Abstract
Chicken acidic leucine-rich EGF-like domain containing brain protein (CALEB) was identified by combining binding assays with immunological screens in the chicken nervous system as a novel member of the EGF family of differentiation factors. cDNA cloning indicates that CALEB is a multidomain protein that consists of an NH2-terminal glycosylation region, a leucine-proline–rich segment, an acidic box, a single EGF-like domain, a transmembrane, and a short cytoplasmic stretch. In the developing nervous system, CALEB is associated with glial and neuronal surfaces. CALEB is composed of a 140/130-kD doublet, an 80-kD band, and a chondroitinsulfate-containing 200-kD component. The latter two components are expressed in the embryonic nervous system and are downregulated in the adult nervous system. CALEB binds to the extracellular matrix glycoproteins tenascin-C and -R. In vitro antibody perturbation experiments reveal a participation of CALEB in neurite formation in a permissive environment.
The differentiation of the nervous system is the result of a complex series of cell communications mediated by molecules shown to participate in different aspects of development such as mitogenic signaling, differentiation of precursor cells into neurons and glial cells, outgrowth and pathfinding of axons, and the establishment of synaptic connections. One form of cell communication involves the release of molecules termed trophic or tropic factors. One class of proteins that are released and display mitogenic and differentiation-inducing properties in the nervous system is the neuregulins (Ben-Baruch and Yarden, 1994; Carraway and Burden, 1995). They belong to a family of membrane-bound growth and differentiation factors that are characterized by an EGF-like domain with a specific cysteine spacing and other invariant amino acids in specific positions. Two well-known members of this protein family are EGF and TGF-α. The neuregulins bind to and activate the receptor tyrosine kinase ErbB3/4 by inducing tyrosine phosphorylation (Carraway and Cantley, 1994), for which the EGF-like domain appears to be necessary and sufficient. Alternative pre-mRNA splicing generates a dozen of related proteins that are expressed in a variety of mesenchymal and neuronal tissues (Meyer and Birchmeier, 1994), and some isoforms of neuregulin contain an Ig-like domain (Peles and Yarden, 1993). Although the specific function of this Ig-like domain is currently unknown, gene targeting experiments have shown it to be essential for developmental processes (Kramer et al., 1996), and studies with mutant forms reveal that it might be required to allow cleavage products of the neuregulins to interact with the extracellular matrix (Loeb and Fischbach, 1995). In general, Ig-modules are thought to mediate protein–protein interactions (Brümmendorf and Rathjen, 1995).
Another family of proteins composed of Ig-like and, in several cases, fibronectin type III–like domains is made up of the axonal members of the immunoglobulin superfamily (IgSF)1 that participate in contact-dependent communications between neural cells during development. These axon-associated IgSF members are implicated in different aspects of neurohistogenesis, e.g., in radial and tangential migration of neuronal precursor cells, in neurite fasciculation, in contact-dependent axonal guidance, as well as in contact-dependent inhibition of axonal growth (Brümmendorf and Rathjen, 1995; Cunningham, 1995). Most of these axon-associated Ig-like glycoproteins are typical multidomain proteins consisting of a number of different and, in most cases, repeated structural and functional units. An important feature of these proteins is their binding to several distinct proteins (Brümmendorf and Rathjen, 1996). For example, the F11 protein is one such multifunctional protein that interacts with at least two IgSF members of the L1 subgroup (NgCAM-related cell adhesion molecule [NrCAM] and neuron–glia cell adhesion molecule [NgCAM]), with two extracellular matrix glycoproteins (tenascin-R [TN-R] and tenascin-C [TN-C]), and with the receptor tyrosine phophatase β/ζ (Rathjen et al., 1991; Zisch et al., 1992; Brümmendorf et al., 1993; Morales et al., 1993; Pesheva et al., 1993; Peles et al., 1995; Brümmendorf and Rathjen, 1996). In particular, the axon-associated extracellular matrix (ECM) glycoproteins of the developing nervous system consist of a plethora of different structural domains and undergo multiple interactions with other proteins. For example, the tenascin family members are composed of a cysteine-rich region, several EGF- and fibronectin type III–like modules, and a fibrinogen-like domain (Chiquet-Ehrismann et al., 1995; Nörenberg et al., 1995).
The multitude of binding activities and the multidomain structure of many of these axon-associated members of the IgSF and the ECM glycoproteins suggested to us that other interactions with so far uncharacterized components might occur during nervous system development. The relatively broad binding specificity of many axon-associated proteins might be used to identify novel cell surface or extracellular matrix proteins implicated in the differentiation of the nervous system. We have therefore combined the binding properties of these components with immunological screens to characterize proteins in the chick nervous system.
In this report we describe molecular, cellular, and functional properties of a novel protein of the developing chick nervous system that was identified by its interaction with the ECM glycoproteins TN-R and TN-C. This protein, termed chicken acidic leucine-rich EGF-like domain containing brain protein (CALEB), is a transmembrane glycoprotein that contains an EGF-like domain close to the plasma membrane–spanning domain. The EGF-like domain resembles those found in other proteins such as EGF itself, neuregulin, or TGF-α and might therefore function as a receptor recognition site. Histological investigations demonstrate that CALEB is restricted to the developing and adult nervous system and is associated with neuronal and glial surfaces. Binding studies support the interaction between CALEB and TN-R and between CALEB and TN-C. In vitro antibody perturbation experiments indicate that CALEB is implicated in neurite formation in a permissive environment provided by F11, NgCAM, laminin, or vitronectin.
Materials and Methods
Generation and Screening of mAbs to Binding Proteins
Immunizations and the generation of hybridomas secreting mAbs to axon-associated glycoprotein fractions by fusions of splenocytes with myeloma cell line P3X63-Ag8.653 were performed as described previously (Rathjen et al., 1987a ,b). Selected mAbs that do not recognize known axon-associated proteins were further tested for whether they recognize an antigen that binds to F11, neural cell adhesion molecule (NCAM), NgCAM, NrCAM, laminin, TN-C, or TN-R. For this purpose these proteins were immobilized on ELISA plates (200 ng per well in 150 μl PBS) followed by blocking of the plates with BSA (1% in PBS supplemented with 0.5% Tween 20). Plates were then incubated overnight at 4°C with detergent extracts (1% Triton X-100 in PBS added with protease inhibitors) of embryonic day (E) 16 retinas (200 μl per well at a protein concentration of 13.8 mg/ml) followed by four washing steps. ELISA plates were then incubated with preselected (see above) hybridoma supernatants (150 μl per well) or with purified mAbs at a concentration of 1 μg/ml for 2 h at room temperature. Binding of antibodies was visualized with peroxidase-conjugated secondary antibodies and the o-phenylenediamine reaction.
Antibodies and Purification of NrCAM, NgCAM, F11, Neurofascin, 3/11-Antigen, 12/36-Antigen, or CALEB
The purification of F11, NgCAM(G4), NrCAM(Bravo), neurofascin, and NCAM from detergent extracts (1% Triton X-100) of plasma membrane preparations of adult chicken brain and the specificity of the corresponding monoclonal and polyclonal antibodies and their Fab fragments have been detailed elsewhere (Rathjen et al., 1987a ,b; Wolff et al., 1987; Brümmendorf et al., 1989; de la Rosa et al., 1990; Morales et al., 1993). TN-R or -C was isolated from urea extracts of adult chicken brains by immunoaffinity chromatography as described using mAb 23-13 or mAb M1, respectively (Chiquet and Fambrough, 1984; Rathjen et al., 1991; Nörenberg et al., 1992). Hybridomas producing mAb M1 directed to TN-C were obtained from the Developmental Studies Hybridoma Bank (John Hopkins University School of Medicine, Baltimore, MD). CALEB was purified either from Triton X-100 extracts of embryonic or adult retinas by immunoaffinity chromatography using mAb 4/1 coupled to CNBr-activated Sepharose (Pharmacia LKB Biotechnology, Inc., Piscataway, NJ). After extensive washing with solubilization buffer, the affinity column was washed with 3 column volumes 1.2 M NaCl in PBS to elute copurifying TN-C. Bound CALEB was then eluted from the affinity column by 0.1 M diethylamine, pH 11.5, followed by neutralization. Polyclonal antibodies to CALEB were generated in rabbits by subcutaneous injection at multiple sites with 10–15 μg of immunoaffinity-purified CALEB obtained from adult retinas at 2-wk intervals. The 12/36- or the 3/11-antigen was isolated from detergent extracts of embryonic chicken brains by immunoaffinity chromatography using mAb 12/36 or 3/11, respectively. (Hybridomas secreting mAb 12/36 or 3/11 were generated using a screen as outlined for mAb 4/1. Each antibody recognizes an as-of-yet unknown axon-associated cell surface protein [our unpublished observations].) Polyclonal rabbit antibodies to the 3/11- or 12/36-antigen were obtained by using immunoaffinity-purified material and the same immunization protocol as described above for CALEB. Fab fragments were produced from the IgG fraction by mercuripapain digestion according to Porter (1959).
Protein Analytical Procedures
Concentrations of protein solutions were determined according to Peterson (1977). SDS-PAGE (Laemmli, 1970) was performed with 7% acrylamide under reducing conditions followed by silver staining (Ansorge, 1985). Western blots of CALEB components were analyzed using polyclonal antibodies or mAb 4/1 to CALEB followed by labeling with alkaline phosphatase–conjugated secondary antibodies and 5-bromo-4-chloro3-indolyl-phosphate/Nitro blue tetrazolium (BCIP/NBT) staining. To obtain internal CALEB amino acid sequences, peptides were generated from carboxamidomethylated CALEB 140- or 80-kD polypeptides by digestion with trypsin or Asp-N and separated by reverse-phase, high-pressure liquid chromatography using a trifluoroacetic-acetonitrile buffer system. Peptides were subjected to Edman degradation on a gas-phase sequenator. NH2-terminal sequences of the 140- or 80-kD CALEB components were obtained from bands blotted on a Problott membrane (Applied Biosystems, Inc., Foster City, CA) according to the instructions of the manufacturer.
Cloning, DNA Sequencing, and Transfection
An adult chicken eye cDNA library randomly primed and inserted into λgt11 phage vector (Clontech, Palo Alto, CA) was screened by mAb 4/1 by standard immunoscreening methods as described by Huynh et al. (1985). cDNA clone D1, which expressed insert-encoded polypeptide reactive with mAb 4/1 to CALEB, was isolated and further analyzed (Ausubel et al., 1994; Sambrook et al., 1989). DNA probes derived from clone D1 were used to isolate additional CALEB-encoding cDNA clones by hybridization using the DIG-technology (Boehringer Mannheim Corp., Indianapolis, IN). Six independent overlapping cDNA clones (D1–D6) were isolated. Insert of clone D1 encodes amino acids (aa) 102–440, of D2 aa 302–551, of D3 aa 1–309, of D4 aa 104–344, of D5 aa 102–387, and of D6 aa 412–551. cDNA inserts from purified phages were subcloned into the plasmid Bluescript KS(+) (Stratagene, La Jolla, CA). Nucleotide sequences were determined on both strands by the dideoxy chain termination method of Sanger et al. (1977) and an automatic fluorescent laser sequencer-DNA sequencer (Pharmacia LKB Biotechnology Inc.). Using standard cloning procedures, the insert of cDNA clone D1 was combined with inserts of cDNA clones D2 and D3 to generate a continuous CALEB open reading frame, which was then cloned into the eukaryotic expression vector pSG5 (Stratagene). Transient transfection and analysis of expression by indirect immunofluorescence using mAb 4/1 were performed with slight modifications as described elsewhere (Brümmendorf et al., 1993). Nucleotide and amino acid sequences were analyzed using the HUSAR program package (German Cancer Research Center, Heidelberg, Germany). Sequence alignments were performed using the programs clustal and bestfit of HUSAR.
Biosynthetic Labeling and Enzymatic Digestions
Biosynthetic labeling of retinal cells in vitro with 200 μCi/ml [35S]sulfate (DuPont/New England Nuclear, Wilmington, DE) was performed in culture medium in which MgSO4 was replaced by MgCl2 (Gibco Laboratories, Grand Island, NY) during the labeling period of 24 h. CALEB immunoaffinity isolate from labeled cells was digested with neuraminidase (Boehringer Mannheim), chondroitinase ABC (Miles, Inc., Kankakee, IL), heparinase (Sigma Chemical Co., St. Louis, MO), or heparitinase (Sigma Chemical Co.) according to the instructions of the manufacturer in the presence of protease inhibitors at various time intervals at 37°C. Samples incubated in parallel without enzyme served as control for proteolytic contaminants. Digests were stopped by addition of SDS-PAGE sample buffer, subjected to SDS-PAGE (7%), and visualized by fluorography (25-diphenyloxazol [PPO] in DMSO).
Flow Cytometric Analysis
Immunoaffinity-purified proteins were coupled to red or green fluorescing microspheres with a diameter of 0.5 μm (Bioclean or Covaspheres; Duke Scientific Corp., Palo Alto, CA) and incubated for binding as detailed elsewhere (Kuhn et al., 1991; Brümmendorf et al., 1993; Morales et al., 1993). In blocking experiments, protein-coated beads were preincubated with Fab fragments of polyclonal antibodies to TN-C or -R. Analysis of aggregated beads by flow cytometry using FACSCalibur® and the Cellquest software package (Becton-Dickinson Immunocytometry Sys., Mountain View, CA) for data acquisition has been outlined previously (Brümmendorf et al., 1993).
Immunohistological Studies and In Vitro Bioassays
For the immunohistological localization of CALEB, TN-C, or TN-R, formaldehyde-fixed cryostat sections of embryonic tissue were incubated with primary and Cy3-conjugated secondary antibodies (Dianova, Hamburg, Germany) as described elsewhere (Rathjen et al., 1987b ). Neurite extension on immobilized F11, NgCAM, laminin-1 (GIBCO BRL, Gaithersburg, MD), or vitronectin (Sigma Chemical Co.) in the presence of Fab fragments of polyclonal antibodies or monoclonal antibodies to CALEB was measured as detailed elsewhere (Chang et al., 1987; Brümmendorf et al., 1993; Morales et al., 1993). To demonstrate specificity of the effects observed with polyclonal antibodies to CALEB, 300-μl Fab fragments of rabbit No. 1 at a concentration of 5 mg/ml were incubated with CALEB. For this purpose, 8 μg of immunoaffinity-purified CALEB was immobilized on nitrocellulose followed by blocking of residual binding sites on the nitrocellulose by FCS. Fab fragments were incubated with immobilized CALEB at 4°C overnight. Fab fragments were then applied to tectal cell cultures at a concentration as indicated in Fig. 6. Alternatively, tectal cells were grown in the presence of Fab fragments of rabbit No. 1 at a concentration of 800 μg/ml supplemented with 10 μg/ml of purified CALEB. Fab fragments of polyclonal antibodies to the 3/11- or 12/36-antigen were used at 1.6 mg/ml in tectal cultures.
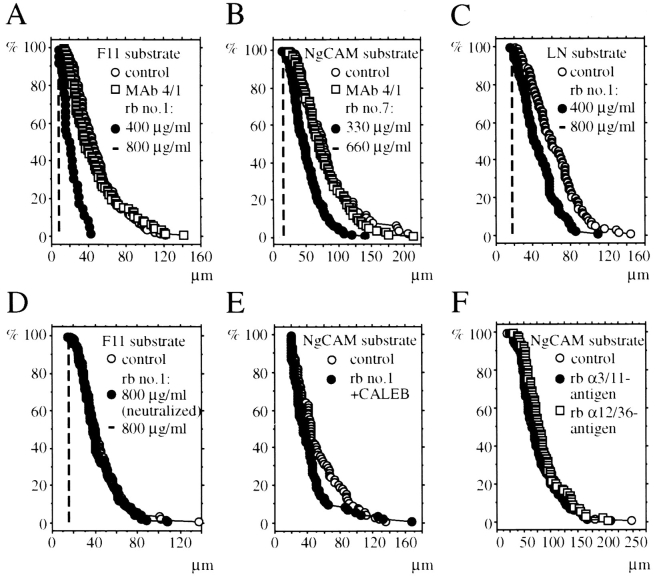
Figure 6.
Distribution of neurite lengths of tectal cells in the presence and absence of antibodies to CALEB. Culture dishes were coated with immunoaffinity purified F11 (A and D), NgCAM (B, E, and F), or laminin-1 (C) and blocked with culture medium containing FCS. Dissociated tectal cells were cultivated on NgCAM or laminin-1 (LN) for 22 h and on F11 for 30 h in culture medium containing FCS. Control cultures were grown without antibodies and test cultures in the presence of Fab fragments of antiCALEB antibodies from four different rabbits (Nos. 1, 4, 6, or 7) using different concentrations as indicated in each panel (300–1,000 μg/ ml). Purified mAb 4/1 was used at 10 μg/ml. In D, tectal cultures were grown on F11 without antibodies, in the presence of Fab fragments of antibodies to CALEB, or in the presence of Fab fragments preincubated with immunoaffinity-purified CALEB immobilized on nitrocellulose (neutralized; for details see Materials and Methods). In E, tectal cells were grown on NgCAM in the absence of Fab fragments or in the presence of both Fab fragments (800 μg/ml) and immunopurified CALEB (10 μg/ml). Cultures were fixed, stained, and analyzed using an inverted microscope and an image analysis system. The percentage of neurons (vertical axis) with neurites longer than 20 μm (horizontal axis) is plotted as introduced by Chang et al. (1987). For each experimental condition, 90–100 neurites were measured. The broken lines in A, B, C, and D indicate that there are no neurons with neurites at the indicated Fab concentration. (The length distributions have been analyzed using the Mann-Whitney U-test and relevant p-values are given: in B, control versus Fab at 330 μg/ml P < 0.0001; in C, control versus Fab at 400 μg/ml P < 0.0001; in E, control versus Fab plus CALEB P < 0.01.)
Results
Screening for Novel Binding Components of Axon-associated Proteins
To identify novel binding proteins, known axon-associated proteins (F11, NgCAM, NrCAM, or NCAM) or ECM glycoproteins (TN-C, TN-R, or laminin-1) were immobilized on ELISA plates (Fig. 1 A). After blocking and washing, ELISA plates were incubated with detergent extracts of embryonic retina tissue to allow putative binding proteins to interact with the immobilized proteins. Plates were then incubated with prescreened supernatants of hybridomas generated against embryonic neural glycoprotein fractions. (The prescreening procedure leads to a selection of mAbs that stain predominantly developing fiber tracts and the surface of extending axons.) The application of these steps resulted in the identification of an mAb, termed 4/1, that recognizes an antigen expressed on the surface of neural cells (see below). The antigen appeared to interact with TN-C and -R, but not with other immobilized axon-associated proteins such as NrCAM, F11, or NCAM (Fig. 1 B and not shown). In comparison to TN-C or -R, weak binding was also observed to NgCAM and laminin-1 on ELISA plates (Fig. 1 B).
Figure 1.
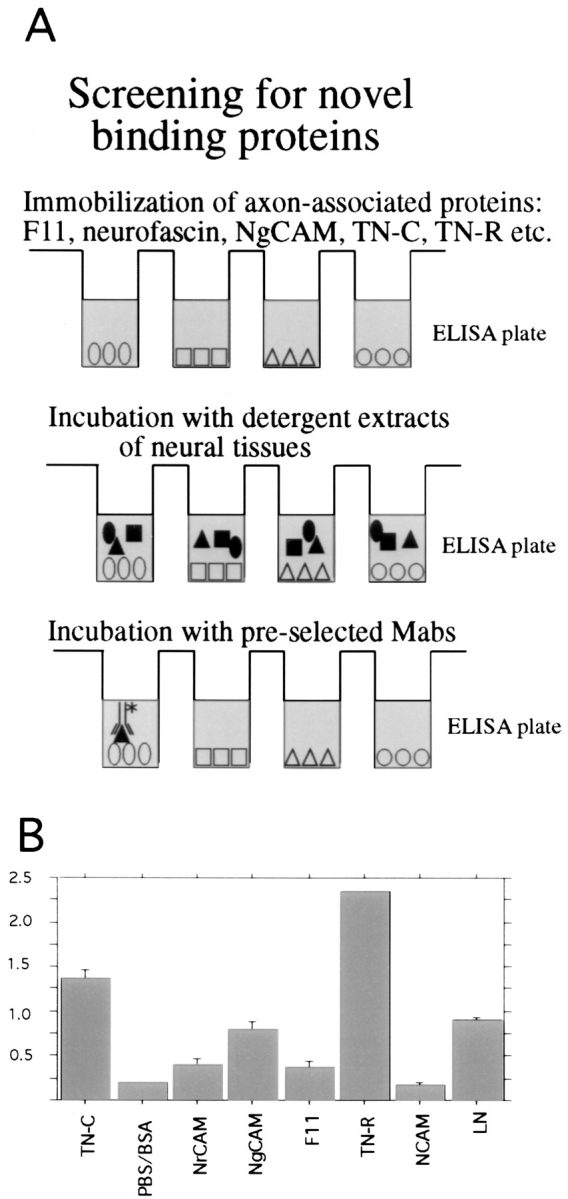
(A) Scheme of the screening procedure applied to identify novel binding proteins of known axon-associated glycoproteins. (B) Identification of binding proteins to immobilized axon-associated proteins. Immunoaffinity-purified proteins were immobilized on ELISA plates (200 ng/well), followed by blocking by BSA and washing. Plates were incubated with detergent extracts of retinas from embryonic day 16, washed several times, and then incubated with supernatants from preselected hybridomas. Binding as indicated by the optical density in ELISA plates is shown for one monolonal antibody, termed 4/1. Bars on top of the columns indicate SEM.
CALEB Is a Transmembrane Protein with an EGF-like Domain Related to Growth and Differentiation Factors
CALEB-encoding cDNAs were obtained by screening an adult chicken eye cDNA library constructed in λgt11 with mAb 4/1. Additional overlapping clones were found in the same library using the 1-kb insert of the initial cDNA clone as a probe. The amino acid sequence deduced from these overlapping cDNA clones predicts a polypeptide with a molecular mass of 61 kD (Fig. 2 A). This calculated mass differs from that of the carbohydrate-depleted polypeptide (110 kD) as analyzed by SDS-PAGE. Such differences between predicted and observed sizes have also been reported for other proteins (Rauch et al., 1992 and references therein) and might be due to low binding of SDS by the highly negatively charged CALEB. Comparison of amino acid sequences obtained from the NH2 termini of the 200-, 140-, and 80-kD CALEB components and from peptides generated from digests with trypsin and proteinase Asp-N of immunopurified CALEB confirm the identity of the sequence predicted by the cDNA clones (Fig. 2 A). The NH2-terminal peptide sequences of the 140- and the 200-kD component of CALEB obtained by Edman degradation are identical (starting at residue 19 in Fig. 2 A), which is in agreement with the observation that the 200-kD component is a proteoglycan form of the 140kD component (see below). The NH2 terminus of the 80kD component represents a sequence that starts at residue 260 (Fig. 2 A, dotted line). It is currently unknown whether the 80-kD polypeptide is generated by proteolytic processing, alternative splicing of the pre-mRNA, or an alternative translation start. (The methionine at position 245 could serve as an initiator site.)
Figure 2.
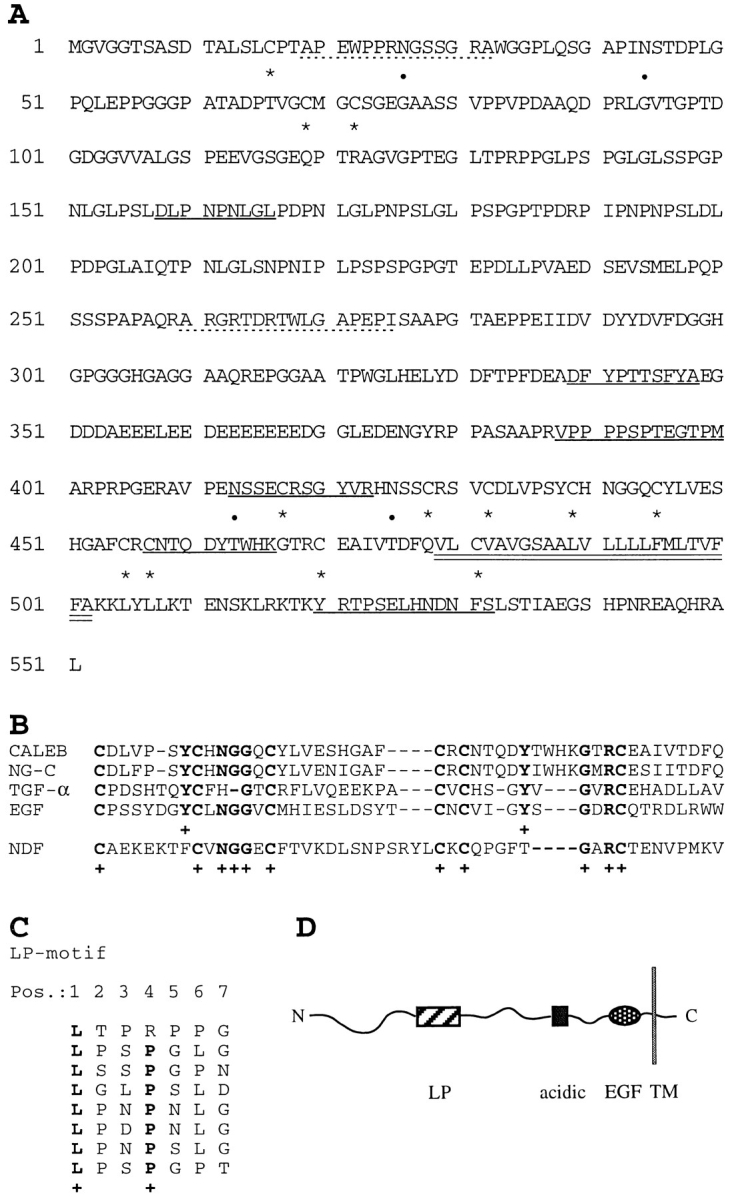
Amino acid sequence, schematic representation of CALEB, and alignments. (A) The amino acid sequence of CALEB deduced from six overlapping cDNA clones is shown using the single letter code. Numbering of amino acids is given at the left. The open reading frame contains 551 amino acids. Dotted lines correspond to the NH2-terminal sequences of the 200-, 140-, and 80-kD components of CALEB obtained by Edman degradation. Sequences obtained from peptides of digests of trypsin or Asp-N of CALEB are underlined and cysteine residues and potential N-glycosylation sites are marked by asterisks and dots, respectively. The putative transmembrane domain is indicated by double underlining. The NH2-terminal peptide sequences of the 200-, 140-, and the 80-kD component are APEWPPRXGXSGRA (aa 19–32) and ARGRXDRTWLGAPEPI (aa 260–275), respectively (X denotes an amino acid not to be defined by peptide sequencing). Sequences from proteolytic digests are DLPNPNLGL (aa 158–166), DFYPTTSFYA (aa 339–348), VPPPPSPXEGTPM (aa 388–400), XSSEXRSGYVR (aa 413–423), XNTQDYTXXK (aa 457–466), and XRTPSEXHNDNFX (aa 520–532). The nucleotide sequence data are available from EMBL/GenBank/DDBJ under accession number Y09264. (B) Alignment of the amino acid sequences of the EGF-like domains and flanking COOHterminal residues of CALEB (chick) with neuroglycan-C (NG-C, rat; Watanabe et al., 1995), transforming growth factor α (TGF-α, rat; Marquardt et al., 1984), epidermal growth factor (EGF, mouse; Gray et al., 1983), or neuregulin-α (NDF, rat; Wen et al., 1992). Alignments obtained by the programs bestfit and clustal of the HUSAR program package and by visual inspection start at the most NH2-terminal cysteine residue of the EGF motifs. Dashes indicate gaps that were introduced for maximal alignment. Identical residues corresponding to amino acids of CALEB are printed in bold and marked by a cross. (C) The amino acid sequence of CALEB at residues 131 to 186 comprises a stretch of primarily uncharged amino acids enriched in leucines and prolines, designated LP-motif. A stretch of seven amino acids repeated eight times contains a leucine at position 1 and a proline at position 4 (one exception). The periodicity of the leucine residues within the LP-motif resembles that of a leucine zipper; however, because of the existence of prolines, it is unlikely that this element of CALEB forms an α-helical structure as defined for leucine zippers. (D) Schematic representation of the overall structure of CALEB. LP, the leucine/proline-motif; acidic, the acidic box that is highly enriched in aspartic and glutamic acids; EGF, the EGF-like domain. A transmembrane segment (TM) separates these segments from a short cytoplasmic stretch.
Analysis of the predicted amino acid sequence reveals that the CALEB-polypeptide can be subdivided into several segments (Fig. 2): The NH2-terminal region contains several potential O-glycosylation sites (Tomita et al., 1978; Gooley et al., 1991) and two asparagines, which may be modified by N-linked sugars (Kornfeld and Kornfeld, 1985). This region contains SG or GS dipeptide sequences, two of which (aa 73 and 74 and aa 115–117) fulfill the criteria for potential attachment sites for chondroitin sulfate chains (Bourdon et al., 1987; Zimmermann and Ruoslahti, 1989). Additional potential sites for N- and O-glycosylation can be found in the COOH-terminal direction of the NH2 terminus of the 80-kD component of CALEB. This fits with our results on deglycosylation experiments of purified CALEB, which indicated that all molecular mass forms contain N- and O-linked sugar chains (data not shown).
Residues 131–186 of the amino acid sequence of CALEB compose a structural motif that, at first sight, resembles a leucine zipper, a dimerization motif known from transcription factors such as GCN4 (Landschulz et al., 1988). The characteristic of a leucine zipper motif is the repetition of a stretch of seven amino acids with a leucine in the first position and a hydrophobic amino acid in the fourth position. CALEB contains an eightfold repetition of seven amino acids with a leucine in every first position and a proline in every fourth position (with one exception) (Fig. 2 C). However, the presence of the prolines makes it unlikely that this stretch of CALEB forms an α-helical structure as proposed for the leucine zipper motif (O\QShea et al., 1991). Because of the regular spacing of leucines and prolines, this structural element of CALEB was designated LP-motif.
Further in the COOH-terminal direction, there is a stretch of sequence containing a cluster of acidic residues (DDD . . . EED from residue 351 to 369). This structure might be involved in cation binding and/or charge-based interactions with other proteins. Human versican, bovine brevican, and the β-amyloid precursor protein contain a similar stretch of acidic residues (Kang et al., 1987; Zimmermann and Ruoslahti, 1989; Yamada et al., 1994).
COOH-terminal of the acidic box, CALEB contains a 40–amino acid–long cysteine-rich segment. The number (six) and the spacing of these cysteines in addition to the conservation of two glycines and one arginine establishes CALEB as a member of the EGF-family of differentiation factors (Campbell and Bork, 1993; Massague and Pandiella, 1993; Ben-Baruch and Yarden, 1994). The cysteine configuration of CALEB is most similar to that found in EGF, TGF-α, and neuregulin as analyzed with the bestfit and clustal programs (Fig. 2 B). The small stalk between the EGF domain and the following putative transmembrane segment does not contain any basic residues (L-R) important for proteolytic cleavage as have been found for all transmembrane forms of neuregulin. It is therefore conceivable that CALEB exists only as a protease-resistant form giving rise to a membrane associated EGF-like domain. The proteolytic processing sequence of TGF-α, however, also does not contain any basic amino acid residues (Massague and Pandiella, 1993).
Hydropathy analysis (Kyte and Doolittle, 1982) of the predicted amino acid sequence revealed a hydrophobic stretch of 24 amino acids in length (residues 479–502) followed by two basic amino acids (KK) that might function as a transmembrane domain (Sabatini et al., 1982) that dissects the CALEB-polypeptide into a glycosylated extracellular part and a short (49 aa long) cytoplasmic stretch that contains several putative serine and threonine phosphorylation sites (Pearson and Kemp, 1991). No strong hydrophobic sequence that could function as a signal peptide was detected at the NH2 terminus. This phenomenon is also observed with other proteins, including some forms of the neuregulins that also contain no classical signal peptide (Wen et al., 1992). Despite the lack of a recognizable strong hydrophobic sequence at the NH2 terminus, CALEB under the control of the SV-40 promoter is expressed on the surface of transfected COS7 cells as detected by cell surface immunostaining using the mAb 4/1 (data not shown). On the basis of the reactivity of mAb 4/1 to λ cDNA clones and to the molecular mass forms of CALEB, its epitope can be allocated most likely between residues 260 to 442, indicating that the epitope is within the extracellular region of CALEB.
Data bank searches indicate that CALEB is a novel protein. However, the EGF-like domain and the cytoplasmic segment are highly related to neuroglycan-C (85% amino acid identity), a recently described proteoglycan of the rat nervous system of unknown function (Watanabe et al., 1995). The residual two thirds of the amino acid sequences of both proteins do not reveal any sequence homology, and, furthermore, neuroglycan-C contains a stretch of basic residues instead of an acidic box and has no recognizable LP-motif. This comparison suggests that CALEB and neuroglycan-C might be products of different genes or are different isoforms of the same gene generated by alternative pre-mRNA splicing.
Taken together, we have described a novel transmembrane protein composed of a highly glycosylated domain, a leucine/proline-rich domain, an acidic box, and an EGFlike domain dissected from the cytoplasmic stretch by a transmembrane domain as indicated in Fig. 2 D.
CALEB Is Composed of Several Molecular Mass Components, Including a Chondroitinsulfate-containing Form
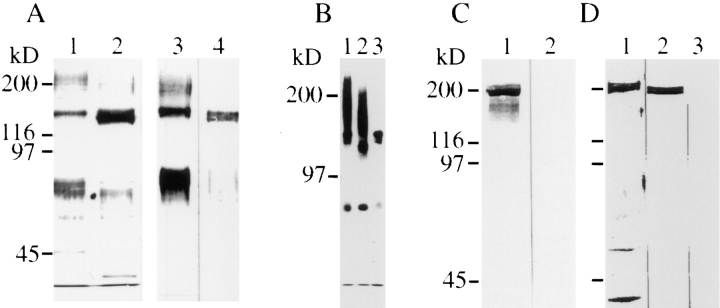
mAb 4/1 was used to purify the antigen from detergent extracts of embryonic or adult retinas by immunoaffinity chromatography (Fig. 3 A). The isolate from embryonic retinas is composed of the following molecular mass components: a band at 140 kD, and broad migrating bands at 80 and 200 kD (lane 1). The latter two components were not or only weakly found in isolates from adult retinas, while the 140 kD components appear as a doublet (140 and 130 kD) (lane 2). All these components observed in silver-stained gels were recognized by mAb 4/1 in Western blots, indicating that they are immunologically related (lanes 3 and 4). To characterize the CALEB components further, CALEB was purified from cultivated retinal cells that were biosynthetically labeled with [35S]sulfate. This isolate is composed of identical molecular mass components as that from embryonic retinal tissue (Fig. 3 B, lane 1). Digestions with chondroitinase ABC, but not with heparatinase or heparinase, completely eliminated the 200-kD smear (Fig. 3 B, lane 3 and not shown). When the 200-kD band was excised from the gel followed by chondroitinase ABC digestion and then analyzed by SDS-PAGE, a 140kD band appeared (not shown). These results indicate that the broad band at 200 kD contains chondroitinsulfate chains attached to the 140-kD core polypeptide. Neuraminidase treatment results in a slightly increased electrophoretic mobility of the 200- and 140-kD bands but not of the 80kD band (Fig. 3 B, lane 2). The finding that a substantial portion of CALEB, the 140- and 80-kD forms, exists on retinal cells without glycosaminoglycan chains attached suggests that CALEB is a “part-time” proteoglycan, as it has also been found for brevican (Yamada et al., 1994).
Figure 3.
CALEB components in neural tissues. (A) Polypeptides isolated by immunoaffinity chromatography using mAb 4/1 from embryonic (lane 1) or adult retinas (lane 2) analyzed by 7% SDSPAGE followed by silver staining. Immunotransfer analysis of CALEB isolates from embryonic (lane 3) or adult retinas (lane 4) using mAb 4/1. (B) CALEB components after biosynthetical labeling with [35S]sulfate of retinal monolayer cultures in vitro (lane 1) followed by digestion with neuraminidase (lane 2) or chondroitinase ABC (lane 3). Bands were visualized by fluorography. (C) TN-C copurifies with CALEB from embryonic retinas. CALEB was isolated from embryonic retinas by immunoaffinity chromatography by omitting the high-salt washing step, subjected to SDSPAGE (7%), and analyzed in immunotransfers using mAb 68 directed to TN-C (lane 1) or mAb 23-13 to TN-R (lane 2). (D) The highsalt fraction of anti-CALEB columns consists of TN-C. The CALEB immunoaffinity column loaded with detergent extracts of embryonic retinas followed by washing with solubilization buffer was preeluted with three column volumes of 1.2 M NaCl in PBS. The highsalt fraction was analyzed in SDS-PAGE followed by silver staining (lane 1) or analyzed in immunotransfers using mAb 68 to TN-C (lane 2) or mAb 23-13 to TN-R (lane 3). Molecular mass markers are indicated at the left of each panel.
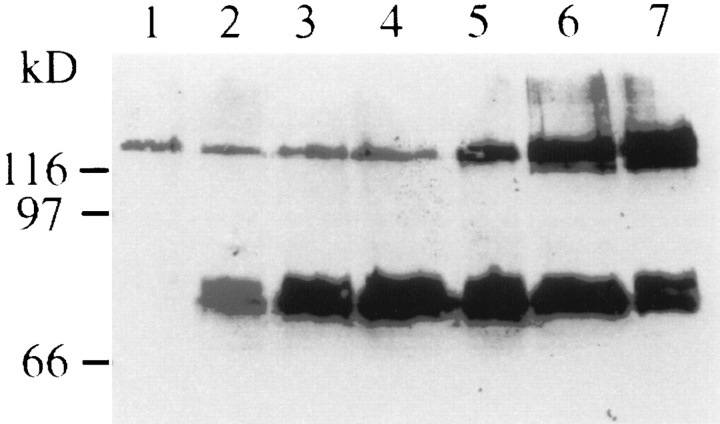
Analysis of Western blots of retinal tissues from different embryonic ages revealed that the expression of the CALEB components is regulated during embryonic development of the retina (Fig. 4). The 140-kD component appears very early in retinal development (at E7) and reaches its maximum at E20. In comparison to the 140-kD component, the 130-kD band appears delayed in its expression but also reaches its maximum at E20. In contrast, the lower molecular mass component at 80 kD reveals its strongest expression at E14/E15 and then gradually declines and is not detectable in adult stages (Fig. 4 and not shown).
Figure 4.
The expression of CALEB components in the retina is developmentally regulated. Retinas of different embryonic ages were solubilized, and equal amounts of proteins were resolved by SDS-PAGE (7%) followed by immunotransfer analysis using mAb 4/1 to CALEB. E8 (lane 1), E10 (lane 2), E12 (lane 3), E14 (lane 4), E16 (lane 5), E18 (lane 6), and E20 (lane 7). Molecular mass markers are indicated at the left of the panel.
CALEB Binds to TN-C and -R
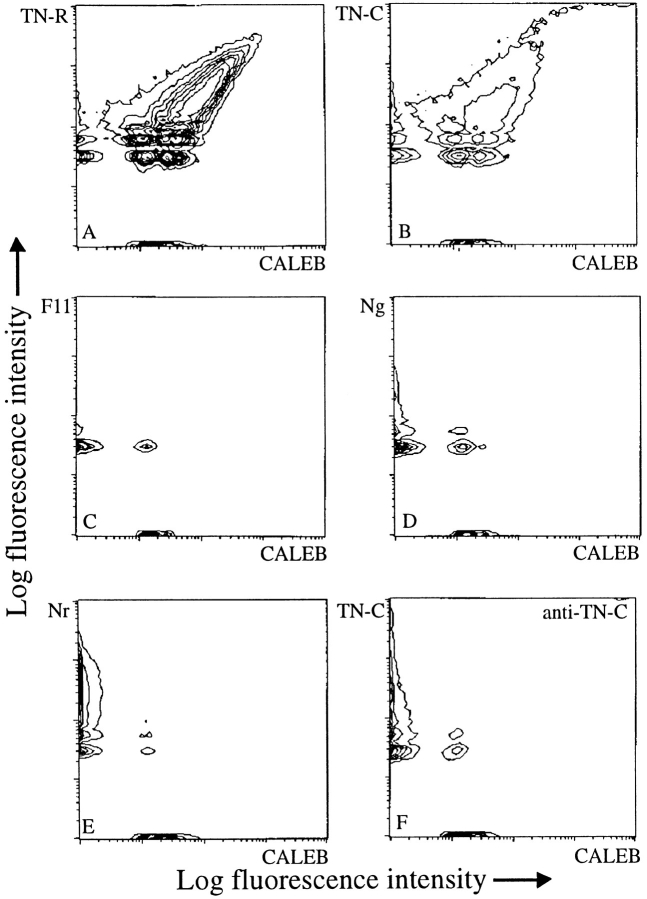
To further substantiate the finding of the mutual affinity of CALEB and TN-C or CALEB and TN-R, we examined the binding of protein-coated microspheres, an assay previously found suitable and reliable to analyze protein–protein interactions. Affinity-purified CALEB and other axon-associated proteins were coupled to fluorescent microspheres of red or green fluorochromes. Mixtures of beads were then incubated for 1 h at room temperature followed by an analysis with the flow cytometer. In dual combinations, only the TN-R– and TN-C–conjugated beads were found to be associated with CALEB beads (Fig. 5, A and B). Specificity of these heterophilic CALEB-TN-R or -C binding could be demonstrated by incubation of different combinations of microspheres coated with other axonal glycoproteins. CALEB was found not to bind to NgCAM, NrCAM, NCAM, F11, or laminin-1 (Fig. 5, C–E and not shown). The binding between CALEB and TN-R or between CALEB and TN-C could be blocked by Fab fragments of polyclonal antibodies to TN-R or -C, respectively (Fig. 5 F and not shown). The weak interactions between NgCAM and CALEB or between laminin-1 and CALEB observed on ELISA plates only could not be confirmed with these methods. It is therefore conceivable that the NgCAM-CALEB binding is an artifact since NgCAM immobilized on ELISA plates binds nonspecifically to many other proteins (our unpublished observations).
Figure 5.
Binding of CALEB to TN-R or to TN-C analyzed by flow cytometry. CALEB, TN-R, or TN-C were coupled to fluorescent microspheres, mixed, and incubated for 1 h at a 1:1 ratio. Aliquots were analyzed by flow cytometry. Two parametric measurements are documented as contour plots using a logarithmic scale for both axes. The fluorescence intensity corresponds to the size of aggregates. Only beads coated with TN-R (A) or TN-C (B), but not F11 (C), NgCAM (D), or NrCAM (E) formed large mixed aggregates with CALEB. The binding between CALEB and TN-C could be completely blocked by Fab fragments of polyclonal antibodies to TN-C (F).
To analyze whether TN-C or -R copurifies with CALEB, affinity isolates of CALEB were subjected to Western blotting using mAbs to TN-C or -R. Isolates of CALEB from embryonic but not from adult retinas, which were not washed with high salt, were found to contain TN-C but not TN-R (Fig. 3 C, lanes 1 and 2, respectively). The coisolating TN-C could be eluted from the column by 1.2 M NaCl (Fig. 3 D, lanes 1–3), suggesting a charge-based interaction between CALEB and TN-C. One reason for the coisolation of TN-C on anti-CALEB affinity columns in contrast to TN-R might be that TN-C expression dominates over the TN-R expression within the developing retina (see also below). In summary, the currently available binding data support the notion that CALEB is a high-affinity TN-C– or TN-R–binding protein, while the interactions between CALEB and NgCAM or laminin-1 need further investigations.
Antibodies to CALEB Interfere with Neurite Formation on Different Substrates In Vitro
To study the biological function of CALEB, neurite outgrowth assays in a permissive environment provided by specific IgSF members, by laminin-1 or vitronectin in the presence of antibodies to CALEB, were performed. (The ECM glycoproteins TN-R and -C are nonpermissive for neurite extension for most neurons.) While the density of E6 tectal cells attached to the substratum was increased in the presence of Fab fragments of polyclonal antibodies to CALEB (data not shown), neurite length on either an F11, NgCAM, laminin-1, or vitronectin substratum was dramatically diminished, suggesting that the effects of antiCALEB antibodies are substrate independent (Fig. 6, A–C, and not shown). We used antisera from four different rabbits that appear functionally identical; however, the degree of reduction in neurite length varied and was dependent on the Fab concentration applied. (The tested concentration range was 300–1,000 μg/ml.) The effects on F11- or vitronectin-mediated neurite extension appeared generally stronger than on NgCAM- or laminin-1–dependent extension. To demonstrate specificity, tectal cells were grown in the presence of Fab fragments that have been preincubated with immobilized CALEB (Fig. 6 D) or in the presence of both Fab fragments and purified CALEB. In both cases, the activity of the Fab fragments was completely neutralized, indicating that the effects observed are due to antibodies recognizing CALEB. Furthermore, Fab fragments of polyclonal antibodies to two recently characterized neuronal cell surface antigens, the 12/36 or the 3/11antigen, did not affect neurite extension of tectal cells even when used at a much higher concentration, indicating that Fab preparations generated under identical conditions have no general toxic effect on tectal cells and that binding of Fabs to axonal surface alone does not cause a reduction of neurite length. mAb 4/1 was not found to affect neurite formation on all substrates tested (Fig. 6, A and B). Our observations therefore suggest that Fab fragments of polyclonal antibodies to CALEB specifically impair the ability of tectal neurons to form neurites in a permissive environment and might identify CALEB as a competence factor for neurite extension.
CALEB Is Associated with Glial and Neuronal Surfaces In Vivo and In Vitro
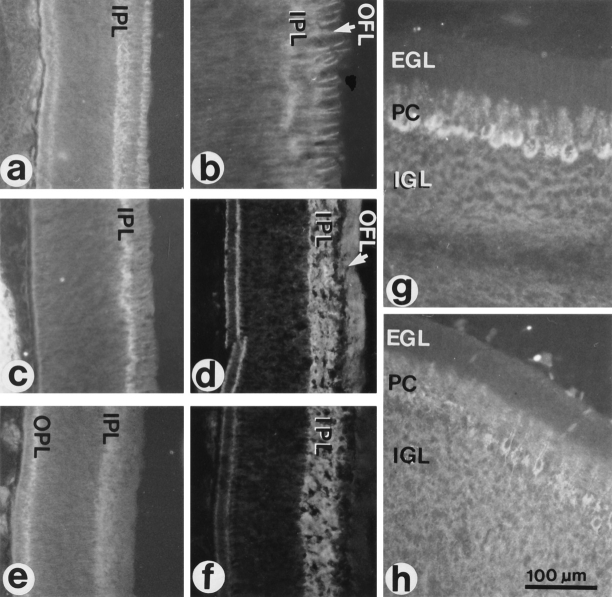
In the embryonic retina, CALEB immunoreactivity coincides with the formation of the inner plexiform layer (IPL) in the central retina at E7/E8 (Fig. 7 a). At later stages, the intensity of staining gradually increases in the IPL, which is then uniformly labeled (Fig. 7 d). In addition, homogeneous CALEB staining was observed in the outer plexiform layer (OPL) and in the optic fiber layer (OFL). At E8, a prominent labeling of Müller cell processes transversing the ganglion cell layer and the optic fiber layer was detected (Fig. 7 b). In the GCL and the OFL, the processes of these glial cells are thicker and ramify, and the pattern of staining reflects this (Prada et al., 1989, 1991). In the inner nuclear layer, the staining is faint. At E8, the immunostaining pattern of TN-C is similar to that of CALEB dominating in the developing IPL and on the Müller cell processes (Fig. 7, c and f). TN-R immunoreactivity has a slightly different pattern of localization than CALEB. It is present in the IPL and OPL, but weak in the OFL (Fig. 7 e).
Figure 7.
Localization of CALEB in sections of the developing retina and cerebellum. Cryostat sections were stained indirectly by mAb 4/1 directed to CALEB (a, b, d, and g), by mAb M1 to TN-C (c, f, and h) or mAb 23-13 to TN-R (e). Staining was visualized by fluorescence. Retinas were from 8-d-old (a, b, c, and e) or from 12-d-old chicken embryos (d and f). Cerebella were from 18-d-old chicken embryos (g and h). b shows the distribution of CALEB in the OFL at a higher magnification as shown in a. EGL, external granular layer; IGL, internal granular layer; PC, Purkinje cells.
The staining pattern in the developing retina indicates an association with glial surfaces and suggests an association with neuronal surfaces in the plexiform layers. A clear association of CALEB immunoreactivity with neuronal surfaces, however, was observed in the developing cerebellum where antibodies to CALEB stain primarily the Purkinje cells and their dendritic trees (Fig. 7 g), and at more advanced stages, CALEB expression is dominant throughout the molecular layer (not shown). Weaker labeling is observed in the developing internal granular layer, and very faint staining is seen on structures transversing the external granular layer, probably Bergmann glia cells. In comparison, TN-C shows a more widespread and uniform distribution in the developing cerebellum (Fig. 7 h). Taken together, in vivo CALEB immunoreactivity is associated with astroglial and neuronal surfaces in different parts of the embryonic brain. Furthermore, Western blot analysis and protein purifications indicate that the 140-kD form of CALEB is also found in the adult retina and brain (Fig. 3 A, lane 4 and not shown). CALEB labeling was not detected outside the nervous system. In in vitro cultures, CALEB was associated with the surface of processes of retinal and tectal neurons, of astrocytes, and of oligodendrocytes of the optic nerve (data not shown).
Discussion
By combining the sensitivity of binding detection in ELISAs with immunological screens, we were able to establish CALEB as a novel transmembrane protein of neurons and glial cells of the developing nervous system that interacts with the ECM glycoproteins TN-R and -C. The most remarkable structural motif identified in this protein is an EGF-like domain. Alignment analysis showed that the EGF-like domain is most similar to EGF, to that found in TGF-α or in neuregulin, and fits with the criteria used to group the latter three and some other proteins together into the family of transmembrane growth and differentiation factors. One subgroup of this family comprises the different neuregulin isoforms, while the other subfamily is composed of EGF, TGF-α, HBEGF, amphiregulin, and betacellulin (Massague and Pandiella, 1993; Peles and Yarden, 1993; Carraway and Burden, 1995). All members of the latter subgroup bind to the receptor tyrosine kinase ErbB1, while the EGF-like domains of the neuregulins are ligands of ErbB3 and ErbB4. It is assumed that the different distance between cysteine 3 and 4 in the EGF-like domain of these two subgroups causes a different loop length that might contribute to binding specificity to a particular receptor (Peles and Yarden, 1993). The interval between cysteine 3 and 4 in the EGF-like domain of CALEB is different from that in the neuregulins but similar to that in EGF and TGF-α. In contrast, the spacing between cysteine 5 and 6 is longer in CALEB than in any other member of these EGF-like domains. If one assumes a ligand function for CALEB, it is therefore likely that CALEB interacts with a receptor distinct from ErbB1 and ErbB3/4.
All known members of the family of growth and differentiation factors can generate a diffusible EGF-like domain from the transmembrane precursor by proteolytic processing with cleavages on both sides of the EGF-like domain (Massague and Pandiella, 1993). TGF-α is able to activate its receptor both as transmembrane and as released form (Brachmann et al., 1989). TGF-α does not contain basic amino acid residues at the cleavage sites surrounding the EGF-like domain as have been found for neuregulin and EGF. Instead, there are small apolar amino acids. The short stalk in the sequence of CALEB between the EGFlike domain and the transmembrane segment also does not contain basic residues but does contain the tripeptide AIV (aa 472–474), which resembles the cleavage site sequence found in TGF-α (AVV). Currently, we have no biochemical data indicating that the EGF-like domain of CALEB can be released by proteolytic processing. Nevertheless, we are able to isolate different molecular mass forms of CALEB, and we demonstrate that the 80-kD component of CALEB is specifically regulated during development. For example, in the retina the 80-kD form is found predominantly during stages when synaptic connections are being established (Hughes and LaVelle, 1974; Hering and Kröger, 1996) and is downregulated in the adult. Immunohistochemical analysis reveals the presence of CALEB in the synapse-rich layers IPL and OPL of the retina. In this context, it is interesting that one isoform of neuregulin, termed ARIA, was identified as inducing activity of acetycholine receptor synthesis (Falls et al., 1993; Chu et al., 1995; Jo et al., 1995). Loeb and Fischbach (1995) presented data showing that ARIA binds to extracellular matrix molecules, and they postulated a model that suggests that the access of ARIA to the ErbB3/4 receptors on the postsynaptic membrane is regulated by proteolytical release from the extracellular matrix. We have demonstrated an interaction between CALEB and two large ECM molecules, TN-C and -R. We further showed by immunohistochemical analysis that all three molecules (CALEB, TN-C, and TN-R) have an overlapping distribution in synapse-rich areas in the retina. In analogy to the results presented by Loeb and Fischbach, it is possible that because of their interaction with CALEB, TN-C and -R regulate the accessibility of CALEB to its corresponding receptor. It is currently not possible to decide whether the 80-kD component is generated by proteolytic processing of the 140-kD polypeptide, by splicing events or by the use of an alternative translation start site. However, the combined data indicate that the 80-kD component is not a diffusible form of CALEB and suggest that both major forms of CALEB, the 140- and 80-kD components, may be able to exert their biological action via a juxtacrine mechanism like TGF-α.
In addition to this EGF motif, CALEB contains the LPmotif, a leucine-rich sequence resembling a leucine zipper, which might enable CALEB to interact with other proteins. Leucine zipper structures are known from many transcription factors and allow them to homo- or heterodimerize. Whether the LP-motif within the sequence of CALEB is able to fold into a regularly secondary structure remains to be determined. Connectin, a cell adhesion molecule of Drosophila with a repulsive function during growth cone guidance and synapse formation, also possesses leucine-rich repeats (Nose et al., 1992). The spacing of the leucines, however, is different from the LP-motif of CALEB, and there are no regularly spaced prolines within these repeats of connectin. At present nothing is known about the function of these repeats in this Drosophila protein. In addition to several potential glycosylation sites, the CALEB sequence contains two potential tyrosine sulfation sites (aa 289–294 and 327–331; Huttner, 1987), and there are biochemical data that indicate tyrosine sulfate in the protein core of CALEB (Roth, S., and F.G. Rathjen, unpublished data). One very interesting possible function of tyrosine sulfate was elucidated by Sako et al. (1995) and Pouyani and Seed (1995), who demonstrated that a sulfated peptide segment of PSGL-1 is neccessary to build up the high-affinity binding site for P-selectin.
Neuroglycan-C, a recently described proteoglycan of the rat nervous system, has an EGF-like and a cytoplasmic domain that is 85% identical in the amino acid sequence with the corresponding region of CALEB (Watanabe et al., 1995). The remaining two thirds of both sequences are completely unrelated. In particular, in neuroglycan-C there is no leucine/proline-rich segment (LP-motif), and in contrast to CALEB, it contains a highly basic stretch of sequence. Neuroglycan-C expresses a classical signal peptide that is not seen in CALEB. CALEB and neuroglycan-C might therefore be products of different genes or different isoforms of equivalent genes in two different species. It will be interesting to see in the future whether there exists a family of related genes or multiple isoforms as it has been described for the neuregulins. Interestingly, we found a short piece of EST sequence (human) in data banks (These sequence data are available from GenBank/EMBL/DDB under accession number h05182.emnew.) that, when translated, yields a peptide that is highly related to the EGFlike domain of CALEB. This might indicate that the type of EGF domain present in CALEB is highly conserved during evolution.
In addition to their roles as glial growth, maturation, and survival factor, the neuregulins are implicated in a variety of biological processes during the development of the nervous system (Marchionni et al., 1993; Pinkas-Kramarski et al., 1994; Dong et al., 1995). Gene targeting experiments have revealed that the neuregulins are essential for the neurogenic lineage of the cranial neural crest (Meyer and Birchmeier, 1995). Furthermore, Bermingham-McDonogh et al. (1996) presented evidence that the neuregulins stimulate survival and neurite extension of retinal cells in culture in a dose-dependent manner. These multiple responses might be due to different isoforms of the neuregulins or different combinations of receptors. We were interested in whether CALEB might be implicated in the process of neurite formation and have therefore tested if antibodies to CALEB disturb neurite formation of tectal cells grown on NgCAM or F11, two axonal members of the IgSF; or laminin-1 or vitronectin, two ECM glycoproteins. Fab fragments of four different antisera were able to suppress neurite formation, and this effect is independent of the substrate used. The effect could be neutralized by preincubation of the Fab fragments with CALEB, excluding the possibility of nonspecific or general toxic side effects of the Fabs. The impairment of neurite formation caused by antibodies to CALEB could be the result of at least four different mechanisms: (a) CALEB might be a general axonal receptor for the substrates tested. No direct interaction between CALEB and the substrates used was observed that could be supported by different independent binding assays. Therefore, it is unlikely that CALEB serves as a receptor protein on the axonal surface for these substrates. Furthermore, previous experiments have shown that neurite outgrowth on F11 is mediated by NrCAM (Morales et al., 1993), on NgCAM by an homophilic interaction (Lemmon et al., 1989), and on laminin-1 or on vitronectin by integrins (Venstrom and Reichardt, 1993). (b) The transmembrane form of CALEB itself might be a signaling receptor, and binding of antibodies to CALEB mimicks binding of an unknown ligand, resulting in intracellular changes that culminate in the blockade of neurite formation. By analogy to TGF-α and neuregulin, however, the probability is low—although not excluded—that CALEB itself is a signaling receptor protein. (c) Antibodies to CALEB might interfere with the proteolytical processing of CALEB, preventing the release of a CALEB form active in a paracrine or autocrine manner. (d) Antibodies to CALEB might directly block the EGF-like domain of CALEB. The latter two cases assume that the EGF-like domain contains a receptor recognition site that activates an unknown tectal receptor protein to transmit a signal to tectal cells. This receptor would then generate an intracellular differentiation signal that might make tectal neurons competent for neurite formation in an appropriate environment, as provided by the axonal IgSF members or ECM proteins. If this interpretation can be supported by future studies, it is likely that CALEB is of interest in studies of neurodegenerative diseases and in the regeneration of axons after injury. The downregulation of specific molecular mass forms of CALEB during development of the central nervous system might be in line with such assumptions.
In summary, this study has established CALEB as a member of the EGF differentiation factors that is implicated in neurite formation in vitro in a permissive environment. Further understanding of the function of CALEB will require the identification of its cellular receptor(s) and of biologically active forms that are released from the cell surface.
Acknowledgments
We thank Dr. G. Lewin and B. Hassel for helpful comments on the manuscript and B. Cloos for secretarial assistance. We are grateful to Dr. R. Chiquet-Ehrismann for providing us antibody 68 to TN-C and Drs. U. Schwarz and G. Morales for antibodies to NrCAM. We appreciate the technical help of Mechthild Henning and Dörte Cörlin and thank the institute for clinical immunology (University of Leipzig) to let us use the flow cytometer.
These studies were supported by Deutsche Forschungsgemeinschaft grant No. Ra424/2-2.
Abbreviations used in this paper
- aa
amino acid
- CALEB
chicken acidic leucine-rich EGF-like domain containing brain protein
- E
embryonic day
- ECM
extracellular matrix
- IgSF
immunoglobulin superfamily
- IPL and OPL
inner and outer plexiform layer
- NCAM
neural cell adhesion molecule
- NgCAM
neuron-glia cell adhesion molecule
- NrCAM
N gCAM-related cell adhesion molecule
- OFL
optic fiber layer
- TN-C and -R
tenascin-C and -R
Footnotes
Address all correspondence to Fritz G. Rathjen, Max-Delbrück-Centrum für Molekulare Medizin, Robert-Rössle-Str. 10, D-13122 Berlin, Germany.
References
- Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985;11:13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, editors. 1994. Current Protocols In Molecular Biology. John Wiley and Sons, Inc., New York.
- Ben-Baruch N, Yarden Y. Neu differentiation factors: a family of alternatively spliced neuronal and mesenchymal factors. Proc Soc Exp Biol Med. 1994;206:221–227. doi: 10.3181/00379727-206-43746. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, McCabe K, Reh TA. Effects of GGF/ neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development (Camb) 1996;122:1427–1438. doi: 10.1242/dev.122.5.1427. [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Krusius T, Cambell S, Schwartz NB, Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci USA. 1987;84:3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann R, Lindquist PB, Nagashima M, Kohr W, Lipari T, Napier M, Derynck R. Transmembrane TGF-α precursors activate EGF/ TGF-α receptors. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Protein Profile. 1995;2:963–1108. [PubMed] [Google Scholar]
- Brümmendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Hubert M, Treubert U, Leuschner R, Tarnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Campbell ID, Bork P. Epidermal growth factor-like modules. Curr Opin Struct Biol. 1993;3:385–392. [Google Scholar]
- Carraway KL, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Cantley LC. A Neu acquaintance for ErbB3 and ErbB4: a role for receptor heterodimerization in growth signaling. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol. 1984;98:1937–1946. doi: 10.1083/jcb.98.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Hagios C, Schenk S. The complexity in regulating the expression of tenascins. [review] Bioessays. 1995;17:873–878. doi: 10.1002/bies.950171009. [DOI] [PubMed] [Google Scholar]
- Chu GC, Moscose LM, Sliwkowski MX, Merlie JP. Regulation of the acetylcholine receptor ε subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Cunningham BA. Cell adhesion molecules as morphoregulators. [review] Curr Opin Cell Biol. 1995;7:628–633. doi: 10.1016/0955-0674(95)80103-0. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, Kayyem JF, Roman JM, Stierhof YD, Dreyer WJ, Schwarz U. Topologically restricted appearance in the developing chick retinotectal system of Bravo, a neural surface protein: experimental modulation by environmental cues [published erratum appears in J. Cell. Biol.1991. 112:1049] J Cell Biol. 1990;111:3087–3096. doi: 10.1083/jcb.111.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Gooley AA, Classon BJ, Marschalek R, Williams KL. Glycosylation sites identified by detection of glycosylated amino acids released from edman degradation: the identification of Xaa-Pro-Xaa-Xaa as a motif for Thr-O-glycosylation. Biochem Biophys Res Commun. 1991;178:1194–1201. doi: 10.1016/0006-291x(91)91019-9. [DOI] [PubMed] [Google Scholar]
- Gray A, Dull TJ, Ullrich A. Nucleotide sequence of epidermal growth factor predicts a 12,800 molecular weight protein precursor. Nature (Lond) 1983;303:722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Hering H, Kröger S. The formation of synaptic specializations in the inner plexiform layer of the developing chick retina. J Comp Neurol. 1996;375:393–405. doi: 10.1002/(SICI)1096-9861(19961118)375:3<393::AID-CNE4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hughes WF, La A, Velle On the synaptogenic sequence in the chick retina. Anat Rec. 1974;179:297–302. doi: 10.1002/ar.1091790302. [DOI] [PubMed] [Google Scholar]
- Huttner WB. Protein tyrosine sulfation. Trends Biochem Sci. 1987;12:361–363. [Google Scholar]
- Huynh, T.V., R.A. Young, and R.W. Davies. 1985. Constructing and screening cDNA libraries in λgt10 and λgt11. In DNA Cloning Techniques: A Practical Approach. D. Glover, editor. IRL Press at Oxford University Press, Oxford. 49–78.
- Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature (Lond) 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature (Lond) 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci USA. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science (Wash DC) 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Fischbach GD. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol. 1995;130:127–135. doi: 10.1083/jcb.130.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl DJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature (Lond) 1993;362:313–317. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Marquardt H, Hunkapiller MW, Hood LE, Todaro GJ. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science (Wash DC) 1984;223:1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Distinct isoforms of neuregulins are expressed in mesenchymal and neuronal cells during mouse development. Proc Natl Acad Sci USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature (Lond) 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Morales G, Hubert M, Brümmendorf T, Treubert U, Tárnok A, Schwarz U, Rathjen FG. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and NrCAM/ Bravo. Neuron. 1993;11:1113–1122. doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- Nörenberg U, Wille H, Wolff JM, Frank R, Rathjen FG. The chicken neural extracellular matrix molecule restrictin: similarity with EGF-, fibronectin type III-, and fibrinogen-like motifs. Neuron. 1992;8:849–863. doi: 10.1016/0896-6273(92)90199-n. [DOI] [PubMed] [Google Scholar]
- Nörenberg U, Hubert M, Brümmendorf T, Tárnok A, Rathjen FJ. Characterization of functional domains of the tenascin-R (restrictin) polypeptide—cell attachment site, binding with F11, and enhancement of F11-mediated neurite outgrowth by tenascin-R. J Cell Biol. 1995;130:473–484. doi: 10.1083/jcb.130.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Mahajan V, Goodman CS. Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. . Cell. 1992;70:553–567. doi: 10.1016/0092-8674(92)90426-d. [DOI] [PubMed] [Google Scholar]
- O'Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science (Wash DC) 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Peles E, Yarden Y. Neu and its ligands: from an oncogene to neural factors. BioEssays. 1993;15:815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwarz M, Yarden Y. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci USA. 1994;91:9387–9391. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RR. The hydrolysis of rabbit γ-globulin and antibodies with crystalline papain. Biochem J. 1959;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Prada C, Puga J, Pérez-Méndez L, López R, Ramirez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Prada FA, Magalhaes MM, Coimbra A, Genis-Galvez JM. Morphological differentiation of the Müller cell: golgi and electron microscopy study in the chick retina. J Morphol. 1989;201:11–22. doi: 10.1002/jmor.1052010103. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Chang S, Bonhoeffer F, Raper JA. Neurofascin: a novel chick cell-surface glycoprotein involved in neurite-neurite interactions. Cell. 1987a;51:841–849. doi: 10.1016/0092-8674(87)90107-3. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987b;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Chiquet R, Ehrismann Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development (Camb) 1991;113:151–164. doi: 10.1242/dev.113.1.151. [DOI] [PubMed] [Google Scholar]
- Rauch U, Karthikeyan L, Maurel P, Margolis RU, Margolis RK. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992;267:19536–19547. [PubMed] [Google Scholar]
- Sabatini DD, Kreibich G, Morimoto T, Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982;92:1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chainterminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Furthmayer H, Marchesi VT. Primary structure of human erythrocyte glycophorin a. Isolation and characterisation of peptides and complete amino acid sequence. Biochemistry. 1978;17:4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF. Extracellular matrix 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB (Fed Am Soc Exp Biol) J. 1993;7:996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Maeda N, Matsui F, Kushima Y, Noda M, Oohira A. Neuroglycan-C, a novel membrane-spanning chondroitin sulfate proteoglycan that is restricted to the brain. J Biol Chem. 1995;270:26876–26882. doi: 10.1074/jbc.270.45.26876. [DOI] [PubMed] [Google Scholar]
- Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbinger SM, Levy RB, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Wolff JM, Rathjen FG, Frank R, Roth S. Biochemical characterization of polypeptide components involved in neurite fasciculation and elongation. Eur J Biochem. 1987;168:551–561. doi: 10.1111/j.1432-1033.1987.tb13453.x. [DOI] [PubMed] [Google Scholar]
- Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem. 1994;269:10119–10126. [PubMed] [Google Scholar]
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO (Eur Mol Biol Organ) J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, D'Alessandri L, Ranscht B, Falchetto R, Winterhalter KH, Vaughan L. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J Cell Biol. 1992;119:203–213. doi: 10.1083/jcb.119.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]