Abstract
Receptor protein tyrosine phosphatase β (RPTPβ) is expressed as soluble and receptor forms with common extracellular regions consisting of a carbonic anhydrase domain (C), a fibronectin type III repeat (F), and a unique region called S. We showed previously that a recombinant Fc fusion protein with the C domain (βC) binds to contactin and supports neuronal adhesion and neurite growth. As a substrate, βCFS was less effective in supporting cell adhesion, but it was a more effective promoter of neurite outgrowth than βCF. βS had no effect by itself, but it potentiated neurite growth when mixed with βCF. Neurite outgrowth induced by βCFS was inhibited by antibodies against Nr-CAM and contactin, and these cell adhesion molecules formed a complex that bound βCFS. NIH3T3 cells transfected to express βCFS on their surfaces induced neuronal differentiation in culture. These results suggest that binding of glial RPTPβ to the contactin/Nr-CAM complex is important for neurite growth and neuronal differentiation.
Abetter understanding of molecular mechanisms of cell–cell interactions in the nervous system is emerging from studies of neural cell adhesion molecules (CAMs)1 and other receptors that transmit signals across the plasma membrane to control cell behavior (Edelman and Crossin, 1991; Doherty and Walsh, 1994). Neurons express various CAMs, including several members of the Ig superfamily that also contain fibronectin type III repeats (Grumet, 1991; Rathjen and Jessel, 1991). Most proteins in this family such as Ng-CAM (Grumet, 1992) or its likely mammalian homolog L1 (Schachner et al., 1990) are expressed after neurons become postmitotic. Nr-CAM is closely related to Ng-CAM, consisting of six Ig domains and five fibronectin type III repeats, as well as a single transmembrane region and a cytoplasmic domain (Grumet et al., 1991; Kayyem et al., 1992) that can bind to the cytoskeletal protein ankyrin (Davis and Bennett, 1994). Despite structural similarities between these two proteins, Ng-CAM/L1 is a much more potent promoter of neurite growth than Nr-CAM (Grumet and Sakurai, 1996), whereas Nr-CAM appears to be important for axonal guidance in regions such as the floor plate of the spinal cord (Stoeckli and Landmesser, 1995). In contrast to these transmembrane CAMs, contactin/F11/F3, which contains six Ig domains and four fibronectin type III repeats, is anchored in the plasma membrane by a glycophosphatidylinositol (GPI) linkage (Ranscht, 1988; Brummendorf et al., 1989; Gennarini et al., 1989). Although contactin lacks a cytoplasmic domain, it has been implicated in transmembrane signaling in neurons (Pesheva et al., 1993), most likely by associating with transmembrane and cytoplasmic proteins (Olive et al., 1995; Zisch et al., 1995).
Phosphorylation of proteins inside cells on tyrosine is an important mechanism for mediating transmembrane signaling that is regulated by the balanced actions of protein tyrosine kinases and protein tyrosine phosphatases (Schlessinger and Ullrich, 1992). Receptor-like protein tyrosine phosphatase β (RPTPβ; Krueger and Saito, 1992; Levy et al., 1993) is expressed primarily in the nervous system and is synthesized by glial progenitors, radial glial cells, and astrocytes (Canoll et al., 1993; Milev et al., 1994; Engel et al., 1996; Meyer-Puttlitz et al., 1996; Sakurai et al., 1996). It consists of a carbonic anhydrase domain (C) in its NH2-terminal region, followed by a fibronectin type III repeat (F), a long cysteine-free spacer region (S) in its extracellular region, and two phosphatase domains in its intracellular part (Barnea et al., 1994b ; Maurel et al., 1994). There are three splicing isoforms of RPTPβ, one that is secreted and two that are membrane bound, which differ by a sequence of 860–amino acid residues. Proteins corresponding to all three forms have recently been detected in the developing nervous system and in glioma cell lines (Sakurai et al., 1996). The secreted form, also named phosphacan (Rauch et al., 1991; Maurel et al., 1994), as well as the long receptor form, are chondroitin sulfate proteoglycans (Barnea et al., 1994a ; Shitara et al., 1994), while the short receptor form is detected primarily without glycosaminoglycan (Sakurai et al., 1996). Receptor forms are expressed on glial progenitors and radial glial cells, while the soluble form is detected in developing fiber tracts associated with more mature astroglia (Canoll et al., 1993; Milev et al., 1994; Canoll et al., 1996). The expression of receptor forms changes moderately during neural development, while expression of the soluble form increases dramatically (Meyer-Puttlitz et al., 1995; Sakurai et al., 1996).
We have previously identified contactin as a neuronal receptor for the C domain of RPTPβ, raising the possibility that the interaction between contactin and RPTPβ may generate bidirectional signals between neurons and glia (Peles et al., 1995). All three isoforms of RPTPβ contain the C domain, suggesting that they all interact with contactin. We also showed that the soluble proteoglycan form of RPTPβ, phosphacan, can bind to neurons and inhibits neurite outgrowth in culture (Milev et al., 1994). This binding was shown to be mediated, at least in part, by Ng-CAM and N-CAM (Milev et al., 1994). Thus, it is reasonable to hypothesize that neuron–glia interactions mediated by the different isoforms of RPTPβ involve different CAMs and receptors on neurons.
To gain further insight into the function of the individual isoforms of RPTPβ on cell interactions, we began a functional analysis of the short receptor form, which is the major receptor form expressed on astrocytes and glioma cells (Canoll et al., 1996; Sakurai et al., 1996). Astrocytes can support neurite outgrowth and modulate neuronal differentiation, and our data suggest that some of these activities may be mediated by specific interactions of RPTPβ on astrocytes with contactin and Nr-CAM expressed on neurons.
Materials and Methods
Cells
COS7 cells, 293 cells, and an NIH-3T3 subline, 2.2 cells, which do not express endogenous EGF receptors (Honegger et al., 1987), were maintained in DME medium (Biowhittaker, Inc., Walkersville, MD) with 10% FCS (GIBCO BRL, Gaithersburg, MD). The βCFS/EK chimera construct, which has the extracellular region of RPTPβ short form fused with the intracellular region of the EGF receptor kinase, was retrovirally infected into 2.2 cells, and stable transformants were established by G418 selection (Peles et al., 1995). For transient expression of βCFS/EK, COS7 cells were transfected with the cDNA using lipofectamine reagent (GIBCO BRL) and used for analysis 72 h after transfection. IMR32 cells, a human neuroblastoma cell line, were cultured as described (Peles et al., 1995). Primary chick tectal neurons were prepared from embryonic day 9 chick embryos, and rat cerebellar cells and cortical cells were prepared from postnatal 2–4-d-old rats (Grumet and Edelman, 1988).
Proteins and Antibodies
Chick Ng-CAM and N-CAM were purified from membrane fractions of embryonic day 14–16 chick embryo brains using immunoaffinity chromatography columns as described (Grumet and Edelman, 1988); Nr-CAM was purified from the same extract using the anti–Nr-CAM monoclonal antibody, C3B23A7 (generously provided by Dr. Jeffrey Denburg, University of Iowa, Iowa City, IA; Denburg et al., 1995). Rat L1 was purified from membrane fractions of postnatal 7 d rat brains (Friedlander et al., 1994). Polyclonal antibodies against N-CAM, Ng-CAM (Grumet and Edelman, 1988), Nr-CAM (Grumet et al., 1991), and their Fab′ fragments were prepared as described. Purified human contactin and anti–human contactin polyclonal antibody were prepared as described (Reid et al., 1994). Fab′ fragments of rabbit antibodies and monoclonal antibodies against chick contactin were generously provided by Dr. Fritz Rathjen (Max-Delbrueck-Centrum for Molecular Medicine, Berlin-Buch, Germany; Rathjen et al., 1987).
Human Fc Fusion Proteins
Recombinant human Fc fusion proteins of the extracellular regions of RPTPβ were expressed in either COS7 cells (for transient transfection) or 293 cells (for stable) and purified from culture supernatants using protein A beads (Zymed Labs., Inc., S. San Francisco, CA). βC (amino acids from 1 to 313), βCF (amino acids from 1 to 415), and βF (amino acids from 301 to 414) fusion proteins were prepared as described (Peles et al., 1995). cDNAs encoding βCFS (amino acids from 1 to 630), βFS (amino acids from 301 to 630), and βS (amino acids from 415 to 630) were synthesized by PCR and ligated with cDNA of human Fc portion in pCDM8 vector in frame. Nucleotide sequences were confirmed by sequencing. Protein concentrations were determined by Western blotting using anti–human Fc antibody (Jackson ImmunoResearch Labs., Inc., West Grove, PA) with kit-Fc fusion protein as a control. Human contactin–Fc fusion protein was prepared from culture supernatant of COS7 cells transiently transfected with human contactin-Fc cDNA using lipofectamine reagent (Peles et al., 1995).
Neurite Outgrowth Assay
Neurite outgrowth assays were performed using 35-mm petri dishes coated with different protein substrates (Friedlander et al., 1994). Chick primary tectal neurons (5 × 104 cells) or IMR32 cells (2.5 × 104 cells) were incubated for 48 h in DME/F12 medium (Biowhittaker, Inc.) supplemented with ITS+ (Collaborative Research Inc., Lexington, MA) and fixed with Hanks/balanced salt solution (GIBCO BRL) /2% paraformaldehyde/3% sucrose. Treatment with phospho-inositol specific phospholipase C (PIPLC; generous gift of Dr. J. Salzer, New York University Medical Center, New York) was performed as described (Peles et al., 1995). When antibodies were used, they were added to cultures 3 h after seeding the neurons. After fixation, pictures were taken using a microscope (Diaphot; Nikon Inc., Melville, NY), and neurite lengths were measured on the pictures. Neurites that were more than one cell diameter were measured, and only the longest neurite for each cell was considered. More than 100 cells for each substrate were quantified. Data were analyzed with Microsoft Excel, and the percentage of neurons with neurites longer than a given length was plotted in Figs. 3, 4, and 8.
Figure 3.
Inhibition by anti-contactin antibodies of neurite outgrowth induced by βCFS. Primary chick tectal cells were plated on dishes coated with βCFS fusion protein (80 μg/ml) and cultured for 48 h in the presence of Fab′ fragments (500 μg/ml) of nonimmune (A) or anti-contactin polyclonal antibody (B). The plates were fixed and photographed. Quantification of lengths of tectal cell neurites on βCFS fusion protein (C) in the presence of 100 μg/ml Fab′ fragments of nonimmune and anti-contactin polyclonal antibodies, or no added Fab′. The percentage of neurons with neurites longer than a given length in μm was determined as described in Materials and Methods. The average neurite lengths without Fab′ were 67 ± 8 μm (n = 100), with normal rabbit 73 ± 7 μm (n = 57), and with anti-contactin 16 ± 2 μm (n = 82). This level of anti-contactin produced maximal inhibition insofar as higher levels of anti-contactin did not yield greater levels of inhibition; the average lengths at 300 and 500 μg/ml were 22 ± 2 (n = 99) and 25 ± 3 μm (n = 119), respectively. Bar, 100 μm.
Figure 4.
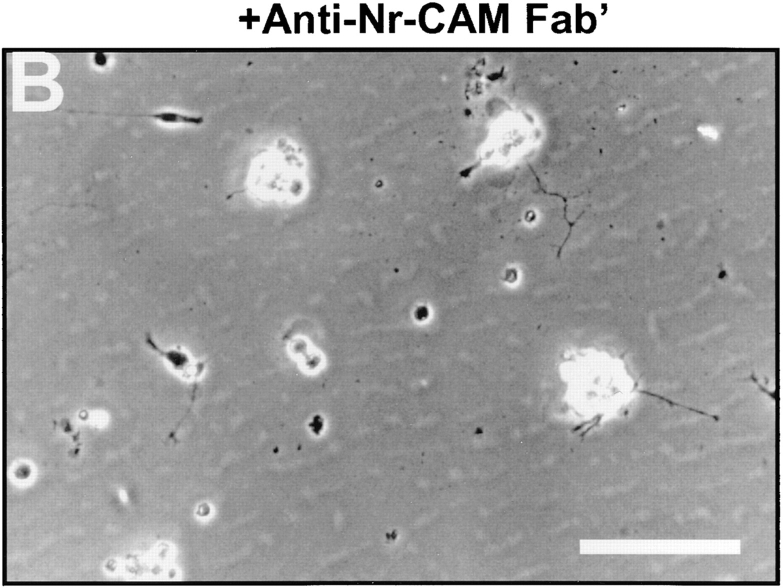
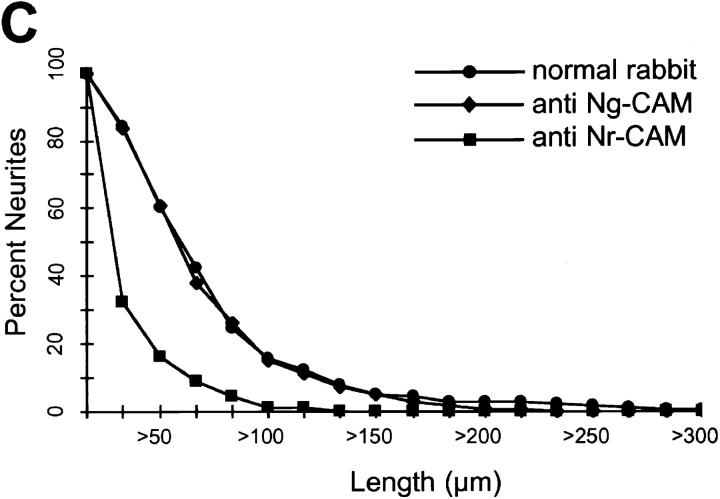
Inhibition by anti– Nr-CAM antibodies of neurite extension induced by βCFS. Primary chick tectal cells were plated on dishes coated with βCFS fusion protein (80 μg/ml) and cultured for 48 h in the presence of Fab′ fragments (500 μg/ml) of nonimmune (A) or anti– Nr-CAM polyclonal antibody (B). The plates were fixed and photographed. Quantification of lengths of tectal cell neurites on βCFS fusion protein (C) and on Ng-CAM (D) in the presence of Fab′ fragments. The average neurite lengths on βCFS (C) treated with Fab′ were 59 ± 4 μm (n = 193) for normal rabbit, 55 ± 2 μm (n = 302) for anti–Ng-CAM, and 16 ± 3 μm (n = 93) for anti–NrCAM. The average neurite lengths on Ng-CAM (D) treated with Fab′ were 83 ± 5 μm (n = 198) for normal rabbit, 34 ± 2 μm (n = 118) for anti–Ng-CAM, and 80 ± 5 μm (n = 187) for anti–Nr-CAM. The percentage of neurons with neurites longer than a given length in micrometers was determined as described in Materials and Methods. The results of one representative experiment are shown, and similar results were obtained in five different experiments. Anti–Nr-CAM polyclonal antibody, inhibited neurite outgrowth on Nr-CAM substrate completely at the concentration of 500 μg/ml (data not shown). Bar, 100 μm.
Figure 8.

βS induces neurite growth when combined with βCF. Primary chick tectal cells (5 × 104 cells) were plated on purified βCFS, βCF, βS (40 μg/ml), or on combinations (40 μg/ml final concentration) of the latter two as noted in the right panels of the figure. After 48 h, cultures were fixed and photographed. The graph shows quantification of the lengths of neurites on βCF and βCFS and on mixtures of βCF + βS at different ratios. Neurite lengths were measured and analyzed as described in Materials and Methods. The average neurite lengths were: βCFS, 73 ± 7 μm (n = 109); βCF, 26 ± 2 μm (n = 148); 1X[CF] + 3X[S], 67 ± 6 μm (n = 161); 2X[CF] + 2X[S], 39 ± 3 μm (n = 153); and 3X[CF] + 1X[S], 27 ± 2 μm (n = 192). Representative data are shown, and similar results were obtained in three experiments. Bar, 100 μm.
Immunofluorescence
Tectal neurons that adhered and extended processes on substrates coated with βCFS, or with 10 μg/ml polylysine followed by 40 μg/ml laminin (Becton Dickinson, Bedford, MA), were fixed as described above, treated with PBS/100 mM glycine, and blocked with PBS/2% goat serum. Double staining was performed using 30 μg/ml monoclonal anti-F11 (Rathjen et al., 1987) and 1:100 dilution of polyclonal anti–Nr-CAM antiserum (Grumet et al., 1991) for 1 h, followed by treatment for 1 h with 1:250 dilutions of lissamine-labeled anti–mouse Ig and fluorescein labeled anti–rabbit Ig (Jackson ImmunoResearch Labs., Inc.). After washing, fluorescence for lissamine and fluorescein was visualized in a microscope (Diaphot; Nikon Inc.) and recorded with appropriate filters using a Sony CCD camera interfaced with a frame grabber (AG-5; Scion Corp., Frederick, MD) in a PowerMacintosh 7100 computer.
Protein Binding Assay
1 μl of various proteins (10 μg/ml in PBS) was incubated on 35-mm petri dishes for 1 h. The dishes were washed with PBS and blocked with 1% BSA for 1 h and then incubated with culture supernatant from COS7 cells expressing RPTPβ fusion proteins (0.5–1 μg/ml) for 1 h. After washing the dishes with PBS for 5 min, the proteins were fixed by treatment with 3% paraformaldehyde/PBS. To visualize the bound fusion protein, incubations were performed with biotinylated anti–human Fc followed by avidin conjugated with alkaline phosphatase and the alkaline phosphatase substrate, according to the manufacturer's specifications (Vector Labs, Inc., Burlingame, CA). All incubations were performed at room temperature. Reaction products are seen as blue/black deposits on the petri dish and were scanned using Silverscan III, analyzed with Adobe photoshop and NIH Image 1.54 software. Anti–human Fc antibody was spotted and detected as a control substrate.
DNA Constructs, Transfection, and Co-precipitation Studies
Full length chick Nr-CAM cDNA was constructed from λgt 11 clones (Mauro et al., 1992) using Bluescript II KS (Stratagene, La Jolla, CA) as a cloning vector; the cDNA was inserted into pCMP1 using BamHI and EcoRV. This cDNA encodes an isoform of Nr-CAM lacking amino acids, inserted after the second Ig domain and half of the fifth fibronectin type III repeat as a result of alternative RNA splicing (Grumet et al., 1991). Human contactin cDNA was cloned into pCMP1 (Peles et al., 1995).
Nr-CAM or contactin cDNAs were transfected into COS7 cells using lipofectamine. After 72 h, the cells were lysed in extraction buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 20 mM PMSF, 10 μg/ml leupeptin, 0.1 M NaF, 10 mM sodium tetraphosphates, and 0.6 mM sodium vanadate) and cleared for 30 min in a microfuge. For the mixtures of the individual cell lysates, separate cultures of COS7 cells transfected to express Nr-CAM or contactin were extracted and then mixed in a 1:1 ratio. For experiments involving mixtures of cells expressing either Nr-CAM or contactin, the cells were removed from tissue culture dishes with 0.25% trypsin and 1 mM EDTA (GIBCO BRL) 24 h after transfection and combined in new dishes at a 1:1 ratio. After incubation for an additional 48 h, the cultures were extracted as described. In all cases, protein was precipitated by protein A that had been preincubated with antibodies against Nr-CAM or contactin, or with Fc fusion proteins.
Coprecipitation of Nr-CAM with contactin from detergent extracts of E14 chick embryo brain membranes was performed using Pansorbin (Calbiochem Corp., La Jolla, CA; Zisch et al., 1995); binding of Fc fusion proteins (5 ml of ∼1 μg/ml) was performed with 100 μl of a 50% suspension of Pansorbin in PBS, and the complexes were washed with HNTG buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, and 10% glycerol). The immunoprecipitates were resolved on SDS-PAGE, immunoblotted with antibodies, and analyzed with the ECL detection system (NEN Dupont, Wilmington, DE; Peles et al., 1995).
Binding of various Fc fusion proteins to COS7 cells transiently transfected with contactin or Nr-CAM was measured by scintillation counting after incubation with 125I-protein A (Peles et al., 1995).
Neurite Outgrowth on Transfected Cell Monolayer
Monolayer cultures of βCFS/EK transfectant and parental 2.2 cells were grown to confluence in DME medium with 10% FCS on 2.5-mm wells of 24-well slides (Cel-line Assoc., Inc., The Sea Ranch, CA) coated with polylysine and fibronectin (Friedlander et al., 1989). After overnight culture, each well was gently washed with DME medium/F12/ITS+, and dissociated primary tectal neurons (1,000 cells/10 μl) were added. After 24 h, the slides were fixed, and the neurons were incubated with anti–Ng-CAM polyclonal antibodies followed by biotinylated anti–rabbit IgG (Vector Labs, Inc.) and avidin–rhodamine (Molecular Probes, Inc., Eugene, OR) and visualized by fluorescence microscopy. Neurite outgrowth was measured on photographs and analyzed as described.
Results
βCFS Protein Induces Neurite Outgrowth and Neuronal Differentiation
To analyze functions of the extracellular region of RPTPβ, we made several recombinant Fc fusion proteins containing different subdomains of the extracellular region of RPTPβ (Fig. 1). We showed previously that the carbonic anhydrase domain (βC) of RPTPβ alone or together with the fibronectin type III repeat (βCF) induces adhesion and neurite growth of primary chick neurons and IMR32 neuroblastoma cells (Peles et al., 1995). Similarly, a fusion protein called βCFS, containing most of the extracellular region of the short receptor form of RPTPβ including the S region (Fig. 1), also promoted adhesion of chick primary neurons. The number of neurons that adhered to βCFS was, however, lower than those that adhered to βCF substrates (Table I). Remarkably, the average length of neurites on βCFS (73 ± 7 μm; n = 110) was much longer than those on βC and βCF (26 ± 2 μm; n = 149) and resembled the morphology of neurites induced on Ng-CAM, which on average were longer (118 ± 7 μm; n = 165; Fig. 2). In contrast, neurons extended short thick processes on βC (Peles et al., 1995) and βCF fusion proteins, as well as on anti–Ng-CAM antibodies (average length of 48 ± 2 μm; n = 110) and on Nr-CAM (Grumet and Sakurai, 1996; Fig. 2). Fc fusion proteins with βF and βS (see Fig. 8) did not promote neuronal adhesion or neurite growth, and an Fc fusion protein of the extracellular region of MCK10 containing the discoidin I domain (Alves et al., 1995) supported neuronal adhesion but not neurite growth (data not shown), indicating that the Fc region itself does not significantly affect neuronal adhesion and neurite growth.
Figure 1.
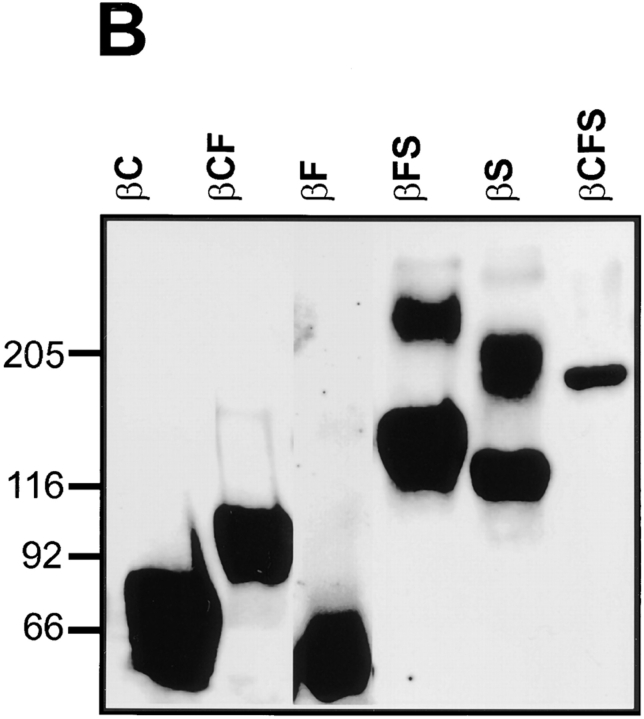
Fusion proteins representing extracellular region of RPTPβ. (A) Schematic representations of the short form of RPTPβ and different subdomains used to construct fusion proteins with human IgG-Fc. The short receptor form (the deletion variant) consists of an NH2-terminal carbonic anhydrase domain (C), fibronectin type III repeat (F), a spacer region (S), and cytoplasmic protein tyrosine phosphatase domains (PTP). Vertical bar represents the transmembrane region (TM). (B) Expression of the chimeric IgG molecules in COS cells. Various RPTPβ fusion proteins containing different combinations of C, F, and S regions as illustrated in A, were purified, separated on an SDS gel, and immunoblotted with antibodies against human IgG. The diffuse higher molecular weight components in βFS and βS probably represent proteoglycans insofar as they were not observed after chondroitinase treatment (Sakurai et al., 1996), indicating that the short form of RPTPβ is a “part time” proteoglycan. Molecular weight makers are shown in kD.
Table I.
Binding of Neurons to βCFS and βCF
| Fc fusion protein | Cells bound | |
|---|---|---|
| βCF | 343 ± 56 | |
| βCFS | 89 ± 2 |
Chick primary tectal neurons (5 × 104 cells) were incubated for 1.5 h on dishes coated with Fc fusion proteins at 80 μg/ml. The number of cells bound/0.26 mm2 was counted under phase microscopy, and the numbers represent averages ± SE. Similar results were obtained in five independent experiments.
Figure 2.
Extracellular regions of RPTPβ promote neuronal adhesion and neurite outgrowth. Primary chick tectal cells were plated on purified βCFS or βCF fusion proteins (80 μg/ml), Ng-CAM (20 μg/ml), and anti-Ng-CAM monoclonal antibody 4B9 (100 μg/ml of IgG) and incubated for 48 h. After fixation with paraformaldehyde, photographs were taken. In each case the average length of neurites was measured (see text). Bar, 100 μm.
Studies using βC and βCF indicated that the carbonic anhydrase domain of RPTPβ binds to the GPI-linked neuronal cell recognition molecule contactin (Peles et al., 1995). We have confirmed that βCFS also binds to contactin and induces neurite growth (Figs. 2 and 3). As shown previously on βC-coated substrates (Peles et al., 1995), when chick tectal neurons were pretreated with PI-PLC, neuronal adhesion and neurite outgrowth on βCFS was reduced dramatically (data not shown), indicating that these activities involve GPI-linked molecules on the neuronal cell surface. To verify the involvement of the GPI-linked protein contactin, we tested the effect of antibodies on neurite growth after allowing chick tectal neurons to adhere to substrates coated with βCFS. Neurite growth was inhibited by Fab′ fragments of polyclonal antibodies against chick contactin (Fig. 3). βCFS also promoted adhesion of human neuroblastoma IMR32 cells which express contactin, and anti–human contactin antibodies inhibited neuronal adhesion and neurite growth on substrates coated with βC (Peles et al., 1995) and βCFS (data not shown). These results indicate the involvement of contactin in neuronal adhesion and neurite growth induced by βCFS.
Neurite Outgrowth Promoted by βCFS Is Inhibited by Anti–Nr-CAM Antibodies
The striking observation that neurite outgrowth on βCFS differs from that seen on βC and βCF provided a clue that molecules other than contactin may also be involved in neurite outgrowth on RPTPβ. Indeed, we showed previously that Ng-CAM and N-CAM (Milev et al., 1994) as well as Nr-CAM (Milev et al., 1996) bind to phosphacan, the soluble form of RPTPβ. Interestingly, Ng-CAM and Nr-CAM can also bind to contactin (Brummendorf et al., 1993; Morales et al., 1993). Therefore, we investigated whether any of these CAMs are also involved in the promotion of neurite outgrowth by RPTPβ. Whereas polyclonal antibodies against Ng-CAM and N-CAM had no effect on neurite outgrowth on βCFS substrates, anti–Nr-CAM antibodies inhibited it (Fig. 4). Quantitation of neurite outgrowth from tectal cells on βCFS substrates revealed strong inhibition by anti–Nr-CAM antibodies, whereas the effects of anti–N-CAM (data not shown) and anti–NgCAM antibodies were negligible (Fig. 4 C). In control experiments, anti–Ng-CAM antibodies inhibited neurite outgrowth on Ng-CAM, as expected, whereas anti–Nr-CAM antibodies did not (Fig. 4 D).
The results implicate Nr-CAM as well as contactin in neurite outgrowth of chick tectal neurons induced by RPTPβ. Consistent with this idea, we found that the majority of neurons that adhered to and extended neurites on βCFS expressed both Nr-CAM and contactin (Fig. 5). Most tectal neurons were stained positively for Ng-CAM, while only a few of these cells were Nr-CAM and contactin positive when cultured on substrates coated with laminin and polylysine (data not shown). These results suggest that the majority of tectal neurons is not stained with antibodies against Nr-CAM and contactin, and a subpopulation of tectal neurons that adhere selectively to βCFS are NrCAM and contactin positive.
Figure 5.
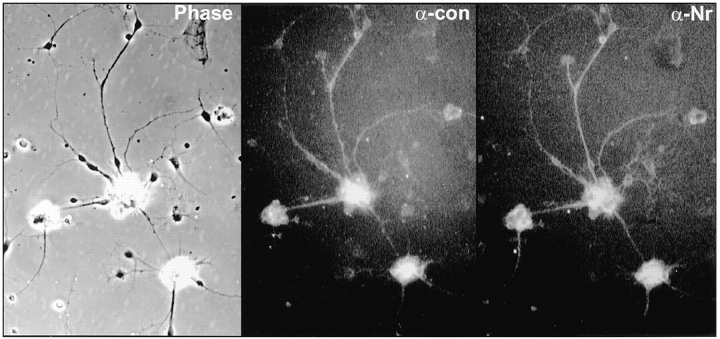
Coexpression of Nr-CAM and contactin on tectal neurons. Primary chick tectal cells were isolated on dishes coated with βCFS fusion protein, as described in the legend to Fig. 3, and stained by immunofluorescence with monoclonal antiF11 (α-con) and polyclonal anti–Nr-CAM antibodies (αNr) as described in Materials and Methods. Note that the majority of cells that bound to βCFS were stained by both antibodies to contactin and Nr-CAM.
Nr-CAM and Contactin Bind to Distinct Domains in RPTPβ
In view of the interactions of RPTPβ/phosphacan with NgCAM, N-CAM, and Nr-CAM, it was of interest to define the binding properties of its different extracellular domains. We showed previously that the C domain of RPTPβ contains a binding site for contactin (Peles et al., 1995). A solid phase protein binding assay was used to analyze binding of various combinations of extracellular domains of RPTPβ as Fc fusion proteins (Fig. 6). βC as well as βCF bound to contactin but not to Ng-CAM, N-CAM, NrCAM, fibronectin, or laminin. βCFS binding was detected to contactin, Nr-CAM, Ng-CAM, and N-CAM, confirming our previous findings that RPTPβ binds to these CAMs. Moreover, βS (containing only the S domain) alone or in combination with the fibronectin type III repeat of RPTPβ (i.e., βFS) also bound to Nr-CAM, Ng-CAM, and N-CAM but not to contactin, laminin, or fibronectin. These adhesion molecules did not bind βF, which consists of only the fibronectin type III repeat. These data suggest that different domains of RPTPβ interact with different CAMs; the C domain binds to contactin while the S domain interacts with Nr-CAM, Ng-CAM, and N-CAM.
Figure 6.
Binding of domains in RPTPβ to various adhesion molecules. Proteins (10 μg/ml) were coated on dishes and incubated with different RPTPβ fusion proteins (0.5–1 μg/ml). Binding of the Fc fusion proteins was detected by biotinylated anti–human Fc antibody, followed by streptavidin-alkaline phosphatase, and visualized by NBT/BCIP alkaline phosphatase substrates. Only fusion proteins containing the S domain bound to Ng-CAM (Ng), Nr-CAM (Nr), and N-CAM (N), while fusion proteins containing the C domain bound to contactin (Con). None of the fusion proteins tested bound to laminin (Lm) or fibronectin (Fn). Similar results were obtained in three independent experiments.
βCFS Binds to a Complex of Contactin with Nr-CAM
Given that tectal neurite outgrowth on RPTPβ involves both contactin and Nr-CAM, it was of interest to study interactions among these proteins. To analyze the interaction of RPTPβ with contactin and Nr-CAM when these CAMs are expressed on the cell surface, we transfected COS7 cells with plasmids encoding these proteins alone or in combination. The Fc fusion proteins βCF and βCFS both bound to cells expressing contactin (Table II). No significant change in binding was detected when Nr-CAM was coexpressed with contactin. In control experiments, γCF, an Fc fusion protein containing C and F domains of RPTPγ (Barnea et al., 1993), exhibited no binding. Negligible binding was also observed with the βFS fusion protein (Table II), and with βF and βS, containing only the F and S domains of RPTPβ, respectively (data not shown). The results indicate that the C domain of RPTPβ is important for binding to contactin when expressed on COS7 cells in the absence or presence of Nr-CAM. Although the S domain bound to purified Nr-CAM in the solid phase assay (Fig. 6), no interaction was detected when Nr-CAM was expressed on cells, suggesting that binding of RPTPβ to cells expressing contactin and Nr-CAM is mediated primarily through contactin and not through Nr-CAM.
Table II.
Binding of βCF and βCFS Fc–Fusion Proteins to Cells Expressing Contactin and Nr-CAM
| Transfected cDNAs | Fusion proteins | |||||||
|---|---|---|---|---|---|---|---|---|
| βCF | βCFS | βFS | γCF | |||||
| None | 153 ± 7 | 293 ± 25 | 132 ± 9 | 215 ± 1 | ||||
| Contactin | 3,406 ± 6 | 1,357 ± 46 | 133 ± 8 | 204 ± 27 | ||||
| Nr-CAM | 250 ± 20 | 256 ± 69 | 268 ± 57 | 223 ± 2 | ||||
| Contactin + Nr-CAM | 2,929 ± 17 | 1,641 ± 246 | 164 ± 1 | 139 ± 31 | ||||
COS7 cells transfected with cDNAs of contactin, Nr-CAM, or both were incubated with conditioned media containing equal amounts of Fc fusion proteins (0.5 μg/ml), and bound proteins were detected after incubation with 125I-protein A. Numbers indicate cpm, and the average of duplicate experiments ± SE are shown. γCF is an Fc- fusion protein containing the carbonic anhydrase domain and fibronectin type III repeat of RPTPγ which is closely related with RPTPβ (Barnea et al., 1993). Similar results were obtained in three independent experiments.
Previous studies using immobilized forms of Nr-CAM showed that it binds to contactin/F11 (Morales et al., 1993). Using solid phase protein binding assays, we confirmed that a contactin Fc chimera binds to Nr-CAM (Fig. 7 A). Ng-CAM has also been found to bind to contactin (Brummendorf et al., 1993), however, we found that the contactin fusion protein bound to Nr-CAM but not to Ng-CAM, L1, and N-CAM.
Figure 7.
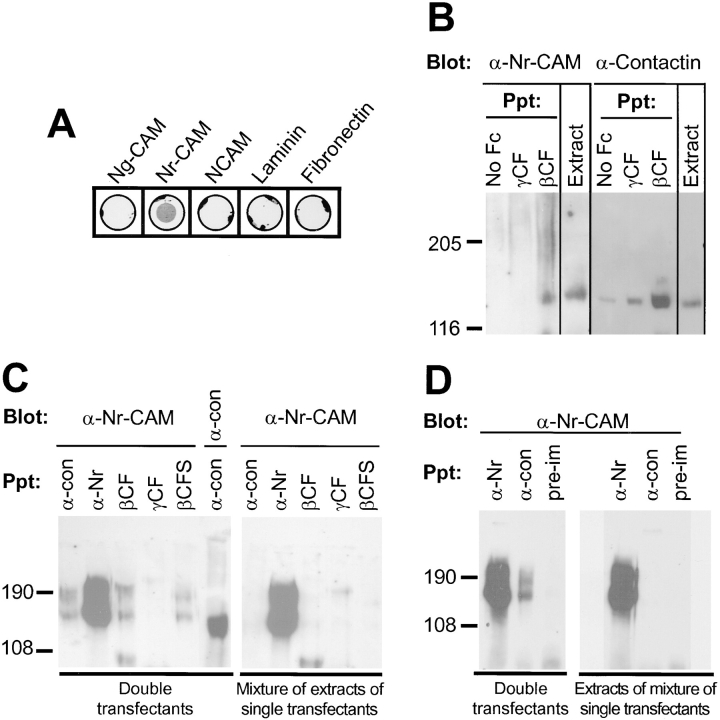
Interactions among contactin, Nr-CAM, and RPTPβ. (A) Binding of human contactin–Fc fusion protein to adhesion molecules was detected as described in Fig. 6. Contactin–Fc fusion protein bound most strongly to Nr-CAM but not to other purified CAMs, including rat L1 (data not shown). (B) Coprecipitation of Nr-CAM with contactin. Proteins were precipitated (Ppt) from detergent extracts of E14 chick embryo brain membranes (extract) with Pansorbin that had been incubated with culture medium (no Fc) as a control, or with conditioned medium containing βCF or γCF Fc fusion proteins. Precipitated proteins were resolved on 7.5% SDS-PAGE and immunoblotted with polyclonal antibodies against chick Nr-CAM and monoclonal antibodies against chick F11/contactin. The specificity of the anti– Nr-CAM and anti-contactin antibodies was demonstrated in immunoblots of the unfractionated brain extracts which revealed species of ∼140 and 130 kD, respectively. Note that strong signals for Nr-CAM and contactin were only seen in association with βCF. By comparison with the βCF precipitates, much lower levels of contactin were consistently found in the controls with no Fc and with γCF, indicating the nonspecific level of binding of contactin to Pansorbin. (C) COS7 cells were transiently transfected with cDNAs of human contactin and chick Nr-CAM alone (single transfectants) or in combination (double transfectants). After 72 h, the cells were lysed and extracts were precipitated with protein A beads coated with anti-contactin (α-con) or anti– Nr-CAM (α-Nr) antibodies, or with Fc fusion proteins with βCF, γCF, and βCFS, as indicated. The eluted proteins were resolved on SDS-PAGE and immunoblotted (Blot) with polyclonal antibodies against chick Nr-CAM and human contactin. Contactin was detected as a 130-kD protein, and Nr-CAM appeared as two species of ∼190 and 140 kD representing the full length protein and its major cleavage product (Kayyem et al., 1992). (D) COS7 cells transfected as described in C, with plasmids encoding either Nr-CAM or contactin, were released from the dishes with trypsin and cocultured on tissue culture dishes for 48 h, and then extracted. The extracts were immunoprecipitated using antibodies against Nr-CAM or contactin or with preimmune (pre-im) sera and immunoblotted with antibodies against Nr-CAM. Note the presence of Nr-CAM in the anti-contactin precipitates only in the double transfectants. Molecular weight markers are shown in kD.
To determine whether contactin interacts with Nr-CAM in nervous system tissue during development, we analyzed these proteins in membrane extracts prepared from embryonic day 14 chick brains following precipitation with Fc fusion proteins. Immunoblotting with specific antibodies showed that Nr-CAM and contactin coprecipitated with βCF but not with γCF used as a control (Fig 7 B). Insofar as βCF binds to contactin but not directly to Nr-CAM, these results suggest that Nr-CAM can bind to contactin.
To distinguish whether contactin can interact with NrCAM laterally on the same cell or between apposing cells, we used the transient transfection strategy described above (Table II). When COS7 cells were double transfected with cDNAs encoding for contactin and Nr-CAM, both proteins were expressed (Fig. 7 C). Immunoprecipitates of cell extracts with antibodies against contactin were found to contain Nr-CAM, as detected by immunoblotting with anti-Nr-CAM antibodies. When Fc fusion proteins were linked to protein A beads, Nr-CAM coprecipitated with βCF and βCFS Fc fusion proteins but not with the γCF Fc fusion protein. These results confirm an interaction between Nr-CAM and contactin and suggest that a complex of these proteins in cells binds specifically to extracellular regions of RPTPβ.
The interaction between Nr-CAM and contactin was only detected when both proteins were expressed in the same cells and not when mixtures were prepared from cells transfected with each protein individually. When extracts of COS7 cells individually transfected to express either Nr-CAM or contactin were mixed and analyzed by immunoprecipitation and immunoblotting, Nr-CAM did not coprecipitate with anti-contactin antibodies or with any of the Fc fusion proteins (Fig. 7 C). In contrast, contactin coprecipitated with βCF and βCFS but not with γCF Fc fusion protein (data not shown), as expected from its ability to bind to the C domain of RPTPβ (Peles et al., 1995). In addition, we could not detect an interaction between Nr-CAM and contactin following coculture for 48 h of mixtures of COS7 cells that had been transfected individually to express either Nr-CAM or contactin (Fig. 7 D). The observation that Nr-CAM coprecipitated specifically with contactin only from extracts of doubly transfected cells suggests that Nr-CAM and contactin interact laterally in the plane of the plasma membrane to form a complex.
Neurite Outgrowth Promoted by RPTPβ Involves the S Region
Although βCF bound to contactin and could coprecipitate Nr-CAM as a complex (Fig. 7), βCFS was more potent than βCF in promoting neurite growth (Fig. 2), suggesting the importance of the S region in neurite growth. This idea was tested directly using an Fc fusion protein with the S region. βS by itself was a very poor substrate for neuronal adhesion, and when cells adhered to substrates coated with βS, they did not extend processes (Fig. 8). Interestingly, when combined with βCF, βS potentiated neurite growth in a dose-dependent manner as determined by measurements of neurite lengths (Fig. 8). The potentiation of neurite growth by βS occurred specifically in combination with the CF domains of RPTPβ and not with other adhesive substrates including laminin, fibronectin, and antibodies against Ng-CAM. For example, the S domain was found to be slightly inhibitory for neurite outgrowth on substrates coated with 4B9 monoclonal antibody to NgCAM reducing the average outgrowth from 48 ± 2 μm (n = 149) to 40 ± 6 μm (n = 106). The ability of the S region to potentiate neurite extension was only observed in combination with the C domain.
Fibroblasts Expressing βCFS Promote Neurite Outgrowth and Neuronal Differentiation
Given that extracellular regions of the short form of RPTPβ promote neurite outgrowth when presented as substrates, it was of interest to test their effects as cell surface proteins. For this purpose, we expressed on NIH3T3 cells a chimeric receptor protein consisting of extracellular domains of RPTPβ and intracellular regions of EGF receptor kinase called βCFS/EK. Initial experiments using transiently transfected cells indicated that βCFS/EK promoted neurite growth. Therefore, stable transfectants were isolated and their expression of the chimeric protein was confirmed by immunoblotting (data not shown). Chick tectal neurons cultured on monolayers of stable βCFS/EK transfectants for 24 h had on average longer neurites than neurons found on monolayers of the parental cells (Fig. 9). Moreover, on the βCFS/EK transfectant, some cells developed very long neurites that were >300 μm in length, while such highly differentiated tectal neurons were never seen on the parental cells. Similar results were observed using COS7 cells stably transfected with βCFS/EK. Fab′ fragments of antibodies against Nr-CAM inhibited neurite growth on βCFS/EK transfectants, indicating a role for Nr-CAM in neurite growth for these tectal cells (data not shown). The cell bodies of these differentiated neurons were large and oval shaped (Fig. 9, A–C), but we could not definitively identify these cells because molecular markers for distinguishing tectal neurons are not available. In contrast to the tectal neurons, cerebellar neurons did not extend long neurites on the βCFS/EK transfectant (data not shown), suggesting that different types of neurons respond differently to the short form of RPTPβ, which is the major receptor form expressed on astrocytes (Sakurai et al., 1996).
Figure 9.
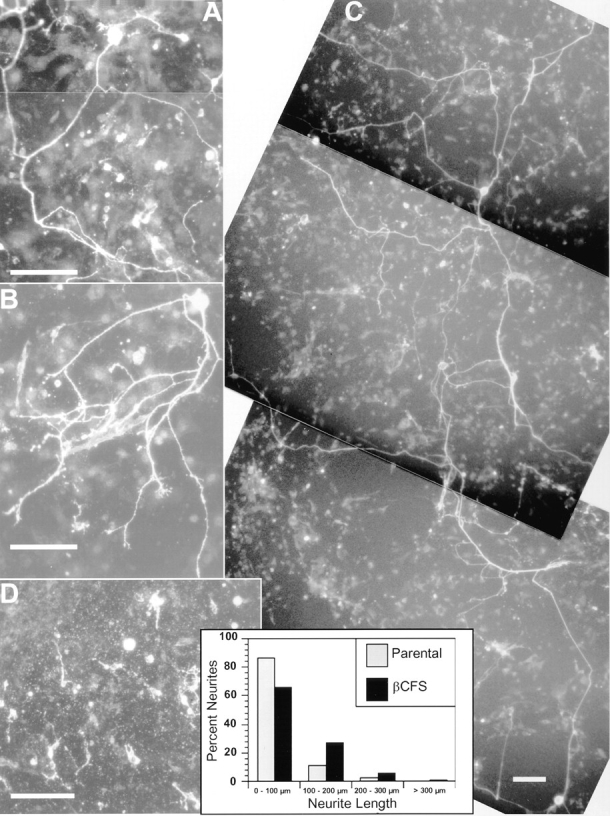
βCFS transfectants promote neurite outgrowth and differentiation of chick primary neurons. Chick tectal cells (1,000 cells/ well) were cultured for 24 h on confluent monolayers of 2.2 fibroblasts stably transfected with the βCFS/EK chimera (A–C) or on the parental cells (D). After fixation, neurons were stained with anti–Ng-CAM polyclonal antibodies; staining with anti–Nr-CAM antibody was essentially the same as that observed for anti–NgCAM antibody (data not shown). On monolayer of the βCFS/EK chimera (A–C) but not on the parental cells (D), 5–10 cells/well had long neurites. Note that neurons with long processes have large, oval-shaped cell bodies. Neurite lengths were measured on monolayers of the parental cells and the cells expressing βCFS/EK, as described in Materials and Methods, and plotted as histograms. The average neurite lengths were 46 ± 5 (n = 73) on the parental cells and 82 ± 6 (n = 172) on the βCFS/EK transfectants. Bars, 100 μm.
Discussion
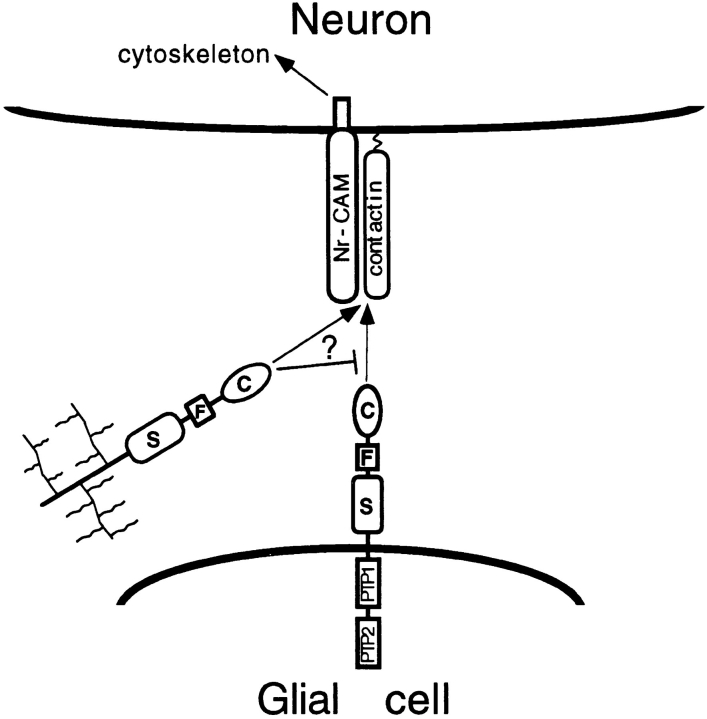
A major conclusion of this study is that the extracellular region of the short receptor form of RPTPβ (βCFS) is a potent promoter of neurite growth acting through the neuronal CAMs, contactin and Nr-CAM. These CAMs are expressed on overlapping subsets of developing axons, and the combined observations have led us to hypothesize that they form a complex in a subset of neurons by interacting laterally in the same plasma membrane (Fig. 10). βCFS can bind to the contactin–Nr-CAM complex, and this interaction is critically dependent on the binding of contactin to the C domain. In addition, maximal neurite growth induced by RPTPβ involves the S domain.
Figure 10.
Model of interaction of RPTPβ with contactin and NrCAM. Our results support the hypothesis that contactin and NrCAM bind to each other laterally in the plasma membrane and interact with RPTPβ which is expressed by glia as receptor forms (only the short form is shown) and as a secreted proteoglycan (wavy branches represent glycosaminoglycan chains). The CFS region of RPTPβ induces neurite outgrowth through interaction with contactin and Nr-CAM. Neurite outgrowth might involve signaling mediated by Nr-CAM or other transmembrane receptors. The cytoplasmic region of Nr-CAM binds to ankyrin (Davis and Bennett, 1994) and contains a COOH-terminal SFV amino acid sequence that has been predicted to bind to PDZ domains (Kornau et al., 1995). The secreted form of RPTPβ may also interact with neuronal receptors or inhibit binding of the glial receptor forms.
CAMs were initially characterized as ligands or counter receptors on apposing cells that mediate cell adhesion by homophilic and heterophilic mechanisms (Edelman, 1984). While many CAMs can support cell adhesion and induce neurite growth, adhesiveness is a poor predictor of neurite growth (Lemmon et al., 1992). This has stimulated the hypothesis that in addition to adhesion per se, specific signaling events are involved in CAM-mediated neurite growth (Doherty and Walsh, 1994; Doherty et al., 1995). Accordingly, there may be two different modes of neurite growth: one that is permissive, being adhesion dependent, and the other that involves specific signaling. Ng-CAM/L1 may be particularly complicated because it can act both as a ligand to promote neurite growth (Lagenaur and Lemmon, 1987; Fig. 2) and as a neuronal receptor that mediates signaling (Schachner, 1993). As a substrate, the anti–Ng-CAM antibody appears to act in an adhesion-dependent manner and supports only limited process extension. We found striking morphological similarities between the robust responses of neurons to βCFS and Ng-CAM and the weaker responses to βCF and anti–Ng-CAM antibody (Fig. 2). These results suggest that βCF mediates initial adhesion-dependent processes while βCFS provides additional signals that promote neurite growth and that the S region may be important for the signaling.
Contactin has been implicated as a neuronal receptor that mediates repulsion by a mechanism involving binding of tenascin-R (Pesheva et al., 1993), and RPTPβ functions as a ligand for contactin that promotes neurite outgrowth. Given that contactin is not a transmembrane protein, its ability to modulate neurite growth may be adhesion dependent, but it probably also involves other receptors that can transmit signals to the cytoplasm. Therefore, modulation of neurite outgrowth via contactin in particular, and possibly by CAMs in general, is likely to involve a hierarchy of lateral interactions with other CAMs and/or receptors in the neuronal membrane. Similarly, axonin-1 is another GPI-linked protein that may interact laterally with Ng-CAM to regulate axonal growth in certain neurons (Rader et al., 1996), and both of these CAMs can bind to RPTPβ/phosphacan (Milev et al., 1994, 1996), suggesting that other complexes of neuronal CAMs may also interact with RPTPβ.
The promotion of neurite growth by glial RPTPβ requires interactions with contactin and Nr-CAM, which probably form a complex on the responding neuron (Fig. 10). This activity is dependent on binding of the C domain of RPTPβ to contactin (Peles et al., 1995). The involvement of the S domain was suggested by comparing βCF with βCFS (Fig. 2) and was demonstrated in mixing experiments where it was able to potentiate neurite growth by βCF (Fig. 8). Therefore, we propose that the extracellular region of RPTPβ has at least two distinct domains that are important for neurite growth: the C domain that mediates adhesion-dependent events via contactin and the S domain that is necessary but not sufficient for extension of long neurites, probably by transmembrane signaling via Nr-CAM and possibly other CAMs and/or receptors. Whereas the binding studies using purified proteins indicated an interaction of the S domain of RPTPβ with NrCAM, this may be a weak interaction insofar as it was not detected when Nr-CAM was expressed on cells. The action of the S domain may involve intramolecular interactions with other extracellular domains in RPTPβ that modulate its binding affinity for Nr-CAM. These other domains (i.e., C and F) are not simply acting to promote adhesion because other adhesion molecules could not substitute for their ability to potentiate neurite growth by βS. It is possible that the CF domains interact with the S region of RPTPβ and modulate its conformation and its ability to promote neurite growth. Inasmuch as βCF bound neurons more robustly than βCFS did (Table II), it is unlikely that the S region is important for binding, per se. An excess of βS over βCF was required for maximal neurite growth in the mixing experiments (Fig. 8), possibly because of its low affinity of binding to Nr-CAM alone or in complexes with other membrane proteins such as contactin. In any case, it appears that simultaneous binding of C and S domains to complexes of neuronal receptors may be important for promoting neurite growth.
Both Ng-CAM and Nr-CAM can bind to contactin (Brummendorf et al., 1993; Morales et al., 1993), but only contactin and Nr-CAM have been implicated in neurite growth promoted by the short form of RPTPβ. These results combined with the coprecipitation studies suggest that a lateral interaction between Nr-CAM and contactin (Fig. 10) in certain cells, such as a subset of tectal neurons, may be particularly important for neuronal differentiation and neurite growth that is promoted by RPTPβ. Significant levels of contactin, Nr-CAM, as well as RPTPβ/phosphacan are found during neural development, but it has been difficult to compare their patterns of expression in a single species because of limitations with the available antibodies. The available data suggest that RPTPβ/phosphacan (Milev et al., 1994; Meyer-Puttlitz et al., 1996), contactin/ F11/F3 (Ranscht, 1988; Faivre-Sarrailh et al., 1992), and Nr-CAM/Bravo (Grumet et al., 1991; Krushel et al., 1993; Denburg et al., 1995) have overlapping distributions in vertebrate fiber tracts in the retina, optic nerve, spinal cord, and tectum (Lustig, M., and M. Grumet, unpublished observations). Therefore, it is of interest to determine whether changes in the expression and interactions between RPTPβ and various combinations of neuronal CAMs during development modulate the behavior of neurons and glia locally.
Receptor complexes containing contactin and Nr-CAM may be able to relay specific transmembrane signals or recruit additional proteins for neurite outgrowth (Fig. 10). The FGF receptor has been implicated in neurite growth involving transmembrane CAMs such as Ng-CAM/L1 (Doherty and Walsh, 1994), and Nr-CAM has a peptide sequence between its third and forth Ig domains that is similar to that found in Ng-CAM/L1 (Grumet et al., 1991; Williams et al., 1994). It is possible that Nr-CAM, as a complex with contactin, mediates signaling via the FGF receptor. Alternatively, a recently identified contactinassociated transmembrane protein of 190 kD that coprecipitates with RPTPβ (Peles et al., 1995, 1997) may be involved in transducing neurite growth signals generated by RPTPβ. Nr-CAM might also act in signaling by linking to the cytoskeleton via ankyrin (Davis and Bennett, 1994; Dubreuil et al., 1996).
The short form of RPTPβ induced neurite outgrowth both as a cell surface molecule (βCFS/EK) and a substrate bound to plastic (βCFS), suggesting that this activity of RPTPβ is biologically relevant. Previous studies have shown that astrocytes and Schwann cells support neurite outgrowth in culture by mechanisms involving integrins, cadherins, N-CAM, and Ng-CAM/L1 (Bixby et al., 1988; Tomaselli et al., 1988). The ability of RPTPβ to interact with neural CAMs in the Ig family (Milev et al., 1994, 1996) suggests that it might induce regulatory signals in neurons. In addition, binding of contactin and Nr-CAM to RPTPβ may generate signals in glia via this receptor, but, as yet, candidate substrates in glia for the tyrosine phosphatase have not been detected. Recently, it has been proposed that binding of extracellular regions of RPTPμ to its ligand does not induce any change in phosphatase activity. Rather, ligand binding may regulate the localization of phosphatases on the cell surface (Gebbink et al., 1995). Similarly, binding to neural CAMs and extracellular matrix molecules might induce changes in localization of RPTPβ on glial cells that modulate local signaling by clustering of phosphatases. While receptor forms of RPTPβ expressed on glia may mediate communication between neurons and glia, the secreted form has the potential for blocking these activities (Fig. 10). Interestingly, the level of expression of the secreted form increases dramatically during development (Meyer-Puttlitz et al., 1995; Sakurai et al., 1996)
Remarkably, a subset of neurons bearing long neurites with oval-shaped cell bodies were observed when tectal cells were incubated on βCFS transfectants (Fig. 9). The characteristics of these cells are similar to those seen for the chick tectobulbar neurons which extend efferent fibers from the tectum and have large oval-shaped cell bodies (Kroger and Schwarz, 1990). Bundles of axons in the tectobulbar tracts grow through the tectum without contacting axons growing in from the retina. Perhaps, glial cells in the tectum expressing RPTPβ provide cues for neuronal differentiation, and axonal growth. Using other chick neurons such as cerebellar cells, we did not detect dramatic effects on βCFS, but we did observe that cortical neurons from P2 rat brain extended long neurites on βCFS (Sakurai, T., and M. Grumet, unpublished observations). While this manuscript was in preparation, it was reported (Maeda and Noda, 1996) that protein moieties in the NH2terminal half of phosphacan can promote differentiation of certain cortical neurons which is consistent with our observations.
RPTPβ/phosphacan is also found in certain locations during development that axons avoid such as the roof plate, and phosphacan has been found to inhibit neurite growth in culture (Milev et al., 1994; Maeda and Noda, 1996). In addition to the C, F, and S domains, phosphacan and the long receptor form of RPTPβ contain an 860– amino acid domain that has been postulated to function as an inhibitor of neurite growth (Grumet et al., 1996). It is interesting that the roof plate of the spinal cord is deficient in adhesion molecules in general while the fiber tracts have a rich assortment of CAMs (see references in Brummendorf and Rathjen, 1995). Therefore, it is likely that RPTPβ/phosphacan differentially affects neurite growth depending on the relative abundance of the different forms of the molecule. Responses may also vary depending on the receptors available in particular neurons as well as on extracellular binding proteins such as tenascin (Grumet et al., 1994), which may modulate interactions of RPTPβ with other proteins. Additional studies are needed to determine whether contactin and Nr-CAM also act as neuronal receptors mediating inhibition of neurite growth by phosphacan.
In conclusion, RPTPβ has two distinct domains that bind to different neuronal receptors, including contactin and Nr-CAM, that can form a complex in the plasma membrane. These CAMs probably act in the form of complexes possibly including other neuronal receptors to mediate glial signals that control neurite growth.
Acknowledgments
We thank Drs. Fritz Rathjen and Jeffrey Denburg for their generous gifts of antibodies, Dr. James Salzer for PI-PLC, and Drs. David R. Friedlander and Irit Lax for helpful discussions and for critically reading the manuscript.
This work was supported by grants from the National Institutes of Health (NS21629 and NS33921). M. Lustig was supported in part by a National Institutes of Health MD–Ph.D. training grant.
Abbreviations used in this paper
- C
carbonic anhydrase domain
- CAM
cell adhesion molecule
- F
fibronectin type III repeat
- GPI
glycosylphosphatidylinositol
- PI-PLC
phospho-inositol specific phospholipase C
- RPTPβ
receptor-like protein tyrosine phosphatase β
- S
spacer region
Footnotes
Please address all correspondence to Martin Grumet, Department of Pharmacology, New York University Medical Center, New York, NY 10016. Tel.: (212) 263–7126; Fax.: (212) 263-7133; E-mail: grumem01@mcrcr6.med.nyu.edu
References
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- Barnea G, Silvennoinen O, Shannan B, Honegger AM, Canoll PD, D'Estachio P, Levy JL, Laforgia S, Huebner K, Musacchio JM, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP-γ defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–1506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase β is expressed in the form of a proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994a;269:14349–14352. [PubMed] [Google Scholar]
- Barnea G, Grumet M, Sap J, Margolis RU, Schlessinger J. Close similarity between a receptor-linked tyrosine phosphatase and a rat brain proteoglycan. Cell. 1994b;76:205. doi: 10.1016/0092-8674(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988;107:353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Protein Profile. 1995;2:963–1108. [PubMed] [Google Scholar]
- Brummendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Hubert M, Treubert U, Leuschner R, Tarnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Barnea G, Levy JB, Sap J, Ehrlich M, Silvennoinen O, Schlessinger J, Musacchio JM. The expression of a novel receptortype tyrosine phosphatase suggests a role in morphogensis and plasticity of the nervous system. Dev Brain Res. 1993;75:293–298. doi: 10.1016/0165-3806(93)90035-9. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTPβ are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996;44:199–215. doi: 10.1002/(SICI)1097-4547(19960501)44:3<199::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27165. [PubMed] [Google Scholar]
- Denburg JL, Caldwell RT, Marner JM. Developmental changes in epitope accessibility as an indicator of multiple states of an immunoglobulin-like neural cell adhesion molecule. J Comp Neurol. 1995;354:533–550. doi: 10.1002/cne.903540405. [DOI] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994;4:49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Doherty P, Williams E, Walsh FS. A soluble chimeric form of the L1 glycoprotein stimulates neurite outgrowth. Neuron. 1995;14:57–66. doi: 10.1016/0896-6273(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR, MacVicar G, Dissanayake S, Liu C, Homer D, Hortsch M. Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Crossin KL. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Engel M, Maurel P, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. I. Cellular sites of synthesis of neurocan and phosphacan. J Comp Neurol. 1996;366:34–43. doi: 10.1002/(SICI)1096-9861(19960226)366:1<34::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Mege R-M, Cunningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci USA. 1989;86:7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to neural adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink MFBG, Zondag GCM, Koningstein GM, Feiken E, Wubbolts RW, Moolenaar WH. Cell surface expression of receptor protein tyrosine phosphatase RPTPμ is regulated by cell–cell contact. J Cell Biol. 1995;122:647–658. doi: 10.1083/jcb.131.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G, Cibelli G, Rougon G, Mattei MG, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M. Cell adhesion molecules and their subgroups in the nervous system. Curr Opin Neurobiol. 1991;1:370–376. doi: 10.1016/0959-4388(91)90055-c. [DOI] [PubMed] [Google Scholar]
- Grumet M. Structure, expression, and function of Ng-CAM, a member of the immunoglobulin superfamily involved in neuron–neuron and neuron– glia adhesion. J Neurosci Res. 1992;31:1–13. doi: 10.1002/jnr.490310102. [DOI] [PubMed] [Google Scholar]
- Grumet M, Edelman GM. Neuron–glia cell adhesion molecule interacts with neurons and astroglia via different binding mechanisms. J Cell Biol. 1988;106:487–503. doi: 10.1083/jcb.106.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Sakurai T. Interactions of the cell adhesion molecules Ng-CAM and Nr-CAM with neural receptors and extracellular matrix proteins. Semin Neurosci. 1996;8:379–389. [Google Scholar]
- Grumet M, Mauro V, Burgoon MP, Edelman GM, Cunningham BA. Structure and characterization of Nr-CAM, a new member of the Ig superfamily that is closely related to Ng-CAM. J Cell Biol. 1991;113:1399–1412. doi: 10.1083/jcb.113.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon MA, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during development: interactions with adhesion molecules. Perspec on Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Honegger AM, Dull TJ, Felder S, Van Obberghen E, Bellot F, Szapary D, Schmidt A, Ullrich A, Schlessiner J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Kayyem JF, Roman JM, de la Rosa EJ, Schwarz U, Dreyer WJ. Bravo/Nr-CAM is closely related to the cell adhesion molecules L1 and NgCAM and has a similar heterodimer structure. J Cell Biol. 1992;118:1259–1270. doi: 10.1083/jcb.118.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interactions between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science (Wash DC) 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kroger S, Schwarz U. The avian tectobulbar tract: development, explant culture, and effects of antibodies on the pattern of neurite growth. J Neurosci. 1990;10:3118–3134. doi: 10.1523/JNEUROSCI.10-09-03118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger NX, Saito H. A human transmembrane protein-tyrosinephosphatase, PTPζ, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci USA. 1992;89:7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krushel LA, Prieto AL, Cunningham BA, Edelman GM. Expression patterns of the cell adhesion molecules Nr-CAM during histogenesis of the chick nervous system. Neuroscience. 1993;53:797–812. doi: 10.1016/0306-4522(93)90625-p. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates: permissive versus instructive influence and the role of adhesive strength. J Neurosci. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JB, Canoll PD, Silvennoinen O, Barnea G, Morse B, Honegger AM, Huang J-T, Cannizzaro LA, Park S-H, Druck T, et al. The cloning of a receptor-type protein tyrosine phosphatase expressed in the central nervous system. J Biol Chem. 1993;268:10573–10581. [PubMed] [Google Scholar]
- Maeda N, Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Development (Camb) 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V, Krushel L, Cunningham B, Edelman GM. Homophilic and heterophilic binding activities of Nr-CAM, a nervous system cell adhesion molecule. J Cell Biol. 1992;119:191–202. doi: 10.1083/jcb.119.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Milev P, Junker E, Zimmer I, Margolis RU, Margolis RK. Chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of nervous tissue: developmental changes of neurocan and phosphacan. J Neurochem. 1995;65:2327–2337. doi: 10.1046/j.1471-4159.1995.65052327.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Junker E, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. II. Immunocytochemical localization of neurocan and phosphacan. J Comp Neurol. 1996;366:44–54. doi: 10.1002/(SICI)1096-9861(19960226)366:1<44::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptortype tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Maurel P, Haring M, Margolis RK, Margolis RU. TAG1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-ζ/β, and N-CAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- Morales G, Hubert M, Brummendorf T, Treubert U, Tarnok A, Schwarz U, Rathjen FG. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and Nr-CAM/Bravo. Neuron. 1993;11:1113–1122. doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- Olive S, Dubois C, Schachner M, Rougon G. The F3 neuronal glycophosphatidylinositol-linked molecule is localized to the glycolipid-enriched membrane subdomains and interacts with L1 and fyn kinase in cerebellum. J Neurochem. 1995;65:2307–2317. doi: 10.1046/j.1471-4159.1995.65052307.x. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Peles, E., M. Nativ, M. Lustig, M. Grumet, J. Schilling, R.G. Martinez, G.D. Plowman, and J. Schlessinger. 1997. Identification of a novel contactin associated transmembrane receptor with multiple domains implicated in protein–protein interactions. EMBO (Eur. Mol. Biol. Organ.) J. In press. [DOI] [PMC free article] [PubMed]
- Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/F11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- Rader C, Kunz B, Lierheimer R, Giger RJ, Berger P, Tittmann P, Gross H, Sonderegger P. Implications for the domain arrangement of axonin-1 derived from the mapping of its NgCAM binding site. EMBO (Eur Mol Biol Organ) J. 1996;15:2056–2068. [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130–kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen FG, Jessel TM. Glycoproteins that regulate the growth and guidance of vertebrate axons: domains and dynamics of the immunoglobulin/fibronectin type III subfamily. Semin Neurosci. 1991;3:297–307. [Google Scholar]
- Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U, Gao P, Janetzko A, Flaccus A, Hilgenberg L, Tekotte H, Margolis RK, Margolis RU. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J Biol Chem. 1991;266:14785–14801. [PubMed] [Google Scholar]
- Reid RA, Bronson DD, Young KM, Hemperly JJ. Identification and characterization of the human cell adhesion molecule contactin. Mol Brain Res. 1994;21:1–8. doi: 10.1016/0169-328x(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Friedlander DR, Grumet M. Expression of polypeptide variants of receptor protein tyrosine phosphatase β: the secreted form, phosphacan, increases dramatically during embryonic development and modulates glial cell behavior in vitro. J Neurosci Res. 1996;43:694–706. doi: 10.1002/(SICI)1097-4547(19960315)43:6<694::AID-JNR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schachner M. The analysis of neural recognition molecules; benefits and vicissitudes of functional knock-outs using antibodies and gene ablation. Curr Opin Cell Biol. 1993;5:786–790. doi: 10.1016/0955-0674(93)90026-m. [DOI] [PubMed] [Google Scholar]
- Schachner, M., H. Antonicek, A. Fahrig, G. Fischer, V. Künemund, R. Martini, A. Meyer, E. Persohn, E. Pollerberg, R. Probstmeier, et al. 1990. Families of Neural Cell Adhesion Molecules. In Morphoregulatory Molecules. G.M. Edelman, B.A. Cunningham, and J.-P. Thiery, editors. John Wiley & Sons Inc., New York. pp. 443–468.
- Schlessinger J, Ullrich A. Growth factor signalling by receptor tyrosine kinases. Neuron. 1992;9:1–20. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Shitara K, Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Brain-specific receptor-type protein-tyrosine phosphatase RPTPβ is a chondroitin sulfate proteoglycan in vivo. . J Biol Chem. 1994;269:20189–20193. [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neruronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Zisch AH, D'Alessandri L, Amrein K, Ranscht B, Winterhalter KH, Vaughan L. The glypiated neuronal cell adhesion molecule contactin/ F11 complexes with src-family protein tyrosine kinase fyn. Mol Cell Neurosci. 1995;6:263–279. doi: 10.1006/mcne.1995.1021. [DOI] [PubMed] [Google Scholar]