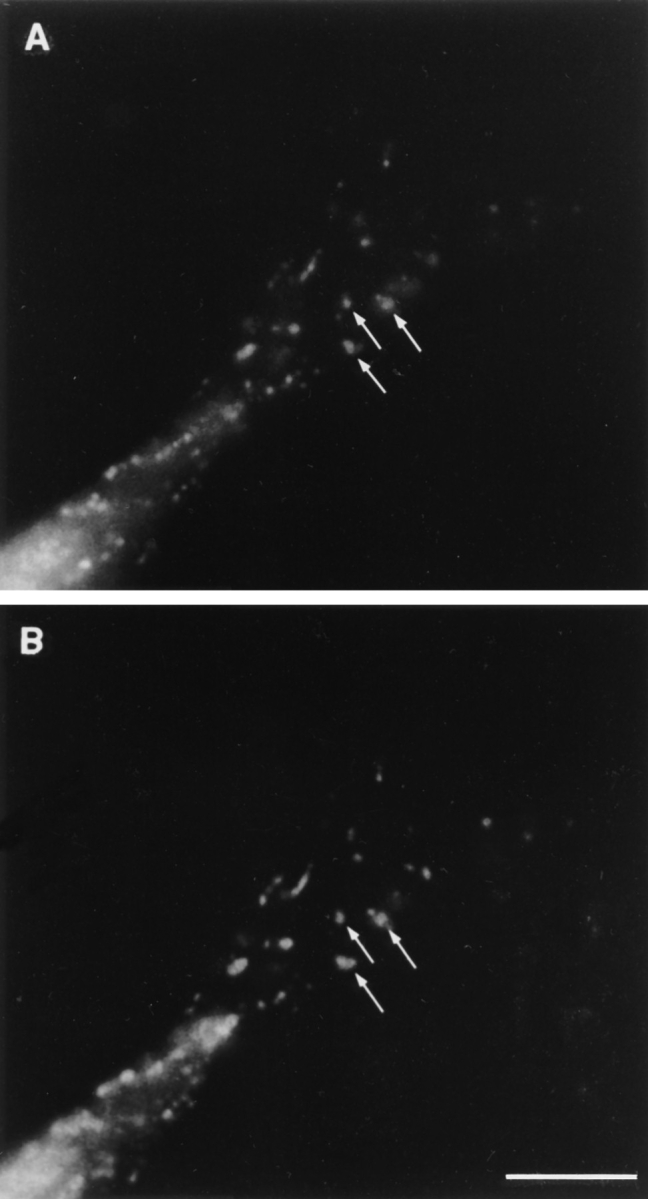
The vesicular location of ankyrin-3 was further substantiated by transient overexpression of HA-tagged ankyrin-3 in COS cells. HA was fused to the COOH terminus of mouse macrophage ankyrin-3 and ligated into the pcDNA3 mammalian expression vector. The fusion protein was visualized using the 12CA5 mAb, which is directed against the HA epitope. This experiment shows that macrophage ankyrin-3 localizes to intracellular vesicle membranes and not plasma membranes (Fig. 6). The vesicular location of macrophage ankyrin-3 (Fig. 7 B) differs dramatically from ankyrin-1, which is found in its characteristic position on the plasma membrane (Fig. 7 A). The presence of ankyrin1 in macrophages was confirmed by immunoblotting (data not shown).
Figure 6.
Localization of epitope-tagged macrophage ankyrin-3 expressed in COS cells. (A) Phase-contrast photomicrographs. (B) COS cell stained with 12CA5 mAb against the HA epitope that has been fused to macrophage ankyrin-3. Note the staining pattern surrounding vesicles shown in phase contrast (arrows). Bar, 10 μm.
Figure 7.
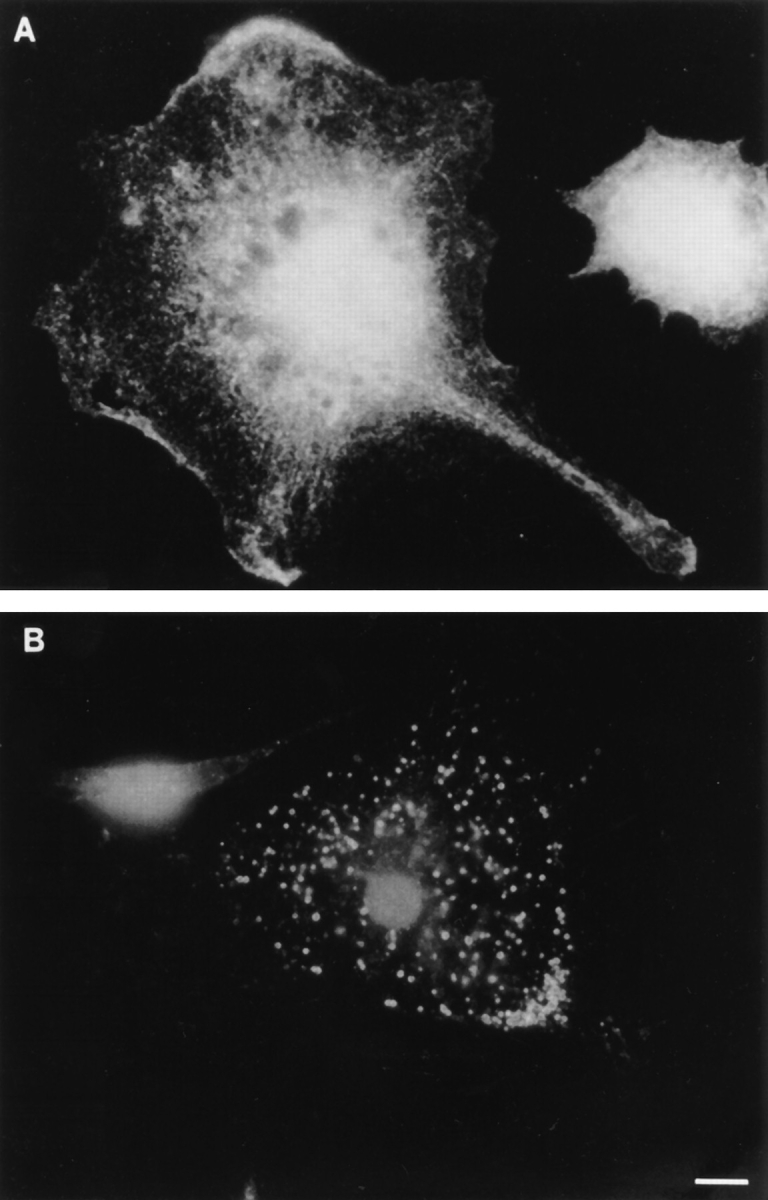
Comparison of ankyrin-3 and ankyrin-1 (erythrocyte ankyrin) localization in mouse bone marrow macrophages. (A) Ankyrin-1 antibody. (B) Ank3-R1 antibody. Note that the ankyrin-1 antiserum does not localize to intracellular membranes and stains most of the surface plasma membrane, while the ankyrin-3 antiserum only labels intracellular vesicles. Bar, 10 μm.
Ankyrin-3–positive Vesicles Are Lysosomes
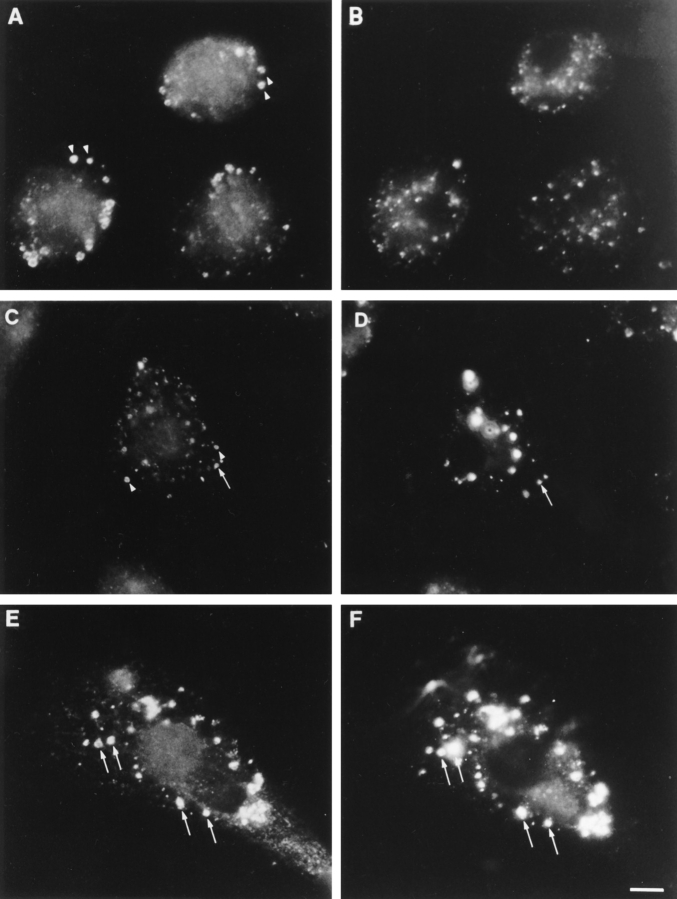
Because the dense appearance of the ankyrin-3–positive vesicles suggested they might be lysosomes (56, 69), we first tested their accessibility to endocytic tracers by incubating macrophages with soluble fluorescein-conjugated dextran (average M r = 10,000 daltons). After a brief 15min labeling pulse, we washed the cells and incubated them for various chase times up to 24 h. We then fixed the macrophages, localized ankyrin-3 with the Ank3-R1 antibody, and visualized it with rhodamine-conjugated antiIgG. None of the ankyrin-3–positive vesicles were filled with fluorescent dextran at very early time points (Fig. 8, A and B, arrowheads), and only a small fraction of the vesicles contained both fluorophores after a 6-h chase (Fig. 8, C and D, arrows). In contrast, after 24 h, all the fluorescent dextran was located in ankyrin-3–positive vesicles (Fig. 8, E and F, arrows). This indicates the vesicles are in the endocytic pathway (5, 23, 24, 30, 38, 57, 72, 73). However, endocytic tracers can be chased out of endosomes and into lysosomes within a few hours (25, 68, 71), while it takes 24 h for dextran-containing endosomes to reach the ankyrin-3–positive compartment. This suggests ankyrin-3– positive vesicles are lysosomes instead of endosomes.
Figure 8.
Simultaneous localization of ankyrin-3 and endocytically incorporated, FITC-labeled dextran. (A, C, and E) Ank3-R1 antibody. (B, D, and F) FITC-dextran. Cells were pulsed for 15 min with 1 mg/ml FITC-dextran, and then washed thoroughly and chased in BMM media for 0 h (A and B), 6 h (C and D), and 24 h (E and F). Arrows and arrowheads indicate ankyrin-3–positive vesicles that do or do not colocalize with FITC-dextran, respectively. Note that at 6 h a small subset of ankyrin-3–positive vesicles contain FITC-dextran, but complete coincidence between ankyrin-3 and vesicular FITC-dextran does not occur until the 24-h chase period. Bar, 10 μm.
To examine this further, we stained macrophages for rab9, a marker of late endosomes (47). Rab9 associates with large, phase-light vesicles that were completely different in appearance from ankyrin-3–positive or dextran-positive vesicles (data not shown). We also stained macrophages with acridine orange, a marker for acidic compartments (53). All phase-dense vesicles were acidic (data not shown), which supports the argument that phase-dense, ankyrin-3– positive vesicles are lysosomes.
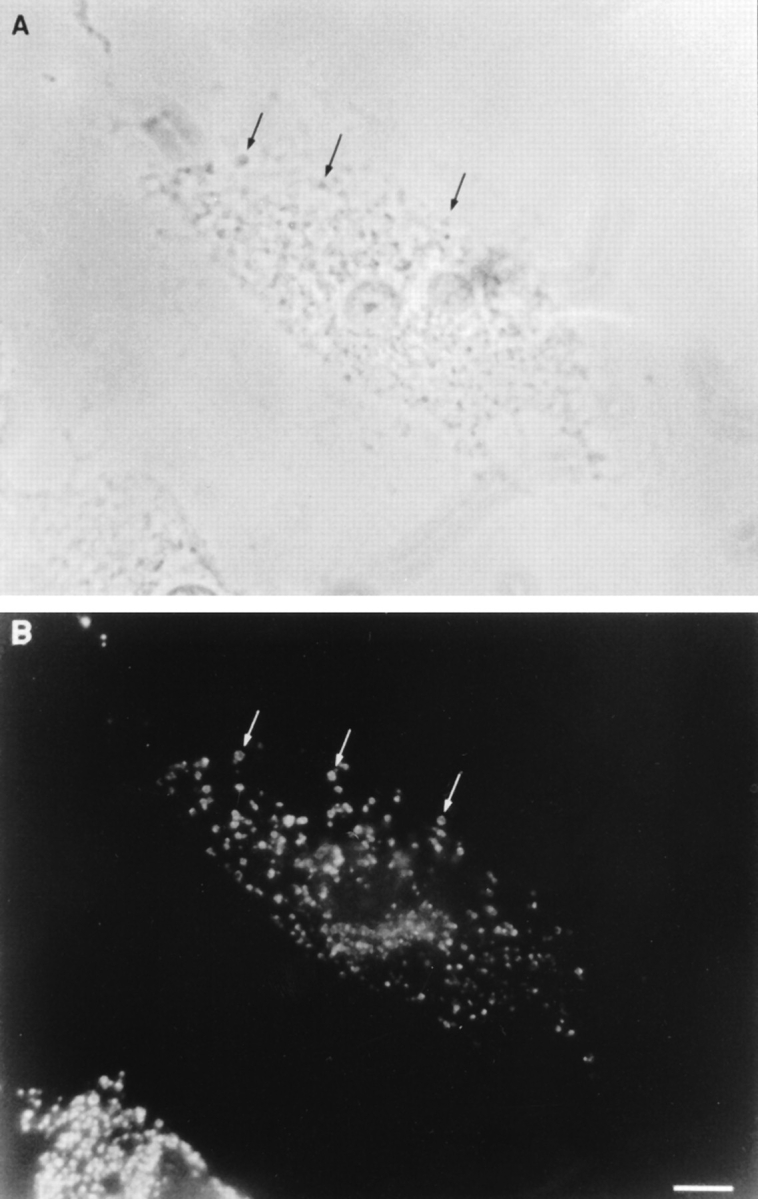
Lastly, we stained macrophages with antibodies to LAMP-1, a well-characterized lysosomal membrane glycoprotein that has been used frequently as a marker for lysosomal compartments (9, 10, 20). After the 24 h chase, a time point at which all ankyrin-3–positive vesicles are filled with dextran, saponin-permeabilized cells reveal that LAMP-1–positive vesicles are also filled with fluorescent dextran (data not shown). Additionally, by simultaneously colocalizing LAMP-1 and ankyrin-3 in macrophages that were not exposed to dextran, we show that both of these proteins associate with the same intracellular vesicles (Fig. 9, A and B, arrows). The LAMP-1 staining shown here is not optimal in its intensity because we had to use higher concentrations of saponin than those normally used for LAMP-1 immunolocalization use to allow ankyrin-3 antibodies to localize simultaneously (see Materials and Methods for more details). Also, under more optimal conditions, LAMP-1 stains many phase-light lysosomes in the perinuclear region of the cytoplasm.
Figure 9.
Colocalization of LAMP-1 and ankyrin-3 in mouse macrophages. (A) Ankyrin-3. (B) LAMP-1. In this representative cell, note the almost identical staining pattern in this region of the cell (A and B, arrows). Bar, 10 μm.
Together these experiments demonstrate that ankyrin-3 is expressed in bone marrow–derived macrophages as two isoforms that lack the NH2-terminal repeats and, within these cells, resides on the intracellular membranes of acidic, phase-dense, LAMP-1–positive lysosomes. The presence of an ankyrin, which is normally associated with the plasma membrane and membrane skeleton, on the surface of lysosomes raises the possibility that structures like the membrane skeleton may also form on intracellular membranes and influence their function.
Discussion
Ankyrin-3 Expression
Ankyrins have been localized to polarized plasma membrane surfaces in a wide range of tissue types, including cardiac and skeletal muscle, intestine, retinal, renal, gastric, and airway epithelium, MDCK cells, initial segments and the node of Ranvier in axons, dendrites, and the Torpedo electrocyte (4, 19, 22, 26, 29, 31, 34–36, 44, 54, 60, 67). In addition to diverse tissue expression, ankyrin-3 is characterized by its unique isoforms, some of which are missing the 89-kD repeat domain (60) responsible for interactions with integral membrane ion channels or adhesive proteins (7, 12, 44, 50–52, 55). Since the isolated 62-kD spectrin-binding domain of ankyrin-1 retains its stability and capacity to bind spectrin (2, 62, 74), it is easy to believe that the 120- and 105-kD isoforms of ankyrin-3, which contain the spectrin-binding and regulatory domains, would be stable and functional as well. It follows that if the domain responsible for binding to plasma membrane–associated proteins is missing, these spliced isoforms might localize to membranes other than the plasma membrane.
Ankyrin-3 is expressed in epithelial cells as multiple isoforms ranging in size from 215 to 100 kD. The major difference between these isoforms is the presence or absence of the NH2-terminal repeat domain. In cells that only express the smaller two isoforms of ankyrin-3 that lack the repeat domain, ankyrin-3 appears to be concentrated in the cytoplasm, not the plasma membrane (60). One of these cells is the macrophage and, as shown here, macrophage ankyrin3 localizes exclusively to the intracellular membranes of lysosomal vesicles. We are confident this is ankyrin-3, and not another ankyrin, since antibodies raised to two different regions of ankyrin-3 localize to the same membrane surface and antibodies to ankyrin-1 show a different (and more typical) plasma membrane staining pattern.
It is interesting that the 5′ end of macrophage ankyrin-3 differs from the NH2-terminal truncated kidney 105- and 120-kD isoforms. Our 5′-RACE analysis reveals that macrophage ankyrin-3 encodes a short 29–amino acid sequence at its NH2-terminal end that corresponds to the final 29 amino acids of the repeat domain. These amino acids do not encode a repeat and are not conserved between ankyrins, suggesting a gene-specific function, but could contain the lysosomal targeting sequence. This hypothesis is supported by the vesicular localization of epitopetagged ankyrin-3 transiently expressed in COS cells. This experiment demonstrates that macrophage ankyrin-3 contains sequence information that can localize it to an intracellular surface in cells other than macrophages. Since all of the sequence of macrophage ankyrin-3 is contained within the full-length (∼210 kD) ankyrin-3, which is found on plasma membranes in various tissues (37, 60), the intracellular targeting sequence(s) must be blocked or inactivated in the larger protein.
Ankyrin-3-positive Lysosomes
It is well established that soluble tracer molecules tagged with fluorescent markers are useful in following the fluid phase through the endocytic pathway over time (23–25, 30, 38, 57, 68, 71–73). Since we can chase one of these endocytic tracers, fluorescent dextran, into ankyrin-3–positive vesicles, the vesicles must be part of the endocytic pathway.
The 24-h chase time required to obtain complete colocalization of endocytically incorporated dextrans and ankyrin-3 (Fig. 8, E and F) suggests the ankyrin-3–positive vesicles are lysosomes (25, 68, 71). This was confirmed by the fact that dextran-containing vesicles are also positive for LAMP-1 at the 24-h chase time and by the fact that LAMP-1 and ankyrin-3 colocalize when the staining conditions for each antigen are carefully adjusted so that both can be stained at the same time.
The colocalization experiment is difficult because immunofluorescence of ankyrin-3 and LAMP-1 staining varies depending on the lysis conditions used. The number of LAMP-1– and/or dextran-positive vesicles in macrophages decreases when high concentrations of saponin or Triton X-100 are used for lysis (conditions that are optimal for ankyrin-3 labeling), while ankyrin-3 cannot be detected when low concentrations of saponin are used (optimal conditions for LAMP-1). In addition, the dark, phase-dense appearance of macrophage lysosomes correlates with the vesicles' ability to retain ankyrin-3 staining (Hoock, T.C., unpublished observations). It is important to note that macrophages grown from bone marrow do not all contain phase-dense vesicles. Anti–ankyrin-3 antibodies do not react with cells that lack phase-dense vesicles. We have tried many cytokines and stimulatory agents to induce their expression: tumor necrosis factor α, interleukin (IL)-1α, IL-4, IL-10, IL-13, gamma interferon, and PMA. While many of these had effects on cell morphology and growth rate, none increased the number of ankyrin-3–positive, phasedense lysosomes.
We do not understand what controls the expression of phase-dense vesicles in macrophages; however, they seem to increase in number when cells become fully differentiated and stop dividing, and when replated from primary culture before cells reach confluent density (Hoock, T.C., unpublished observations). Furthermore, resident macrophages isolated from the peritoneal cavity of mice also show that ankyrin-3 associated with intracellular vesicles, while an immortal macrophage cell line, J774, does not stain for intracellular ankyrin-3 (data not shown). Preliminary data from experiments in which we fed increasing amounts of unlabeled dextran to macrophages show a correlation with increasing numbers of ankyrin-3–positive vesicles (Hoock, T.C., unpublished data). This experiment suggests that cells respond to an increase in endocytic material by increasing the number of endocytic vesicles to house the material. It remains to be seen whether an increase in the expression of ankyrin-3 is observed before or after an increase in endocytic material being ingested.
Intracellular Membrane Skeletons
Using the red cell plasma membrane skeleton as a model, one might expect to find other skeletal proteins on vesicle membranes. Preliminary experiments suggest the vesicles contain β-spectrin (two of 10 antisera tested) (data not shown). One of these antibodies, raised against β-spectrin from canine red cells, also associates with the intracellular membranes of the Golgi apparatus in MDCK cells (1). Macrophage lysosomes were not stained by antisera raised against α-spectrin (0 of nine antisera), fodrin (0 of three antisera), protein 4.1 (0 of two antisera), adducin (0 of one antiserum), or β-actin (0 of one antiserum). Numerous attempts to identify interactions between ankyrin-3 and spectrin by immunoprecipitation combined with Western blotting were unsuccessful, in part because we were unable to completely inhibit macrophage protease activities with multiple combinations of protease inhibitors, including diisopropylfluorophosphate.
Band 3 (32), β-spectrin (1), and, more recently, ankyrin-3 (18) have been localized to Golgi membranes in MDCK cells. The isoform of ankyrin associated with Golgi in kidney and muscle has recently been identified and is a previously unknown isoform of ankyrin-3 (AnkG119). It contains the last 13 of the 24 repeats in the NH2-terminal domain, the complete spectrin-binding domain, and a unique, truncated (5 kD) regulatory domain. AnkG119 binds avidly to β1 (erythrocyte/muscle) spectrins (K d = 4.2 nM) but not to α-spectrin (18). Since the spectrin-binding domains of AnkG119 and lysosomal ankyrin-3 are highly conserved, it is likely that lysosomal ankyrin-3 also binds β-spectrin. In fact, as noted in the previous paragraph, our preliminary experiments show that mouse macrophage lysosomes stain with some β-spectrin antisera, including an antiserum that sees Golgi spectrin (1).
In macrophages, anti–ankyrin-3 antibodies did not stain plasma membranes and were never found in a perinuclear position, suggesting that macrophage ankyrin-3 isoforms, which differ from those expressed in MDCK cells, do not reside in the Golgi apparatus or the ER. Based on these observations, we hypothesize that ankyrin-3 is translated in the cytoplasm on demand and quickly diffuses or is shuttled to lysosomal membranes.
Overall, the presence of these membrane skeleton proteins, previously thought to only associate with the plasma membrane, on intracellular organelles entices speculation about their possible functions. If part of a vesicular membrane skeleton, ankyrin could: (a) strengthen the membrane of the vesicle and prevent “accidental” leakage of its contents into the cytoplasm; (b) prevent spontaneous or promiscuous fusion or exocytosis of vesicles; (c) influence trafficking of endocytic/trafficking vesicles by interacting with motor and/or motor docking proteins; (d) localize a specific membrane channel to establish or maintain the intravesicular milieu; or perhaps (e) contribute to the regulation of vesicle/organelle biogenesis. Continued work on these questions will no doubt yield interesting roles for this class of proteins in the biology of intracellular membranes.
Acknowledgments
We thank Joel Swanson for all his help and suggestions over the course of this study; John Hartwig for facilitating much of the photography shown here and for his encouragement; and Kathryn John, whose assistance in the lab greatly contributed to the success of these experiments.
This work was supported by National Institutes of Health grants DK34083 (to S.E. Lux), HL32262 (to S.E. Lux), and HL55321 (to L.L. Peters), and by a March of Dimes grant 5-FY94-0921 (to L.L. Peters).
Abbreviations used in this paper
- aa
amino acid
- BMM
bone marrow macrophage medium
- GST
glutathione-S-transferase
- HA
hemagglutinin
- IL
interleukin
- LAMP-1
lysosomal-associated membrane glycoprotein 1
- RACE
rapid amplification of cDNA ends
- RT
reverse transcription
Footnotes
Address all correspondence to Samuel E. Lux, Division of Hematology/ Oncology, Children's Hospital, Enders 7, 300 Longwood Avenue, Boston, MA 02115. Tel.: (617) 355-7904. Fax: (617) 355-7262.
L.L. Peter's present address is The Jackson Laboratory, Bar Harbor, ME 04609.
References
- 1.Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: identification of an erythroid β-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978;253:2292–2299. [PubMed] [Google Scholar]
- 3.Bennett V, Stenbuck PJ. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979;254:2533–2541. [PubMed] [Google Scholar]
- 4.Bennett V, Stenbuck PJ. Association between ankyrin and the cytoplasmic domain of band 3 isolated from human erythrocyte membrane. J Biol Chem. 1980;255:6424–6432. [PubMed] [Google Scholar]
- 5.Berthiaume EP, Medina C, Swanson JA. Molecular size-fractionation during endocytosis in macrophages. J Cell Biol. 1995;129:989–998. doi: 10.1083/jcb.129.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkenmeier CS, White RA, Peters LL, Hall EJ, Lux SE, Barker JE. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993;268:9533–9540. [PubMed] [Google Scholar]
- 7.Bourguignon LYW, Lokeshwar VB, He J, Chen X, Bourguignon GJ. A CD44-like endothelial cell transmembrane glycoprotein (GP116) interacts with extracellular matrix and ankyrin. Mol Cell Biol. 1992;12:4464–4471. doi: 10.1128/mcb.12.10.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan W, Kordeli E, Bennett V. 440-kD ankyrinB: structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J Cell Biol. 1993;123:1463–1473. doi: 10.1083/jcb.123.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JW, Pan W, D'Souza MP, August JT. Lysosome-associated membrane proteins: characterization of LAMP-1 of macrophage P388 and mouse embryo 3T3 cultured cells. Arch Biochem Biophys. 1985;239:574–586. doi: 10.1016/0003-9861(85)90727-1. [DOI] [PubMed] [Google Scholar]
- 11.Coetzer TL, Lawler J, Liu SC, Prchal JT, Gualteri RJ, Brain MC, Dacie JV, Palek J. Partial ankyrin and spectrin deficiency in severe, atypical hereditary spherocytosis. N Engl J Med. 1988;318:230–234. doi: 10.1056/NEJM198801283180407. [DOI] [PubMed] [Google Scholar]
- 12.Davis JQ, Bennett V. Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem. 1984;259:13550–13559. [PubMed] [Google Scholar]
- 13.Davis LH, Bennett V. Mapping the binding sites of human erythrocyte ankyrin for the anion exchanger and spectrin. J Biol Chem. 1990;265:10589–10596. [PubMed] [Google Scholar]
- 14.Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267:18966–18972. [PubMed] [Google Scholar]
- 15.Del Sal G, Manfioletti G, Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989;7:514–520. [PubMed] [Google Scholar]
- 16.DeNofrio D, Hoock TC, Herman IM. Functional sorting of actin isoforms in microvascular pericytes. J Cell Biol. 1989;109:191–202. doi: 10.1083/jcb.109.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derick LH, Liu SC, Chishti AH, Palek J. Protein immunolocalization in the spread erythrocyte membrane. Eur J Cell Biol. 1992;57:317–320. [PubMed] [Google Scholar]
- 18.Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, Morrow JS. Identification of a small cytoplasmic ankyrin (AnkG119) in kidney and muscle that binds βIΣ spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133:819–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drenckhahn D, Bennett V. Polarized distribution of M r210,000 and 190,000 analogs of erythrocyte ankyrin along the plasma membrane of transporting epithelia, neurons and photoreceptors. Eur J Cell Biol. 1987;43:479–486. [PubMed] [Google Scholar]
- 20.D'Souza MP, August JT. A kinetic analysis of biosynthesis and localization of a lysosomal-associated membrane glycoprotein. Arch Biochem Biophys. 1986;249:522–532. doi: 10.1016/0003-9861(86)90030-5. [DOI] [PubMed] [Google Scholar]
- 21.Eber SW, Gonzalez JM, Lux ML, Scarpa AL, Tse WT, Dornwell M, Herbers J, Kugler W, Özcan R, Pekrun A, et al. Ankyrin-1 mutations are a major cause of dominant and recessive hereditary spherocytosis. Nat Genet. 1996;13:214–218. doi: 10.1038/ng0696-214. [DOI] [PubMed] [Google Scholar]
- 22.Flucher BE, Morton ME, Froehner SC, Daniels MP. Localization of the α1 and α2subunits of the dihydropyridine receptor and ankyrin in skeletal muscle triads. Neuron. 1990;5:339–351. doi: 10.1016/0896-6273(90)90170-k. [DOI] [PubMed] [Google Scholar]
- 23.Fritsch JE, Buckmaster MJ, Storrie B. Fibroblasts maintain a complete endocytic pathway in the presence of lysosomotropic amines. Exp Cell Res. 1988;175:277–285. doi: 10.1016/0014-4827(88)90192-9. [DOI] [PubMed] [Google Scholar]
- 24.Geisow MJ, D'Arcy P, Hart, Young MR. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981;89:645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geuze HJ, Stoorvogel W, Strous GJ, Slot JW, Bleekemolen JE, Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundersen D, Orlowski J, Rodriguez-Boulan E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol. 1991;112:863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris AS, Green LAD, Ainger KJ, Morrow JS. Mechanism of cytoskeletal regulation (I): functional differences correlate with antigenic dissimilarity in human brain and erythrocyte spectrin. Biochem Biophys Acta. 1985;830:147–158. doi: 10.1016/0167-4838(85)90022-6. [DOI] [PubMed] [Google Scholar]
- 28.Hoock TC, Newcomb PM, Herman IM. β-Actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991;112:653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huotari V, Sormunen R, Lehto V, Eskelinen S. The polarity of the membrane skeleton in retinal pigment epithelial cells of developing chicken embryos and in primary culture. Differentiation. 1995;58:205–215. doi: 10.1046/j.1432-0436.1995.5830205.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang LW, Mitchell BA, Teodoro JG, Rip JW. Uptake and transport of fluorescent derivatives of dolichol in human fibroblasts. Biochem Biophys Acta. 1993;1147:205–213. doi: 10.1016/0005-2736(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 31.Kashgarian M, Morrow JS, Foellmer HG, Mann AS, Cianci C, Ardito T. Na,K-ATPase co-distributes with ankyrin and spectrin in renal tubular epithelial cells. Prog Clin Biol Res. 1988;268B:245–250. [PubMed] [Google Scholar]
- 32.Kellokumpu S, Neff L, Jasma-Kellokumpu S, Kopito R, Baron R. A 115-kD polypeptide immunologically related to erythrocyte band 3 is present in Golgi membranes. Science (Wash DC) 1988;242:1308–1311. doi: 10.1126/science.2461589. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid β-spectrin. J Cell Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kordeli E, Bennett V. Distinct ankyrin isoforms at neuron cell bodies and nodes of Ranvier resolved using erythrocyte ankyrin-deficient mice. J Cell Biol. 1991;114:1243–1259. doi: 10.1083/jcb.114.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kordeli E, Cartaud J, Nghiem HO, Pradel LA, Dubreuil C, Paulin D, Changeux JP. Evidence for a polarity in the distribution of proteins from the cytoskeleton in Torpedo marmorataelectrocytes. J Cell Biol. 1986;102:748–761. doi: 10.1083/jcb.102.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kordeli E, Davis J, Trapp B, Bennett V. An isoform of ankyrin is localized at nodes of Ranvier in myelinated axons of central and peripheral nerves. J Cell Biol. 1990;110:1341–1352. doi: 10.1083/jcb.110.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kordeli E, Lambert S, Bennett V. AnkyrinG: a new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 38.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 39.Kozak M. Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 40.Kunimoto M, Otto E, Bennett V. A new 440-kD isoform is the major ankyrin in neonatal brain. J Cell Biol. 1991;115:1319–1331. doi: 10.1083/jcb.115.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laemmli UK. Cleavage of the structural proteins during the assembly of the head of the bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Lambert S, Yu H, Prchal JT, Lawler J, Ruff P, Speicher D, Cheung MC, Kan YW, Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci USA. 1990;87:1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–643. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li ZP, Burke EP, Frank JS, Bennett V, Philipson RD. The cardiac Na+-Ca2+exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268:11489–11491. [PubMed] [Google Scholar]
- 45.Lin DC, Flannagan MD, Lin S. Complexes containing actin and spectrin from erythrocyte and brain. Cell Motil. 1983;3:375–382. doi: 10.1002/cm.970030505. [DOI] [PubMed] [Google Scholar]
- 46.Liu SC, Derick LH, Zhai S, Palek J. Uncoupling of the spectrin-based skeleton from the lipid bilayer in sickled red cells. Science (Wash DC) 1991;252:574–576. doi: 10.1126/science.2020854. [DOI] [PubMed] [Google Scholar]
- 47.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO (Eur Mol Biol Organ) J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature (Lond) 1990;344:36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- 49.Lux SE, Tse WT, Menninger JC, John KM, Harris P, Shalev O, Chilcote RR, Marchesi SL, Watkins PC, Bennett V, et al. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature (Lond) 1990;345:736–739. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- 50.Michaely P, Bennett V. The membrane-binding domain of ankyrin contains four independently folded subdomains, each comprised of six ankyrin repeats. J Biol Chem. 1993;268:22703–22709. [PubMed] [Google Scholar]
- 51.Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem. 1995;270:22050–22057. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- 52.Morgans CW, Kopito RR. Association of the brain anion exchanger, AE3, with the repeat domain of ankyrin. J Cell Sci. 1993;105:1137–1142. doi: 10.1242/jcs.105.4.1137. [DOI] [PubMed] [Google Scholar]
- 53.Moriyama Y, Takano T, Okuma S. Acridine orange as a fluorescent probe for lysosomal proton pump. J Biochem (Tokyo) 1982;92:1333–1336. doi: 10.1093/oxfordjournals.jbchem.a134053. [DOI] [PubMed] [Google Scholar]
- 54.Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M. Ankyrin links fodrin to the α subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989;108:455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in MadinDarby canine kidney epithelial cells. J Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novikoff, A. 1963. Lysosomes in the physiology and pathology of cells: contributions of staining methods. In Lysosomes. A.V.S de Reuck and M.P. Cameron, editors. Ciba Foundation Symposium, London Churchill. 36–77.
- 57.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otto E, Kunimoto M, McLaughlin T, Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991;114:241–253. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters LL, Birkenmeier CS, Bronson RT, White RA, Lux SE, Otto E, Bennett V, Barker JE. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nbmice. J Cell Biol. 1991;114:1233–1244. doi: 10.1083/jcb.114.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters LL, John KM, Lu FM, Eicher EM, Higgins A, Yialamas M, Turtzo LC, Otsuka AJ, Lux SE. Ank3(epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J Cell Biol. 1995;130:313–330. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters LL, Shivdasani RA, Liu S, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, et al. Anion exchanger (Band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 62.Platt OS, Lux SE, Falcone JF. A highly conserved region of human erythrocyte ankyrin contains the capacity to bind spectrin. J Biol Chem. 1993;268:24421–24426. [PubMed] [Google Scholar]
- 63.Pollard TD. Purification of a high molecular weight actin filament gelation protein from Acanthamoebathat shares antigenic determinants with vertebrate spectrins. J Cell Biol. 1984;99:1970–1980. doi: 10.1083/jcb.99.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrook, J., T. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Second edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 545 pp.
- 65.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savvides P, Shalev O, John KM, Lux SE. Combined spectrin and ankyrin deficiency is common in autosomal dominant hereditary spherocytosis. Blood. 1993;82:2953–2960. [PubMed] [Google Scholar]
- 67.Smith PR, Bradford AL, Joe EH, Angelides KJ, Benos DJ, Saccomani G. Gastric parietal cell H+-K+-ATPase microsomes are associated with isoforms of ankyrin and spectrin. Am J Physiol. 1993;264:C63–C70. doi: 10.1152/ajpcell.1993.264.1.C63. [DOI] [PubMed] [Google Scholar]
- 68.Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976;68:665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Straus, W. 1963. Comparative observations on lysosomes and phagosomes in kidney and liver of rats after administration of horseradish peroxidase. In Lysosomes. A.V.S. de Reuck and M.P. Cameron, editors. Ciba Foundation Symposium, London Churchill. 151–175.
- 70.Swanson JA. Phorbal esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- 71.Swanson J. Fluorescent labeling of endocytic compartments. Methods Cell Biol. 1989;29:137–151. doi: 10.1016/s0091-679x(08)60192-2. [DOI] [PubMed] [Google Scholar]
- 72.Swanson JA, Yirinec BD, Silverstein SC. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985;100:851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang YL, Goren MB. Differential and sequential delivery of fluorescent lysosomal probes into phagolysosomes in mouse peritoneal macrophages. J Cell Biol. 1987;104:1749–1754. doi: 10.1083/jcb.104.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weaver DC, Pasternack GR, Marchesi VT. The structural basis of ankyrin function. II. Identification of two functional domains. J Biol Chem. 1984;259:6170–6175. [PubMed] [Google Scholar]
- 75.White RA, Birkenmeier CS, Lux SE, Barker JE. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nballele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci USA. 1990;87:3117–3121. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]