Abstract
A quantitative assay was developed to study the interaction of Xenopus laevis sperm and eggs. Using this assay it was found that sperm bound in approximately equal numbers to the surface of both hemispheres of the unfertilized egg, but not to the surface of the fertilized egg. To understand the molecular basis of sperm binding to the egg vitelline envelope (VE), a competition assay was used and it was found that solubilized total VE proteins inhibited sperm-egg binding in a concentration-dependent manner. Individual VE proteins were then isolated and tested for their ability to inhibit sperm binding. Of the seven proteins in the VE, two related glycoproteins, gp69 and gp64, inhibited sperm-egg binding. Polyclonal antibody was prepared that specifically recognized gp69 and gp64. This gp69/64 specific antibody bound to the VE surface and blocked sperm binding, as well as fertilization. Moreover, agarose beads coated with gp69/64 showed high sperm binding activity, while beads coated with other VE proteins bound few sperm. Treatment of unfertilized eggs with crude collagenase resulted in proteolytic modification of only the gp69/64 components of the VE, and this modification abolished sperm-egg binding. Small glycopeptides generated by Pronase digestion of gp69/64 also inhibited sperm-egg binding and this inhibition was abolished by treatment of the glycopeptides with periodate. Based on these observations, we conclude that the gp69/64 glycoproteins in the egg vitelline envelope mediate sperm-egg binding, an initial step in Xenopus fertilization, and that the oligosaccharide chains of these glycoproteins may play a critical role in this process.
Eggs of many animal species have a glycoprotein-rich extracellular layer overlying the egg plasma membrane. This layer, called the vitelline envelope or the zona pellucida, depending on the organism, performs multiple functions during fertilization, such as mediating sperm-egg binding, inducing the sperm acrosome reaction, and participating in a block to polyspermy. In recent years, significant progress has been made towards understanding the molecular basis of these functions, especially in mammals. For example, the three main glycoproteins (ZP1, ZP2, and ZP3) of the mouse zona pellucida have been isolated (Bleil and Wassarman, 1980; Shimizu et al., 1983). Considerable in vitro data suggest that the O-linked oligosaccharide chains on ZP3 are responsible for mediating sperm binding and are involved in inducing the sperm acrosome reaction (reviewed by Wassarman, 1990; Snell and White, 1996). ZP2 has been shown to bind acrosomereacted sperm and is presumed to facilitate sperm penetration through the zona (Bleil and Wassarman, 1986).
In amphibians, the egg vitelline envelope (VE)1 also plays an important role in sperm reception and in the block to polyspermy during fertilization (see Schmell et al., 1983; Elinson, 1986; Hedrick and Nishihara, 1991, for reviews). Xenopus laevis, the South African clawed frog that has been widely used for studying animal fertilization and development, has been thoroughly studied with respect to the composition, ultrastructure, and physicochemical properties of its egg envelope (see Hedrick and Nishihara, 1991; Larabell and Chandler, 1991, for excellent reviews). The VE of X. laevis, which is approximately 1 μm in thickness (Dumont, 1972), consists of seven proteins, called gp120, gp112, gp69, gp64, p57, gp41, and gp37, based on their apparent molecular weights on SDS-PAGE (Gerton and Hedrick, 1986a ). These proteins are believed to be synthesized and secreted by the oocyte during oogenesis (Dumont, 1972; Yamaguchi, et al., 1989). Recent studies have demonstrated that the VE is organized into two layers, an inner layer of horizontal filaments and an outer, thicker layer of curled, cable-like fibers (Larabell and Chandler, 1988). The sperm can penetrate the VE and, upon passage through the VE and fusion of the sperm with the egg, cortical granule exocytosis causes elaborate ultrastructural changes of the envelope (see Larabell and Chandler, 1991; Hedrick and Nishihara, 1991, for reviews). These changes involve alteration in the structure of the vitelline envelope and formation of an amorphous fertilization layer (F-layer) between the altered VE (VE*) and the innermost layer of the jelly coat. The VE* and the F-layer together form the fertilization envelope (FE). These ultrastructural changes are accompanied by a decrease in molecular mass of two glycoproteins, gp69 and gp64, to 66 kD and 61 kD, respectively (Gerton and Hedrick, 1986b ). After fertilization, the FE can no longer be penetrated by sperm (Grey et al., 1976).
Presumably, one of the functions of the VE is to provide binding sites for sperm at fertilization. However, the molecular basis of sperm-VE interaction in X. laevis has not been elucidated. In this paper, we describe sperm binding to dejellied Xenopus eggs and the identification of components of the VE that exhibit sperm binding activity. A quantitative and sensitive Xenopus gamete binding assay was developed that enabled us to determine the factors that affect sperm-egg binding. After the demonstration that the isolated mixture of total VE proteins inhibited sperm binding to dejellied eggs, we purified individual VE proteins and showed that only a pair of related proteins, gp69 and gp64, inhibited sperm-egg binding. Polyclonal anti-gp69/64 antibodies also blocked sperm-egg binding, as well as fertilization. These findings, as well as others, including the direct demonstration that sperm specifically bound to beads coated with gp69/64, indicate that these two glycoproteins function in mediating the binding of sperm to the VE. Furthermore, several lines of evidence indicated that the oligosaccharide chains on these proteins play a critical role in the binding process.
Materials and Methods
Preparation of Gametes
Xenopus laevis frogs were purchased from Nasco Biological Supply Co. (Fort Atkinson, WI). Oviposited eggs were obtained as described by Wolf and Hedrick (1971). Briefly, females were injected into the dorsal lymph sac with 600–700 IU/each of human chorionic gonadotropin (Sigma Chem. Co., St. Louis, MO). After 9–10 h eggs were stripped from the females three to four times at 2-h intervals. Jelly was removed by exposure (3–5 min) of the eggs to 45 mM β-mercaptoethanol in MR solution (Modified Ringer: 100 mM NaCl, 1.8 mM KCl, 1.0 mM MgCl2, 2.0 mM CaCl2, 5.0 mM Na-Hepes) adjusted to pH 8.5. The solubilized jelly in the supernatant was decanted, and the eggs were gently rinsed with several changes of MR (pH 6.5).
Jelly extract was prepared according to the procedure described by Heasman et al. (1991). Briefly, freshly released eggs were incubated in 0.3 × MR (pH 7.8) in a ratio of 8 ml solution per 3 grams of eggs in a culture dish on a rocker plate at medium speed (15 cycles/min). After 45 min of incubation at 20°C, the medium was recovered from the culture dish (usually 60% of the originally added volume). For in vitro fertilization of dejellied eggs, solid Ficoll (Sigma 400 DL) was added into the extracted medium to a final concentration of 10% (wt/vol). For the gamete binding assay, Ficoll was omitted, and it was found that without Ficoll in the jelly extract, fertilization did not occur during the time period of the gamete binding assay (a total of 20–25 min from the time of mixing the gametes to fixation, see below). Unless otherwise specified, the “jelly extract” referred to in this paper did not contain Ficoll.
Sperm were prepared by chopping and macerating a freshly excised testis in 1 ml of jelly extract. The mixture was transferred into a microcentrifuge tube and spun at 100 g for 3 min at 4°C to remove tissue debris. The supernatant contained a mixture of intact live sperm at different states of differentiation, with the majority (∼80%) being mature, as determined by their shape when examined microscopically (Reed and Stanley, 1972). The sperm concentration was determined using a hemocytometer. The sperm suspension was then diluted to the indicated concentrations with jelly extract.
Isolation of Egg Envelope Proteins
A sieving method (Wolf et al., 1976) was used to isolate the vitelline envelope. Briefly, the dejellied eggs were lysed by passing through an 18–19 gauge needle. The lysate was poured through a 100-μm nylon screen and the envelopes retained on the screen were washed with ice-cold 10 mM Tris-DeBoers solution (110 mM NaCl, 1.3 mM CaCl2, and 1.3 mM KCl, adjusted to pH 7.5 with NaHCO3) until visually free from contaminating particulate material. The clean envelopes were washed from the screen into a centrifuge tube and collected by centrifugation at 5,000 g, 4°C for 5 min. The envelope pellet was solubilized by different methods depending on their usage. For sperm-egg binding competition assays, the envelope pellet was dissolved in 0.3 × MR (pH 7.8) by heating and repeated vortexing at 80°C for 10 min, followed by centrifugation for 2 min at 14,000 g to remove particulate material. For SDS-PAGE analysis and other purposes, the envelopes were incubated at 95°C for 2 min in 2% SDS, and then centrifuged at 16,000 g for 5 min to remove any particulate material. The envelopes were completely solubilized by this procedure (Wolf et al., 1976). The envelope solutions were stored at −20°C.
To purify individual VE proteins, isolated total VE proteins were subjected to 7.5% SDS-PAGE (Laemmli, 1970). The protein bands on the gel were visualized by staining the gel with 0.3 M CuCl2 for 5 min and washing with distilled water for several times (Harlow and Lane, 1988). Each protein band was excised from the gel and destained in the destain buffer (0.25 M EDTA, 0.25 M Tris, pH 9.0). Then the gel slices were subjected to electroelution in SDS-electrophoresis buffer at 8–10 mA per elution tube for 4–6 h, followed by electrodialysis at 1 watt constant power for 4 h against electrophoresis buffer not containing SDS (Bleil and Wassarman, 1980). The individual proteins were then dialyzed in distilled water at 4°C for 48 h, lyophilized, and dissolved in water.
Preparation and Property of Polyclonal Antibodies Against gp69 and gp64
The purified gp64 and gp69 glycoproteins (500 μg each) were used by Biodesign International (Kennebunk, ME) to generate separate rabbit antisera against each protein. Antisera were enriched for IgG by ammonium sulfate precipitation followed by DEAE-matrix chromatography (Harlow and Lane, 1988). The pre-immunization sera collected from each rabbit was purified using the same procedure and used as control IgGs in the experiments. To obtain polyclonal antibodies specifically directed against gp69 and gp64, the purified antibodies were pre-adsorbed with the other VE proteins that they also recognized. For the pre-adsorption, an affinity column was made with 0.5 ml of Affigel-15 (Bio-Rad Laboratories, Melville, NY) covalently coupled with the following VE proteins, gp120, 112, 41, and 37, following the manufacturer's instructions. After prewashing the column with MR solution (pH 6.5), the IgG preparations of antigp69 or anti-gp64 antibody were passed through separate columns for multiple rounds and the flow-through was collected. The pre-adsorbed antibodies were stored in MR solution (pH 6.5) with 1% BSA (Fraction V, Sigma) at −20°C.
The specificities of the IgG enriched polyclonal antibodies and the pre-adsorbed antibodies were tested by Western blots using horseradish peroxidase–conjugated goat anti–rabbit IgG (Boehringer Mannheim Biochemicals, Indianapolis, IN) as secondary antibody and the LumiGLO detection method (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
In Vitro Gamete Binding Assay and Competition Assay
All of the gamete binding assays were performed in 24-well, flat bottom tissue culture plates (Falcon 3047, Becton Dickinson and Co., Lincoln Park, NJ). Groups of 20 dejellied eggs were added to 0.5 ml of a sperm suspension in jelly extract (1.0 × 107 sperm/ml) and incubated in the wells for 15 min at 18–22°C. Then each group of sperm-treated eggs was collected carefully with a wide bore pipette and washed by allowing the eggs to fall through a 15-cm tall tube filled with 0.3 × MR buffer (pH 7.8) that were connected to the top of the wells of the 24-well tissue culture plate. The eggs quickly fell to the bottom of the well while unbound free sperm settled much more slowly. As the eggs were falling, the buffer above the eggs was quickly aspirated. Using this procedure, most of the unbound sperm were separated from those bound to the eggs. The tubes were disconnected and the eggs were further washed in the wells with three changes of 0.3 × MR buffer (pH 6.5). Finally, the gametes were fixed and stained in 3% formaldehyde in MR buffer (pH 6.5) containing DNA dye HOECHST 33342 (0.2 mg/ml, Molecular Probes, Inc., Eugene, OR) and viewed with an Axioskop-fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) with a Plan-Neofluar 5× or 10× objective. Images were recorded on Kodak 400 film (Eastman Kodak Co.).
Because of the size of the eggs (∼1.2–1.3 mm in diameter), only a limited “ring” of the egg surface was within focus in each plane. Therefore, the number of bound sperm was counted by focusing stepwise through the depth of one half of the egg; the values obtained were recorded as the “number of sperm/hemisphere.” The total number of bound sperm per egg is twice the number of sperm/hemisphere.
For competition assays using VE proteins, 10 μl of solubilized proteins at various concentrations in 0.3 × MR (pH 7.8) were mixed with the sperm suspensions and preincubated for 10 min before addition of eggs into the sperm suspensions. For inhibition assays using polyclonal antibodies, the dejellied eggs were first incubated with the antibody solution at different concentrations in MR (pH 6.5) for 30 min. After washing briefly in fresh 0.3 × MR (pH 7.8) solution, the eggs were transferred to and incubated with the sperm suspension. The subsequent steps of the gamete binding assay were then carried out as described above.
In Vitro Fertilization of Dejellied Eggs and the Antibody Inhibition Assay
Dejellied eggs were prepared as described above. Sperm were prepared by macerating about 1/8 of a testis in 600 μl jelly extract (plus 10% Ficoll). Approximately 100 dejellied eggs contained in a 35 × 10 mm petri dish were rinsed three times with 0.3 × MR (pH 7.8). The solution was slowly removed from the dish until the level was just above but not touching the top of the eggs. The freshly prepared sperm suspension was gently applied onto the eggs. After 5 min another 600 μl of jelly extract was added and mixed. To achieve high rates of fertilization, the final sperm concentration had to exceed 107 sperm/ml. Fertilization usually occurred within 30 min at 18–20°C, as indicated by contraction of the animal hemisphere. For antibody inhibition assays, dejellied eggs were preincubated with pre-adsorbed antibodies or control IgGs in MR (pH 6.5) for 30 min and then rinsed three times in 0.3 × MR (pH 7.8) before mixing with the sperm.
Sperm-bead Binding Assay
Purified VE proteins, gp41, gp37, gp(69/64) (1:1), gp(120/112) (1:1), or BSA (Fraction V, Sigma) were covalently coupled to cyanogen bromide– activated agarose beads (Sigma), according to the manufacturer's instructions. After coupling, the remaining activated groups on the beads were blocked with Tris-HCl (0.5 M, pH 8.0). The beads were then washed with buffer A: 0.1 M sodium acetate (pH 4.0) and 0.5 M NaCl; buffer B: 0.1 M Tris (pH 8.0) and 0.5 M NaCl; and buffer C: MR/3 (pH 7.8) for three times each. To determine the amount of protein coupled to the beads, the protein concentrations before and after coupling were measured and compared. Beads coated with BSA or with no protein coupled were used as negative controls for sperm binding.
The sperm-bead binding assays were performed in 96-well, flat bottom tissue culture plates (Falcon 3072). In each well, ∼100 beads were mixed with 35 μl of sperm suspension (107 sperm/ml) in jelly extract for 30 min with gentle shaking. After incubation, beads were washed with three changes of 0.3 × MR (pH 7.8), fixed in 3% formaldehyde in MR (pH 6.5) for 30 min, further washed five times with the same fixation solution and finally stained with HOECHST 33342 (0.2 mg/ml). The beads were viewed with a Diaphot fluorescence microscope (Nikon, Inc., Melville, NY) with a 20× objective.
To test the effects of anti-gp69/64 antibodies on sperm-bead binding, aliquots of beads coupled with either gp69/64 (1:1), BSA, or no protein were preincubated with a 1:1 mixture of anti-gp69 and anti-gp64 polyclonal antibodies (final antibody concentration: 0.07 mg/ml) or purified pre-immunization IgG fraction for 1 h and washed three times with 0.3 × MR (pH 7.8). The antibody-treated beads, as well as beads not treated with antibody, were then used in the sperm binding assay as described above. For each group of beads, the average number of sperm bound per bead was determined by counting and averaging the numbers of bound sperm/bead on 30 beads of sizes ∼0.10 ± 0.01 mm in diameter. The value was finally normalized by comparing on an equimolar basis of coupled proteins.
Collagenase Treatment of Dejellied Eggs
Dejellied eggs were incubated in MR (pH 6.5) containing 0.5% (wt/vol) crude type I collagenase (315 U/mg of collagen digestion activity) or pure type VII collagenase (1,500 U/ml, Sigma) at 20°C for 2 h with gentle shaking. A control group of eggs was treated in the same manner without any enzyme. The eggs were then transferred to fresh MR solution (pH 6.5) and washed several times. The envelope proteins of the treated eggs were isolated and analyzed by SDS-PAGE as described above. Twenty treated eggs from each group were used in the gamete binding assay.
NH2-Terminal Peptide Sequencing
Preparation of proteins for microsequencing was as described (Baker and Dunn, 1994). Briefly, proteins were separated by 7.5% SDS-PAGE and electroblotted onto Immobilon-PSQ membrane (Millipore Corp., Bedford, MA) in a transfer buffer containing 50 mM Tris base and 50 mM boric acid, pH 8.5. The membrane was stained with 0.2% wt/vol Coomassie blue for 2 min and destained in a destain buffer containing 45% methanol and 10% acetic acid for 15 min. The band to be sequenced was cut out and washed 10 times in HPLC grade water and air dried. Approximately 3 μg of each purified protein was used and microsequencing was performed at the Center for Analysis and Synthesis of Macromolecules at Stony Brook, using a model 475A pulsed liquid protein sequencer with model 900A control/data analysis module and model 120A PTH analyzer (Appl. Biosystems, Inc., Foster City, CA).
Pronase Digestion
Fresh Pronase solution was preheated at 60°C for 30 min to destroy any glycosidase activity. Each protein sample was incubated with 0.1 mg/ml of Pronase in MR buffer (pH 7.8) for 12 h. In the control, no VE protein was included. After incubation, the Pronase in the control and the experimental samples was inactivated by boiling for 10 min.
Metaperiodate Treatment of Live Eggs and Glycopeptides
Groups of 20 dejellied eggs were treated with varying concentrations of sodium metaperiodate (10–40 mM) in 2 ml of MR buffer (pH 6.5) for 10 min in the dark at room temperature. Control (treated with 0 mM of periodate) and treated eggs were then rinsed with MR (pH 7.8) and exposed to 20 mM sodium borohydride in MR (pH 7.8) for 10 min at room temperature. Eggs were washed extensively with MR and added to the sperm suspension for binding assay.
To treat Pronase digested VE glycopeptides with metaperiodate, the samples were incubated with 50 mM sodium metaperiodate in 50 mM sodium acetate buffer (pH 5.5) for 2 h in the dark at room temperature. At the end of this period, glycerol was added to a final concentration of 250 mM and incubated for another hour before being exposed to 0.5 M sodium borohydride in phosphate-buffered saline for 1 h at room temperature. Finally the treated samples were dialyzed in dialysis tubing (MWCO: 1 kD) against distilled water, lyophilized, and dissolved in 0.3 × MR (pH 7.8) for use in sperm binding competition assay.
PNGase F Treatment of Purified VE Proteins
N-linked oligosaccharide chains were removed by treatment with peptide N-glycosidase F (PNGase F, Boehringer Mannheim). Protein samples were heated in 0.5% SDS and 1% β-mercaptoethanol at 100°C for 5 min. The reaction mixture, containing 0.5 mg/ml protein, 5 U/ml PNGase F, 10 mM EDTA, and 0.5% NP-40 in 50 mM Tris-HCl (pH 8.5), was incubated at 37°C for 24 h. The reaction was terminated by boiling at 100°C for 5 min.
Protein Determinations
The bicinchoninic acid (BCA) kit (Pierce Chemical Co., Rockford, IL) was used to determine protein concentrations. BSA was used as a standard.
Results
Gamete Binding Assay
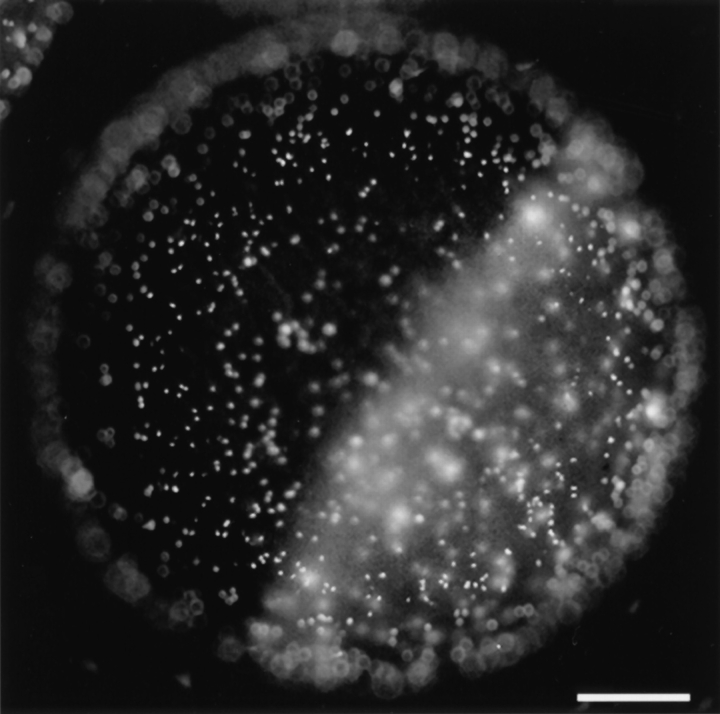
To study the binding of X. laevis sperm to eggs, we developed a quantitative gamete binding assay (see Materials and Methods). A typical example of this assay measuring sperm binding to a dejellied, unfertilized egg is shown in Fig. 1. Under optimal conditions and in the presence of high concentrations of sperm (1.0 × 107 sperm/ml), ∼1,500 ± 200 sperm could bind to a single egg (1.2–1.3 mm in diameter) in the presence of jelly extract. Further increasing the sperm concentration did not increase the density of bound sperm on the egg surface. Low temperature (<16°C) or high salt concentration (1 × MR instead of the standard 0.3 × MR in the jelly extract) reduced sperm motility and resulted in lower levels of binding. We also observed that approximately equal numbers of sperm were present on the animal and the vegetal hemispheres of the egg (Fig. 1). We examined numerous eggs from different females and found that the density of sperm bound on each hemisphere was not statistically different. This finding suggested that the putative protein(s) of the VE responsible for mediating sperm binding was distributed uniformly on both hemispheres of the egg.
Figure 1.
The gamete binding assay demonstrates sperm binding to both the animal (darker half) and the vegetal (lighter half) hemispheres of a dejellied unfertilized egg. Sperm heads are preferentially stained by HOECHST 33342 and appeared as bright dots on the surface of the egg. Bar, 0.2 mm.
Omission of the jelly extract did not significantly change the level of sperm binding. However, the sperm could not fertilize the dejellied eggs in the absence of jelly extract. This is in agreement with the findings of other groups (StewartSavage, 1984; Hedrick and Hardy, 1991). After exposure to jelly no morphological changes could be detected in the sperm as viewed under light or electron microscope (unpublished observation). Similar observations have been made in other amphibian species studied (Raisman et al., 1980; Katagiri et al., 1982), implying that in amphibians the sperm acrosome reaction may not occur or go to completion until the sperm reach the vitelline envelope.
Competitive Inhibition of Sperm-Egg Binding by Solubilized Total or Individual VE Proteins
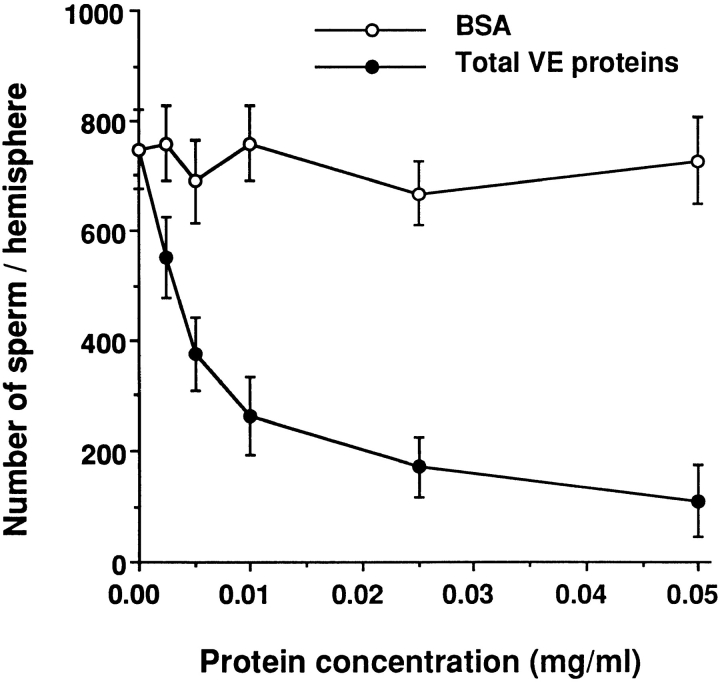
To test if VE proteins could compete with intact vitelline envelopes for binding to sperm, we isolated the total VE proteins and heat treated them in 0.3 × MR (pH 7.8). Although such treatment is known to “solubilize” the envelope proteins, it has been shown that the components of the VE still exist in the form of a supermolecular complex (Nishihara et. al., 1983). As shown in Fig. 2, the solubilized total VE proteins competitively inhibited sperm binding to unfertilized eggs in a dose-dependent manner, whereas the control protein (BSA) had little effect on sperm-egg binding at the same concentrations. This experiment suggested that VE proteins are involved in the sperm-egg interaction.
Figure 2.
Dose-dependent inhibition of sperm-egg binding by heat-solubilized total VE proteins. The sperm binding competition assay was carried out as described in Materials and Methods. Bars indicate the standard deviation (SD) with n = 15 eggs.
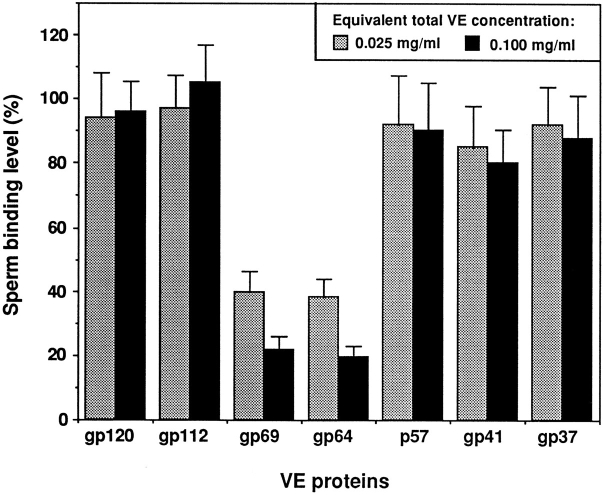
To determine if a particular protein(s) in the isolated total VE protein mixture was responsible for the inhibitory effect described above, we purified individual VE proteins and tested their abilities to inhibit sperm-egg binding. The relative amount of each VE protein tested was proportional to its contribution to the total VE proteins, as reported by Hedrick and Nishihara (1991). The results in Fig. 3 show that gp69 and gp64 had much stronger inhibitory effects than the other five VE proteins tested. Furthermore, the inhibitory effects of gp69 and gp64 were dose dependent and almost indistinguishable from each other. Gp41 was the only other VE protein that appeared to have a weak inhibitory effect at high concentrations. However, in a separate experiment it was found that the inhibitory effect of gp41 on sperm-egg binding was not dose dependent. These experiments suggested that gp69 and gp64 might be the proteins in the VE that are involved in mediating sperm-egg binding. It should be noted that the possible role of other VE proteins in facilitating sperm binding could not be excluded by this in vitro binding competition experiment because of the probability that they, unlike gp69/64, were irreversibly inactivated during purification (see Discussion).
Figure 3.
Effects of purified individual VE proteins on sperm-egg binding. The final concentration of each protein tested was equivalent to its concentration in either 25 or 100 μg/ml of total VE protein. The percentage of each of the proteins in the isolated X. laevis VE has been reported to be: gp120 (6.2 ± 1.5%), gp112 (6.5 ± 1.4%), gp69 (1.9 ± 0.8%), gp64 (2.6 ± 0.6%), p57 (0.9 ± 1.2%), gp41 (43 ± 2%), and gp37 (39 ± 5%) (Hedrick and Hishihara, 1991). Bars represent SD with n = 15 eggs.
Polyclonal Antibodies Specific to gp69 and gp64 Block Sperm-Egg Binding
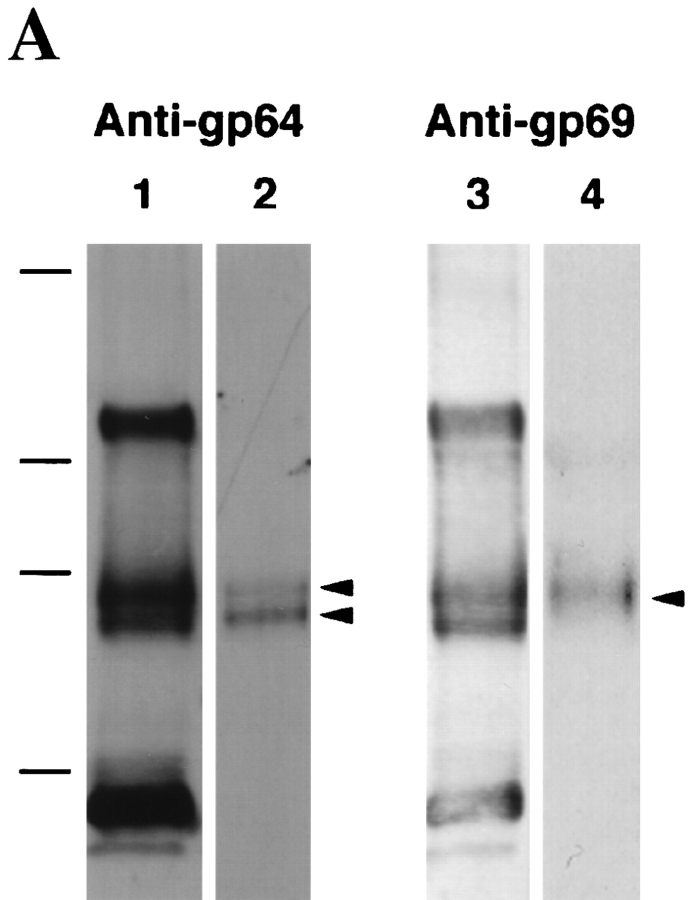
To further study the function of gp69 and gp64, polyclonal antibodies directed against each purified glycoprotein were prepared and purified by ammonium sulfate precipitation and DEAE chromatography. The specificities of the purified antibodies were determined by Western blot analysis of purified total VE proteins (Fig. 4 A). Surprisingly, the results indicate that both polyclonal antibody preparations reacted not only with both gp69 and gp64, but also with gp120/112 and gp41. They also reacted weakly with gp37, but not with p57 (Fig. 4 A, lanes 1 and 3). The control preimmunization IgG fractions purified by the same procedure did not react with any VE proteins. In view of these findings the purity of the gp69 and gp64 preparations used as immunogens was re-examined by 125I-labeling followed by SDS-PAGE and autoradiography. No contaminating proteins were detected within the limits of this method. Therefore, our tentative conclusion is that several of the other VE proteins share epitopes in common with gp69/64. In the case of gp69 and gp64, there already is evidence suggesting that these two glycoproteins may share the same polypeptide sequence, but differ in glycosylation (see below).
Figure 4.
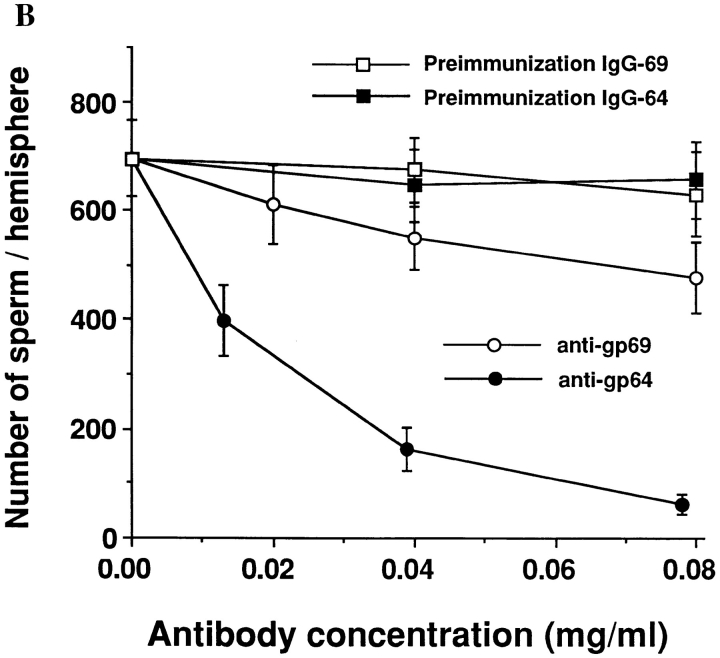
Polyclonal anti-gp64 and anti-gp69 antibodies and their effects on sperm-egg binding. (A) The specificity of the polyclonal anti-gp69 and anti-gp64 antibodies. Total VE proteins (0.8 μg/lane) were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with the DEAE-cellulose purified anti-gp64 (lane 1) or anti-gp69 (lane 3), or with preadsorbed anti-gp64 (lane 2) or anti-gp69 (lane 4). The two bands in lane 2 indicated by arrows are gp69 and gp64, respectively. The band in lane 4 indicated by the arrow is gp69. Molecular weight standards are marked on the left. From top: 216, 97, 71, and 44 kD. (B) The effects of the pre-adsorbed polyclonal anti-gp64 and anti-gp69 antibodies on sperm-egg binding. The horizontal axis represents the antibody concentration in the MR solution in which the eggs were preincubated. Bars represent SD with n = 15 eggs.
To deplete the antibodies that reacted with the common epitopes shared by other VE proteins, the IgG enriched antibodies were pre-adsorbed with gp120/112, gp41, and gp37. After pre-adsorption, the specificities of the antibodies were determined again by Western blot of the total VE proteins. It was found that the pre-adsorbed anti-gp69 only recognized gp69 (Fig. 4 A, lane 4), while the pre- adsorbed anti-gp64 still recognized both gp64 and gp69 (Fig. 4 A, lane 2), but not the other proteins. Both antigp69 and anti-gp64 antibodies bound to the VE surface as demonstrated by FITC-labeled anti-IgG antibody staining (data not shown). The intensity of the fluorescence staining was virtually uniform over the whole egg surface, indicating that gp69 and gp64 are evenly distributed on both hemispheres of the VE. Control pre-immunization IgGs did not bind to the VE.
If gp69 and gp64 are sperm-binding proteins in the VE, one would expect that the polyclonal anti-gp69 and antigp64 antibodies should block sperm-egg binding. Accordingly, the dejellied eggs were preincubated with different concentrations of pre-adsorbed anti-gp69 or anti-gp64. The unbound antibody was removed by washing and then the eggs were tested in the gamete binding assay. It was found that the level of sperm binding to the eggs treated with the antibodies decreased as a function of the antibody concentration used during the pre-incubation (Fig. 4 B). Sperm binding was nearly completely blocked when the eggs were pretreated with sufficient amount of pre-adsorbed anti-gp64 (which recognizes both gp69 and gp64, see Fig. 4 A, lane 2). Anti-gp69, which only reacted with the epitopes unique to gp69 (Fig. 4 A, lane 4), was much less efficient in blocking sperm-binding than anti-gp64, which reacted with both proteins. This finding suggests that the putative additional epitope(s) of gp69 may play only a minor role in the sperm binding function. The purified pre-immunization IgGs had little effect on sperm binding. We also tested another control polyclonal antibody, anti-GST45A, which was specific to a fusion protein consisting of glutathione S-transferase fused with the NH2-terminal half of the sea urchin egg receptor for sperm (Ohlendieck et al., 1993), and found no inhibitory effects on X. laevis sperm binding.
We also found that the antibody that reacted with both gp69 and gp64 (anti-gp64) not only blocked sperm binding, but also blocked fertilization of dejellied eggs. In three control groups (∼50 eggs/group), the average percent of eggs fertilized in the presence of 1.0 × 107 sperm/ml in jelly extract was 90.8 ± 4.5%. After preincubation of the dejellied eggs with pre-adsorbed anti-gp64 (final antibody concentration: 100 μg/ml) for 30 min, the percentage of egg fertilized was zero.
Binding of Individual VE Proteins to Sperm Detected by a Sperm-Bead Binding Assay
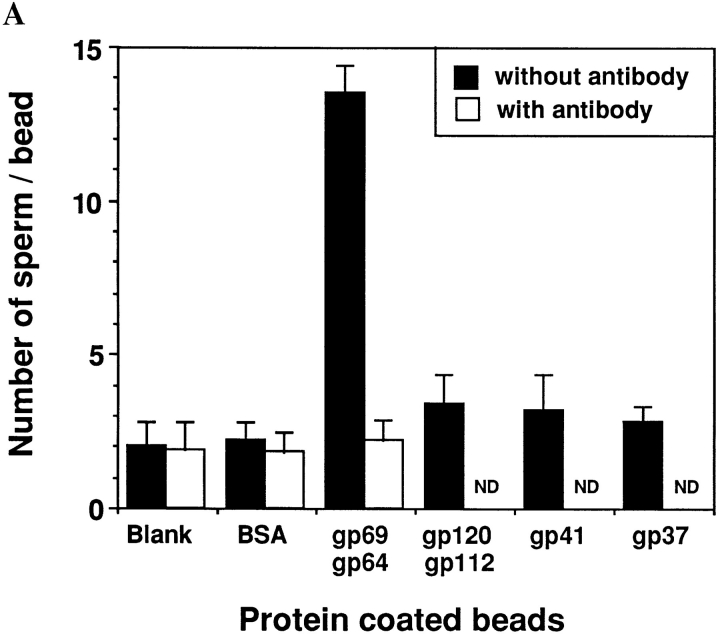
To study the possible interactions between the sperm and the individual VE proteins in a direct, positive assay, we covalently coupled purified VE proteins to cyanogen bromide–activated agarose beads and measured the ability of sperm to bind to these beads. It was determined that ∼0.3–0.4 nmol of protein was covalently linked per mg of beads. The sperm binding assays were performed in the presence of an excess of sperm (107 sperm/ml). Under these conditions, if the VE protein being tested bind to sperm, the number of sperm bound to the bead should be dependent on the amount of protein coupled to the bead. The results in Fig. 5 A show that beads coated with a mixture of gp69 and gp64 on average bound far more sperm than beads coated with other VE proteins or BSA, or blank control beads with no coupled protein. In fact, beads coupled with other VE proteins showed no sperm binding activity above the background level.
Figure 5.
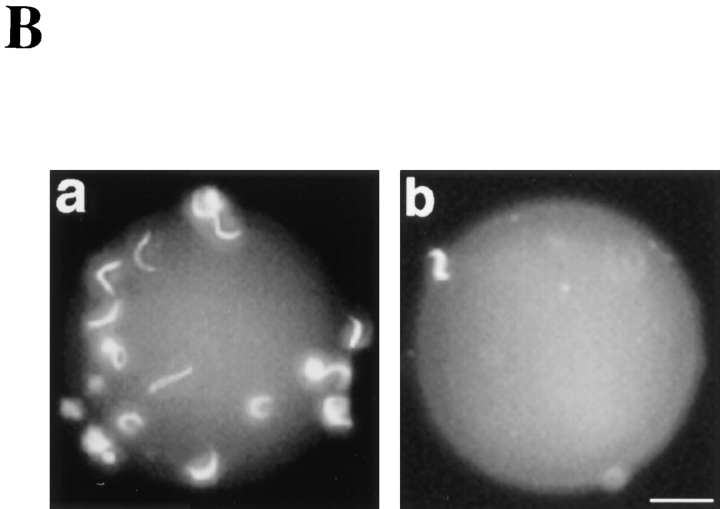
Sperm binding to agarose beads containing coupled proteins. The sperm-bead binding assay was carried out as described in Materials and Methods. (A) Quantitation of the average number of sperm bound to beads containing various covalently coupled proteins. Binding was measured in the absence or presence of 70 μg/ml of polyclonal anti-gp64 antibody (which recognized both gp64 and gp69). Approximately 0.3–0.4 nmol of protein was coupled per mg of beads. Bars represent SD with n = 30 beads. The effects of the anti-gp64 antibody on gp120/112, gp41, gp37 were not determined (ND). (B) Fluorescence micrographs of sperm binding to beads coated with a 1:1 mixture of gp69 and gp64 (a) or BSA (b). Bar, 20 μm.
We also examined the effect of the preadsorbed polyclonal anti-gp64 that reacted with both gp69 and gp64 on sperm-bead binding. Control beads and beads containing a mixture of gp69 and gp64 were incubated with this antibody, washed thoroughly, and then used in the spermbead binding assay. We found that after antibody treatment sperm binding to beads containing gp69/64 decreased to background level, while sperm binding to control beads remained at the background level (Fig. 5 A). Control preimmunization IgGs did not have any inhibitory effect on sperm-bead binding. Fluorescence micrographs showing sperm binding to beads are presented in Fig. 5 B.
Crude Collagenase Treatment Abolishes the Sperm Binding Activity of gp69 and gp64
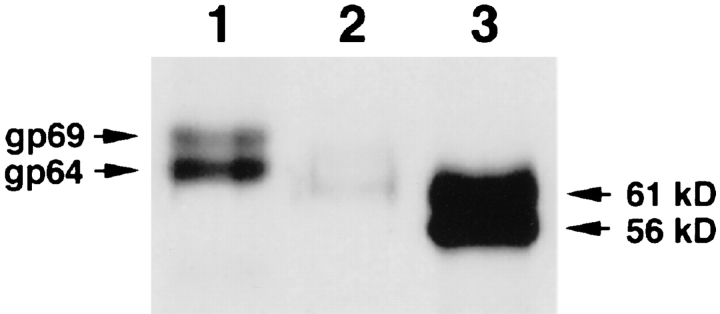
As mentioned in the Introduction, fertilization induces a proteolytic modification of gp69 and gp64, as well as ultrastructural changes in the envelope. After these changes the FE cannot be penetrated by the sperm (Grey et al., 1976). We found that these changes also resulted in a total loss of sperm binding to the fertilized eggs as determined by the sperm binding assay using dejellied fertilized eggs (data not shown). The correlation between the cleavage of gp69/64 at fertilization and the loss of sperm binding to FE prompted us to explore whether modifications of gp69/64 alone could abolish sperm-egg binding to the VE, without the structural changes of the envelope that are known to occur at fertilization. We found that crude type I collagenase, which is commonly used in isolating oocytes from the ovary (Smith et al., 1991), could selectively cleave gp69 and gp64. We observed that when the dejellied oviposited eggs were treated with crude collagenase, the molecular mass of gp69 and gp64 decreased to ∼65 kD and 60 kD, respectively, as determined by SDS-PAGE of the envelopes isolated from the enzyme-treated eggs (Fig. 6 A). None of the other VE proteins were affected by this treatment. We confirmed that the newly formed 65-kD and 60kD bands were derived from gp69 and gp64, respectively, by Western blot analysis using the pre-adsorbed anti-gp69/ 64 antibodies (Fig. 8, lane 2). No morphological changes in the egg or in the VE could be detected by microscopy after crude collagenase treatment, and the treated eggs could still be activated by 5 μM ionophore A23187. However, when we compared the sperm binding level of crude collagenase-treated eggs with that of control eggs not treated with enzyme, we found that sperm binding was almost totally abolished by this treatment (Fig. 6 B). In contrast to the crude type-I collagenase, pure type VII collagenase had no effect on sperm-egg binding and did not process gp69/64 or any other VE proteins, suggesting that a contaminating enzyme in the crude type-I collagenase may be responsible for this processing activity.
Figure 6.
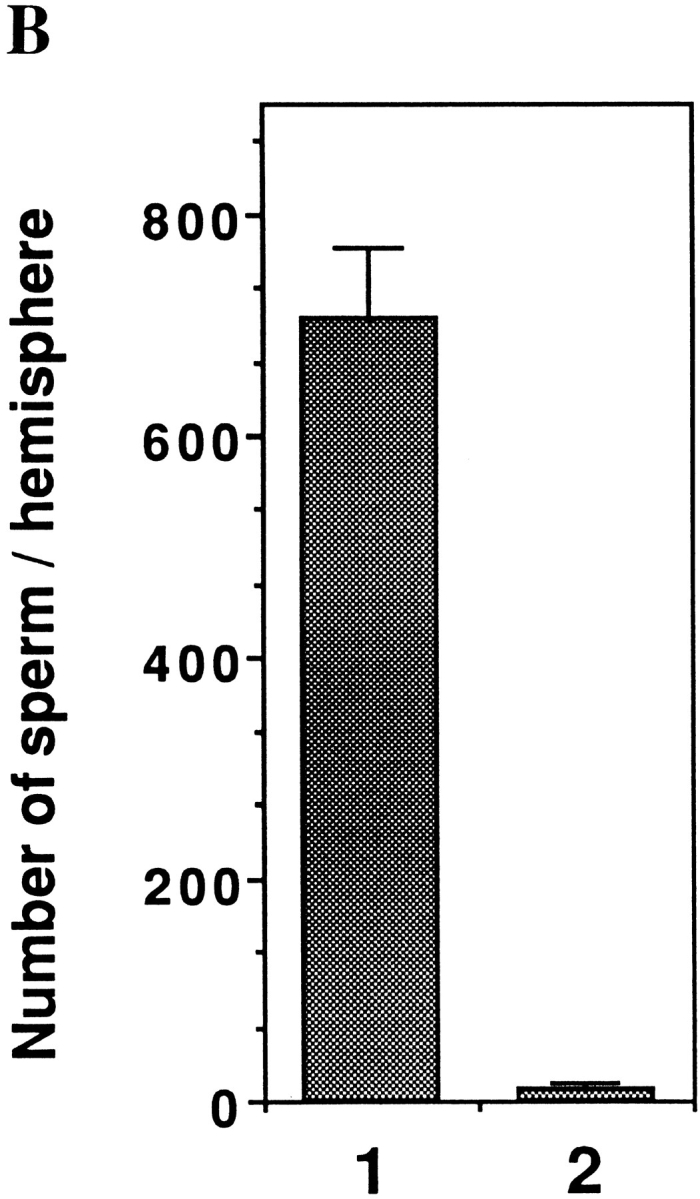
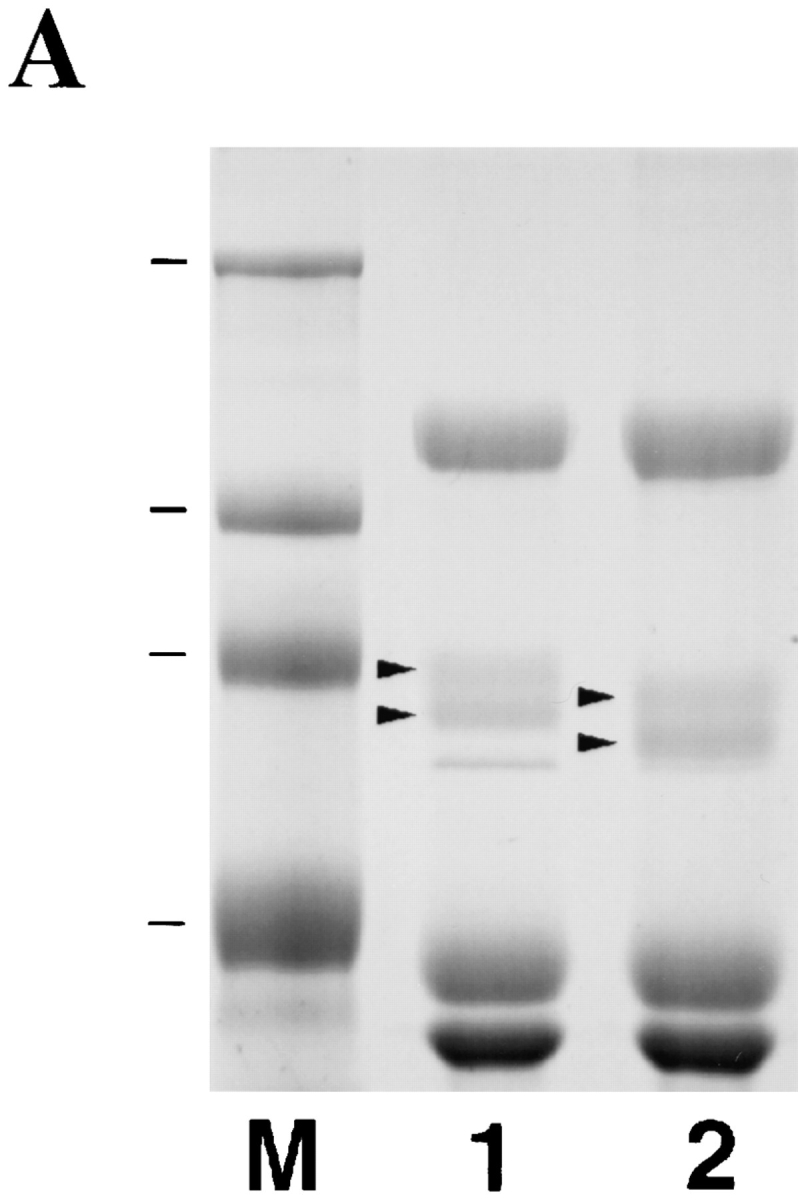
Effect of crude type I collagenase on the VE proteins and on sperm-egg binding. (A) SDS-PAGE analysis of total VE proteins isolated from a control group of eggs (lane 1) or from eggs treated with crude type–I collagenase (lane 2). The gel was stained with Coomassie blue. Molecular weight standards are marked and shown in lane M. From top: 216, 97, 71, and 44 kD. Arrows in lane 1 indicate gp69 and gp64, respectively. Arrows in lane 2 indicate the two bands at ∼65 and 60 kD which are derived from gp69 and gp64, respectively. (B) Sperm binding levels to control eggs (lane 1) or type I collagenase–treated eggs (lane 2). Bars represent SD with n = 15 eggs.
Figure 8.
Effect of PNGase F treatment on gp69/64. Purified gp69 and gp64 (total 1 μg , lane 1) and PNGase F–treated gp69/64 (total 10 μg, lane 3) were separated by 7.5% SDS-PAGE, electrotransferred to membrane, and immunoblotted with specific anti-gp69/64 antibody. The band in lane 3 was much more intense because 10 times as much protein was loaded. The molecular masses of the two bands are marked on the right. Lane 2 shows the 65-kD and 60-kD bands (total 1 μg) generated from crude collagenase digestion of gp69/64.
The cleavage of gp69/64 by crude collagenase was found to be proteolytic in nature. We determined the NH2-terminal peptide sequences of the separated 65-kD and 60-kD bands generated from the crude collagenase treatment of gp69 and gp64 and found that they were identical to each other, but different from the NH2-terminal sequences of gp69 and gp64 (see below). An obvious explanation was that the NH2-terminal region of the two glycoproteins was cleaved off by an unidentified protease. Whether the removed NH2-terminal region included any attached oligosaccharides is currently under investigation. Although no other modification on gp69/64 has been detected, the possibility of other structural modifications, such as limited deglycosylation by contaminating glycosidases has not been excluded yet. However, because we found that treatments that can selectively remove N- or O-linked oligosaccharide chains further reduced the molecular mass of the 65-kD and the 60-kD bands (data not shown), it is clear that the crude collagenase treatment did not totally remove the carbohydrate chains from the two truncated glycopeptides.
Involvement of Carbohydrates on gp69/64 in Sperm Binding
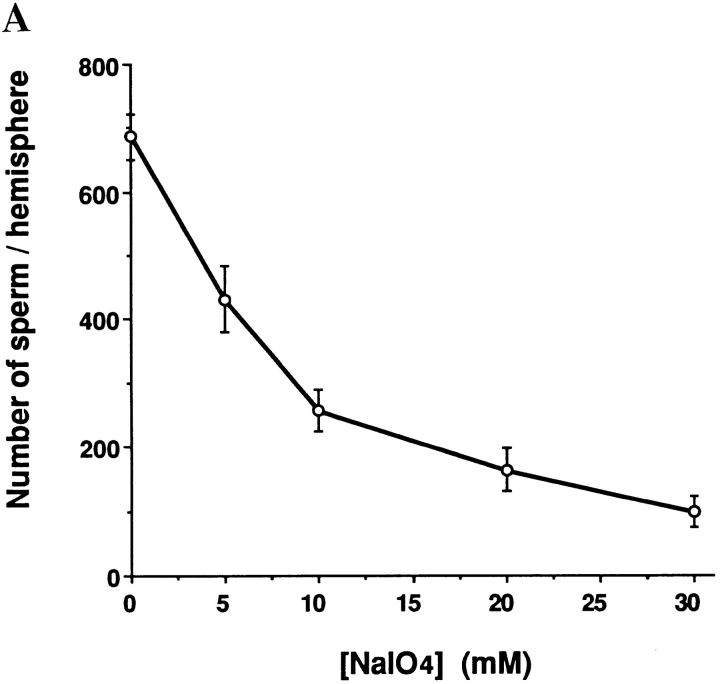
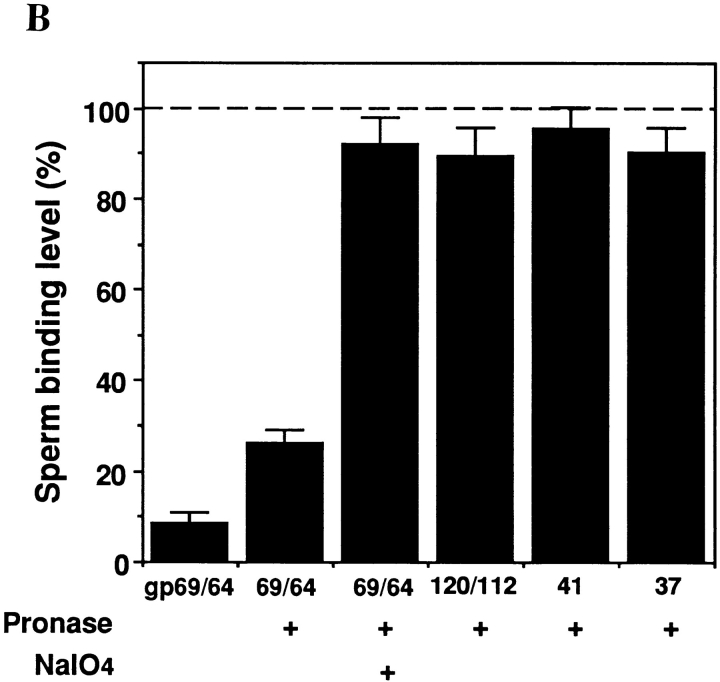
To investigate the role of carbohydrates in mediating Xenopus sperm-egg binding, we first asked whether modification of the sugar structures on the VE surface of live eggs would affect sperm binding. The surface of dejellied eggs were treated with various concentrations of sodium metaperiodate, which selectively cleaves the C-C bonds between vicinal hydroxyl groups of sugars (Bobbit, 1956). Using a concentration and time range for this treatment in which the eggs did not become morphologically altered, i.e., conditions under which the treated eggs could still be activated by 5 μM ionophore A23187, it was found that sperm binding to the treated eggs decreased in a manner dependent on metaperiodate concentration (Fig. 7 A). To determine specifically which purified VE proteins contain periodate sensitive oligosaccharide chains involved in sperm binding, purified gp120/112, gp69/64, gp41, and gp37 were digested extensively with Pronase to generate mixtures of glycopeptides containing a minimal amount of peptide. The digestion was monitored and proved to be complete according to silver staining and Western blot results, since no intact protein or high MW glycopeptides could be detected (data not shown). When these glycopeptides were incubated in the sperm-egg binding assay, only those generated from gp69/64 showed inhibitory activity for sperm binding, although they were slightly less inhibitory than an equivalent amount of intact gp69/64 (Fig. 7 B). Metaperiodate treatment of these small glycopeptides from gp69/64 abolished the inhibitory activity on sperm binding, indicating that the activity resided in the oligosaccharide, not in the peptide moiety of these glycopeptides. Glycopeptides generated from Pronase digestion of the other purified VE proteins did not yield glycopeptides with significant inhibitory activity on sperm binding.
Figure 7.
Possible role of carbohydrates on sperm binding. (A) Effect of metaperiodate treatment on sperm binding to live eggs. (B) Effects of Pronase-digested VE glycopeptides on sperm-egg binding. A total of 5 μg of gp69/64 (1:1) and proportional amounts of other VE proteins were used according to the ratio indicated in Fig. 3. The sperm binding level in the presence of an equal amount of heat-inactivated Pronase was used as a control and designated as 100%. Bars represent SD with n = 15 eggs.
Molecular Relationship Between gp69 and gp64
The available evidence suggests that gp69 and gp64 may be structurally related. They may share the same polypeptide chain but differ in glycosylation (see Discussion). To further investigate the molecular nature of the relationship between the two glycoproteins, we tested the idea that they share the same polypeptide by comparing portions of their amino acid sequence. The NH2-termini of purified gp69 and gp64 were sequenced separately and the result indicated that both proteins had identical NH2-terminal sequences, which were Asp-Glu-Pro-Gly-Ser-. . . . Crude collagenase treatment cleaved off the NH2-termini from both polypeptides and exposed new NH2-termini. The peptide sequences of the newly exposed NH2-termini were also found to be identical: Val-Ala-Ala-Pro-Ser-. . . . The MW of gp69 and gp64 both shifted by ∼4 kD, suggesting that both glycoproteins were similarly processed at the same site by the crude collagenase treatment. To determine the difference in glycosylation between gp69 and gp64, the two glycoproteins were digested with PNGase-F. After this treatment, the MW of gp69 and gp64 both shifted by ∼8 kD to ∼61 and 56 kD, respectively, as detected by Western blot (Fig. 8). Earlier it had been reported that after complete deglycosylation with trifluoromethane sulfonic acid both proteins shifted to a single band of ∼54 kD (Lindsay and Hedrick, 1989). Together the results of the deglycosylation experiments suggested that the MW difference between gp69 and gp64 may be due to the difference in the level of O-glycosylation.
Discussion
Using the Xenopus gamete binding assay that we developed, we have demonstrated the existence of a gamete adhesion step at the level of the egg vitelline envelope. This gamete adhesion step is required for fertilization because if it is blocked (e.g., by antibody), fertilization does not occur. To our knowledge, this is the first quantitative study of X. laevis sperm-egg interaction at the level of the vitelline envelope. We calculate that on the average, a dejellied, unfertilized egg (1.2–1.3 mm in diameter) can bind up to 1,500 ± 200 sperm in the presence of jelly extract and a high concentration of sperm (⩾1.0 × 107 sperm/ml). At this level of binding, the sperm binding sites on the VE surface apparently have been saturated, because further increasing the sperm concentration does not increase the total number of bound sperm, although there is still space on the surface of the VE for more sperm to bind. Several initial observations suggested that this binding process was mediated by receptor-ligand like interactions between the sperm and one or more proteins of the VE. First, the solubilized total VE proteins competitively inhibited sperm binding. Second, the sperm binding was blocked by antibodies directed against VE proteins. Third, the ability of the egg to bind sperm was found to be abolished by treatment of the eggs with crude type I collagenase.
Among the seven VE proteins, only a pair of related glycoproteins, gp69 and gp64, meet a number of criteria for being functional in sperm binding: (a) Purified gp69 or gp64 competitively inhibited sperm-egg binding in a dosedependent manner, while other VE proteins did not. Similar competition assays have been used successfully in identifying the sea urchin egg receptor for sperm (Schmell et al., 1977; Foltz and Lennarz, 1990), and the ZP3 as a mouse sperm-binding protein (Bleil and Wassarman, 1980). (b) Polyclonal antibodies that specifically react with gp69 and gp64 blocked sperm binding to dejellied eggs and also blocked fertilization. This finding also suggests that sperm binding to the VE is a necessary step leading to sperm penetration and fertilization. (c) Coupling of gp69/64 to beads directly showed that these glycoproteins bound sperm. Beads coated with other isolated VE proteins showed little or no sperm binding activity. Furthermore, sperm binding to gp69/64 coated beads could be blocked by polyclonal antibodies directed against gp69/64. (d) Crude collagenase treatment of oviposited eggs selectively cleaved gp69/64, and resulted in greatly decreased sperm binding to treated eggs. (e) gp69 and gp64 were found to be exposed on the outer surface of the VE, because the VE surface was stained by the anti-gp69/64 antibody. Based on the above evidence, we conclude that gp69 and gp64 are the components in the vitelline envelope that are recognized and bound by sperm.
The role of other VE proteins at fertilization, especially in mediating sperm-egg binding is still unclear. In our gamete binding competition assays and sperm-bead binding assay, the validity of the results depend on retention of biological activity after solubilization and purification of the individual VE proteins. The sperm binding activity of gp69/64 that we demonstrated proves that under the conditions used we can successfully recover biological activity in the isolated proteins. Moreover, similar conditions have been used successfully by other investigators to recover biologically active-binding proteins after SDS-PAGE purification (see Bleil and Wassarman, 1980; Foltz et al., 1990). However, the possibility that the sperm-binding activity of some of the other VE protein(s) was irreversibly lost during purification can not yet be excluded.
Several lines of evidence support the hypothesis that gp69 and gp64 share identical polypeptide sequences and only differ in glycosylation. Earlier studies using two-dimensional gel electrophoresis of VE and FE components showed parallel changes in the molecular weights and isoelectric points of gp69/64 in the VE and the corresponding gp66/61 in the FE. They were heterogeneous in the IP direction, and shifted to a more basic direction in the FE. Peptide maps of these two glycoproteins generated by S. aureus V-8 protease showed only one band difference, which corresponded to the MW difference between gp69 and gp64 (Gerton and Hedrick, 1986b ). Chemical deglycosylation that removes both N-linked and O-linked oligosaccharides shifted the 69-kD and 64-kD bands to a single band of ∼54 kD on SDS-PAGE (Lindsay and Hedrick, 1989). Peptide sequencing results from the current study added a most definitive piece of evidence, because it showed that both proteins have the same NH2 terminus, as well as the same internal amino acid sequence that becomes the new NH2 terminus upon crude collagenase treatment. PNGase F treatment decreased the mass of both glycoproteins by the same amount (∼8 kD). Since, as mentioned earlier, complete deglycosylation converted both to the same size (Lindsay and Hedrick, 1989), we conclude that gp69 and gp64 mainly differ in the level of O-glycosylation, not N-glycosylation. Functionally, no difference in the biological activity of the two glycoproteins has been detected. Both seem to be involved in sperm binding. Why two structurally different but functionally identical glycoforms of the same gene product are synthesized is not clear yet.
Of the known sperm receptors or binding proteins, it has been shown by in vitro assays that in most cases the carbohydrate moiety plays a critical role in sperm-egg adhesion; the role of the polypeptide chain is not well understood (De Santis and Pinto, 1987; Litscher and Honegger, 1991; Wassarman and Litscher, 1995; Focarelli and Rosati, 1995). Only in the sea urchin sperm receptor has it been shown that both the oligosaccharide (Dhume and Lennarz, 1995) and the polypeptide chain (Foltz et al., 1993) are directly involved in the binding process and, several peptide binding domains have been mapped (Stears, R., and W.J. Lennarz, manuscript in preparation). The Xenopus sperm binding proteins, gp69/64, offer another example in which the oligosaccharide chains seem to be directly involved in the binding function of the protein. When the oligosaccharide chains on the surface of live eggs were treated with periodate, the level of sperm binding was significantly reduced, indicating that carbohydrates on the VE surface are important structural bases for sperm binding. Specifically, the oligosaccharides on gp69 and gp64 were found to be involved in sperm binding, because when the two inhibitory glycoproteins were digested with Pronase, the resulting small glycopeptides still exhibited inhibitory effects on sperm binding, albeit not as effectively as the intact glycoproteins. Moreover, periodate treatment of these glycopeptides destroyed their inhibitory activity. The glycopeptides from Pronase digestion of the other purified VE proteins, gp120/112, gp41, and gp37, did not inhibit sperm binding. The role of the polypeptide chain of gp69/64 in sperm binding is still not clear. One piece of in vivo evidence suggesting that the polypeptide might be involved in sperm binding is that when the NH2-terminal region of gp69/64 is removed by crude collagenase, the eggs no longer bind sperm. This observation suggests that the polypeptide backbone could play a structural role, even if it may not be directly involved in sperm binding. Perhaps the polypeptide facilitates presentation of the functionally important oligosaccharide chains at the surface of the VE. Molecular cloning of its cDNA and detailed compositional and structural analysis of the carbohydrates are underway. This should aid in determining the relative roles of the carbohydrate and polypeptide moieties in sperm-egg interaction in the frog.
This study has also shed new light on the distribution of the sperm binding sites and the sperm binding proteins in the VE. Using the gamete binding assay, we found that sperm binding occurred virtually uniformly on both hemispheres. This initial observation suggested that the sperm binding sites were evenly distributed on both the animal and vegetal hemispheres. This hypothesis was supported by an antibody staining experiment, in which we found that the polyclonal antibodies that specifically recognize the two sperm binding proteins, gp69 and gp64, bind evenly over the entire egg surface. In Rana pipiens, it has been demonstrated that the sperm can penetrate the vitelline envelope from both hemispheres, but that the sperm entry into the egg cytoplasm is restricted only to the animal hemisphere (Elinson, 1975). This restriction of sperm entry to the animal hemisphere has been generally assumed to be true in X. laevis, but has never been experimentally tested (Grey et al., 1982). The two observations discussed above indicate that both hemispheres of the X. laevis vitelline envelope are equally receptive to sperm. Therefore, if there is a regional restriction of sperm entry to the egg in X. laevis, it is most likely to occur at a step beyond that involving sperm binding the gp69/64 glycoproteins in the VE. This step could be key in gamete fusion and egg activation.
Acknowledgments
We thank all the members of the Lennarz laboratory for critical discussions and Lorraine Conroy for preparation of this manuscript.
This work was supported in part by a Predoctoral Fellowship Award to J.-D. Tian from the Institute for Cell and Developmental Biology and by National Institutes of Health grants HD18590 and GM33184 to W.J. Lennarz.
Abbreviations used in this paper
- FE
fertilization envelope
- MR
modified Ringer solution
- VE
vitelline envelope
Footnotes
Please address all correspondence to W.J. Lennarz, Department of Biochemistry and Cell Biology, SUNY at Stony Brook, Stony Brook, NY 11794-5215. Tel.: (516) 632-8560. Fax: (516) 632-8575. E-mail: wlennarz@life.bio.sunysb.edu
References
- Baker CS, Dunn MJ. Preparation of proteins from gels for protein microsequencing. Methods Mol Biol. 1994;132:177–184. doi: 10.1385/0-89603-268-X:177. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zona pellucida possessing receptor activity for sperm. Cell. 1980;20:873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J Cell Biol. 1986;102:1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbit JM. Periodate oxidation of carbohydrates. Adv Carbohydr Chem. 1956;11:1–14. doi: 10.1016/s0096-5332(08)60115-0. [DOI] [PubMed] [Google Scholar]
- De Santis R, Pinto MR. Isolation and partial characterization of a glycoprotein complex with sperm-receptor activity from Ciona intestinalisovary. Dev Growth Differ. 1987;29:617–625. doi: 10.1111/j.1440-169X.1987.00617.x. [DOI] [PubMed] [Google Scholar]
- Dhume ST, Lennarz WJ. The involvement of O-linked oligosaccharide chains of the sea urchin egg receptor for sperm in fertilization. Glycobiology. 1995;5:11–17. doi: 10.1093/glycob/5.1.11. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis(Daudin) I. Stages of oocyte development in laboratory maintained animals. J Morph. 1972;136:153–180. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Elinson RP. Fertilization in amphibians: the ancestry of the block to polyspermy. Int Rev Cytol. 1986;101:59–100. doi: 10.1016/s0074-7696(08)60246-6. [DOI] [PubMed] [Google Scholar]
- Focarelli R, Rosati F. The 220-kDa vitelline coat glycoprotein mediates sperm binding in the polarized egg of Unio elongatulus through O-linked oligosaccharides. Dev Biol. 1995;171:606–614. doi: 10.1006/dbio.1995.1308. [DOI] [PubMed] [Google Scholar]
- Foltz KR, Lennarz W. Purification and characterization of an extracellular fragment of the sea urchin egg receptor for sperm. J Cell Biol. 1990;111:2951–2959. doi: 10.1083/jcb.111.6.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz KR, Partin JS, Lennarz WJ. Sea urchin egg receptor for sperm: sequence similarity of binding domain and hsp70. Science (Wash DC) 1993;259:1421–1425. doi: 10.1126/science.8383878. [DOI] [PubMed] [Google Scholar]
- Gerton GL, Hedrick JL. The coelomic envelope to vitelline envelope conversion in eggs of Xenopus laevis. . J Cell Biochem. 1986a;30:341–350. doi: 10.1002/jcb.240300407. [DOI] [PubMed] [Google Scholar]
- Gerton GL, Hedrick JL. The vitelline envelope to fertilization envelope conversion in eggs of Xenopus laevis. . Dev Biol. 1986b;11:1–7. doi: 10.1016/0012-1606(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Grey RD, Bastiani MJ, Webb DJ, Schertel ER. An electrical block is required to prevent polyspermy in eggs fertilized by natural mating of Xenopus laevis. . Dev Biol. 1982;89:475–484. doi: 10.1016/0012-1606(82)90335-9. [DOI] [PubMed] [Google Scholar]
- Grey RD, Working PK, Hedrick JL. Evidence that the fertilization envelope blocks sperm entry in eggs of Xenopus laevis: interaction of sperm with isolated envelopes. Dev Biol. 1976;54:52–60. doi: 10.1016/0012-1606(76)90285-2. [DOI] [PubMed] [Google Scholar]
- Harlow, H., and D. Lane. 1988. Antibodies. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. pp. 726.
- Heasman J, Holwill S, Wylie CC. Fertilization of cultured Xenopusoocytes and use in studies of maternally inherited molecules. Methods Cell Biol. 1991;36:214–228. doi: 10.1016/s0091-679x(08)60279-4. [DOI] [PubMed] [Google Scholar]
- Hedrick JL, Nishihara T. Structure and function of the extracellular matrix of anuran eggs. J Electron Microsc Tech. 1991;17:319–335. doi: 10.1002/jemt.1060170306. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larabell CA, Chandler DE. The extracellular matrix of Xenopus laeviseggs: a quick-freeze, deep-etch analysis of its modification at fertilization. J Cell Biol. 1988;107:731–741. doi: 10.1083/jcb.107.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabell CA, Chandler DE. Fertilization-induced changes in the vitelline envelope of echinoderm and amphibian eggs: self-assembly of an extracellular matrix. J Electron Microsc Technique. 1991;17:294–318. doi: 10.1002/jemt.1060170305. [DOI] [PubMed] [Google Scholar]
- Lindsay LL, Hedrick JL. Proteases released from Xenopus laeviseggs at activation and their role in envelope conversion. Dev Biol. 1989;130:37–44. doi: 10.1016/0012-1606(89)90170-x. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Honegger TG. Glycoprotein constituents of the vitelline coat of Phallusia mammillata(Ascidiacea) with fertilization inhibiting activity. Dev Biol. 1991;148:536–551. doi: 10.1016/0012-1606(91)90272-5. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K, Dhume ST, Partin JS, Lennarz WJ. The sea urchin egg receptor for sperm: isolation and characterization of the intact, biologically active receptor. J Cell Biol. 1993;122:887–895. doi: 10.1083/jcb.122.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Stanley HP. Fine structure of spermatogenesis in the South African clawed toad Xenopus laevisDaudin. J Ultrastruct Res. 1972;41:277–295. doi: 10.1016/s0022-5320(72)90070-6. [DOI] [PubMed] [Google Scholar]
- Schmell, E.D., B.J. Gulyas, and J.L. Hedrick. 1983. Egg surface changes during fertilization and the molecular mechanism of the block to polyspermy. In Mechanism and Control of Animal Fertilization. J.F. Hartmann, editor. Academic Press, New York, pp. 365–413.
- Schmell E, Earles BJ, Breaux C, Lennarz WJ. Identification of a sperm receptor on the surface of the eggs of the sea urchin Arbacia punctulata. . J Cell Biol. 1977;72:35–46. doi: 10.1083/jcb.72.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Tsuji M, Dean J. In vitrobiosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983;258:5858–5863. [PubMed] [Google Scholar]
- Smith LD, Xu W, Varnold RL. Oogenesis and oocyte isolation. Methods Cell Biol. 1991;36:45–58. doi: 10.1016/s0091-679x(08)60272-1. [DOI] [PubMed] [Google Scholar]
- Snell WJ, White JM. The molecules of mammalian fertilization. Cell. 1996;85:629–637. doi: 10.1016/s0092-8674(00)81230-1. [DOI] [PubMed] [Google Scholar]
- Stewart-Savage J, Grey RD. Fertilization of investment-free Xenopus eggs. Exp Cell Res. 1984;154:639–642. doi: 10.1016/0014-4827(84)90191-5. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Profile of a mammalian sperm receptor. Development. 1990;108:1–17. doi: 10.1242/dev.108.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Litscher E. Sperm-egg recognition mechanisms in mammals. Curr Top Dev Biol. 1995;30:1–19. doi: 10.1016/s0070-2153(08)60562-1. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Hedrick JL. A molecular approach to fertilization. II. Viability and artificial fertilization of Xenopus laevisgametes. Dev Biol. 1971;25:348–359. doi: 10.1016/0012-1606(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Nishihara T, West DM, Wyrick RE, Hedrick JL. Isolation, physicochemical properties, and the macromolecular composition of the vitelline and fertilization envelopes from Xenopus laeviseggs. Biochemistry. 1976;15:3671–3678. doi: 10.1021/bi00662a005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hedrick JL, Katagiri C. The synthesis and localization of envelope glycoproteins in oocytes of Xenopus laevisusing immunocytochemical methods. Dev Growth & Differ. 1989;31:85–94. doi: 10.1111/j.1440-169X.1989.00085.x. [DOI] [PubMed] [Google Scholar]