Abstract
Eps15 has been identified as a substrate of the EGF receptor tyrosine kinase. In this report, we show that activation of the EGF receptor by either EGF or TGF-α results in phosphorylation of Eps15. Stimulation of cells with PDGF or insulin did not lead to Eps15 phosphorylation, suggesting that phosphorylation of Eps15 is a receptor-specific process. We demonstrate that Eps15 is constitutively associated with both α-adaptin and clathrin. Upon EGF stimulation, Eps15 and α-adaptin are recruited to the EGF receptor. Using a truncated EGF receptor mutant, we demonstrate that the regulatory domain of the cytoplasmic tail of the EGF receptor is essential for the binding of Eps15. Fractionation studies reveal that Eps15 is present in cell fractions enriched for plasma membrane and endosomal membranes. Immunofluorescence studies show that Eps15 colocalizes with adaptor protein-2 (AP-2) and partially with clathrin. No colocalization of Eps15 was observed with the early endosomal markers rab4 and rab5. These observations indicate that Eps15 is present in coated pits and coated vesicles of the clathrin-mediated endocytic pathway, but not in early endosomes. Neither AP-2 nor clathrin are required for the binding of Eps15 to coated pits or coated vesicles, since in membranes lacking AP-2 and clathrin, Eps15 still shows the same staining pattern. These findings suggest that Eps15 may play a critical role in the recruitment of active EGF receptors into coated pit regions before endocytosis of ligand-occupied EGF receptors.
Growth factors such as EGF are involved in many physiological and pathological processes, including cell growth, differentiation, inflammation, and cancer. EGF receptor activation is thought to occur upon ligand-induced receptor dimerization leading to receptor cross-phosphorylation (Schlessinger, 1988; Ullrich and Schlessinger, 1990). The tyrosine-phosphorylated receptor provides for docking sites for SH2 domain containing signal transducing molecules such as Grb2 and phospholipase-Cγ1. Complex formation initiates a signaling cascade that leads to changes in gene expression and cell division. Inactivation of the EGF receptor occurs by several mechanisms such as a reduction in receptor affinity (a process that is called receptor transmodulation [Northwood and Davis, 1990]), by receptor dephosphorylation, by phosphotyrosine phosphatases (Faure et al., 1992), and by receptor downregulation (for review see Sorkin and Waters, 1993). Receptor downregulation includes the endocytosis of activated receptors, resulting in the removal of activated receptors from the cell surface and the subsequent degradation in lysosomes. The importance of downregulation is stressed by the observation that receptors that are unable to undergo ligand-induced internalization can facilitate cellular transformation (Wells et al., 1990) and tumor formation (Masui et al., 1991).
EGF receptor endocytosis is achieved by a constitutive pathway and a ligand-induced pathway (for review see Sorkin and Waters, 1993). The EGF-induced, receptormediated endocytotic pathway occurs via specialized coated pit regions in the plasma membrane. These regions contain a number of proteins, including the adaptor proteins (APs)1 and the heavy and light chains of clathrin that form the clathrin lattice (for review see Schmid, 1992). It has been shown recently that kinase-deficient receptors fail to undergo ligand-induced sequestration into coated pits (Lamaze and Schmid, 1995) and that, as a result, kinasedeficient receptors are not internalized via coated vesicles. Recruitment into coated pits could be restored by the addition of a soluble EGF receptor tyrosine kinase. Therefore, it has been proposed that the phosphorylation of another protein, an as yet unknown EGF receptor substrate, is required for the efficient recruitment of EGF receptors into coated pits (Lamaze and Schmid, 1995).
EGF receptor activation leads to the phosphorylation of various proteins. Recently, two new EGF receptor substrates, Eps15 (EGF receptor pathway substrate clone No. 15) and Eps15R (Eps15 related) have been described (Fazioli et al., 1993; Schumacher et al., 1995). Eps15 and Eps15R are homologous proteins showing 47% identity (Wong et al., 1995). The apparent molecular mass of both proteins is 142 kD, and they consist of three structural domains. Domain I is the putative regulatory domain, which contains a candidate tyrosine phosphorylation site, EF hand-type calcium-binding domains (Fazioli et al., 1993), and three protein binding domains (Wong et al., 1995). Domain II has the features of a coiled-coil structure and domain III exhibits repeated DPF motifs, a motif that is conserved in several methyl transferases, and a prolinerich motif that can bind in vitro to the SH3 domain of c-Crk and v-Crk (Schumacher et al., 1995). Eps15 has homology with the yeast protein End3, which is involved in receptormediated endocytosis of the α factor in Saccharomyces cerevisiae (Benedetti et al., 1994). Recently, further evidence for a possible role of Eps15 in endocytosis originated from the observation that Eps15 is associated with AP-2 (Benmerah et al., 1995). Analysis of the binding sites revealed that domain III of Eps15 binds directly to the COOH-terminal appendage (ear) of α-adaptin (Benmerah et al., 1996).
In this paper, we have investigated the function of Eps15 in the ligand-induced endocytosis of the EGF receptor. We show that in addition to the association of Eps15 to α-adaptin, Eps15 also associates with the EGF receptor and with the clathrin light chain (LC). The interaction of Eps15 with α-adaptin and clathrin LC is not changed by EGF treatment, whereas a strong increase is observed in the association of the EGF receptor with either Eps15 and α-adaptin after EGF stimulation. Furthermore, we demonstrate that the Eps15 binding site of the EGF receptor is located in the regulatory domain of the intracellular part of the EGF receptor. Localization studies using the confocal scanning laser microscope (CSLM) show a strong colocalization of Eps15 with AP-2 and a partial colocalization of Eps15 with clathrin. In contrast, no colocalization of Eps15 with either rab4 or rab5 was found. This suggests a function for Eps15 in coated pits and coated vesicles, but not in early endosomes. Our data may indicate a functional role for Eps15 in ligand-induced endocytosis of EGF receptors.
Materials and Methods
Tissue Culture
Swiss 3T3 fibroblasts, HER14 fibroblasts (NIH 3T3 cells stably transfected with human EGF receptor cDNA), A14 fibroblasts (NIH 3T3 cells stably transfected with insulin receptor cDNA), T963 fibroblasts (NIH 3T3 cells stably transfected with EGF receptor cDNA truncated at amino acid 963), and CHO cells stably transfected with rab4 cDNA tagged with hemagglutinin (NH) and rab5 tagged with vesicular stomatitis virus G protein (G; van der Sluijs, P., manuscript submitted for publication) were cultured in DME (Gibco, Paisley, UK) supplemented with 7.5% vol/vol FCS (Gibco) in a humidified atmosphere at 37°C.
Immunoprecipitation Experiments
Cells were grown in 100-mm dishes (Costar Corp., Cambridge, MA) to 80% confluency. Cells were serum starved in DME–0% vol/vol FCS for 24 h before stimulation with 50 ng/ml EGF, 20 ng/ml PDGF-BB, 50 ng/ml TGF-α, or 1 μg/ml insulin. Cells were lysed in RIPA buffer (20 mM TrisHCl, pH 7.4, 150 mM NaCl, 0.5% Triton X-100. 0.1% SDS, 1 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 100 mM NaF, 1 mM Na3VO4) at 4°C for 10 min and centrifuged for 5 min at 12,000 g in an Eppendorf centrifuge. The supernatants were incubated with 25 μl of a 1:1 suspension of protein A–Sepharose in RIPA buffer for 1 h at 4°C. The samples were centrifuged and incubated with either anti-Eps15 antibody (rabbit polyclonal; Schumacher et al., 1995), anti-Eps15R antibody (rabbit polyclonal; Schumacher et al., 1995), anti–clathrin LC antibody (rabbit polyclonal, a gift from Dr. E. Ungewickell, Max-Planck Institut Für Biochemie, Martinsried, FRG), or anti–EGF receptor (mouse monoclonal clone No. 528; Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C. Protein A–Sepharose was subsequently added, and after a further 2-h incubation, the immunoprecipitates were washed three times, once with RIPA buffer, once with high salt buffer (20 mM Tris-HCl, pH 7.4, 0.5 M NaCl, 1% Triton X-100, 1 mM PMSF, 1 mM benzamidine, 1 mM Na3VO4), and finally with low salt buffer (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 1% Triton X-100, 1 mM PMSF, 1 mM benzamidine, 1 mM Na3VO4). The samples were boiled in 20 μl Laemmli sample buffer for 5 min. For the preparation of total cell lysates, cells were immediately lysed in RIPA buffer. The samples were separated by 8% SDS-PAGE and the Western blot was probed with rabbit polyclonal antibodies against Eps15 and anti–clathrin LC, or with mouse mAbs against α-adaptin (a gift from Dr. F. Brodsky, University of California, San Francisco, CA), anti–EGF receptor, anti–PDGF receptor (mouse monoclonal; Upstate Biotechnology, Inc., Lake Placid, NY), or antiphosphotyrosine (mouse monoclonal; Transduction Laboratories, Lexington, KY). Immunocomplexes were detected using enhanced chemiluminescence (Renaissance; DuPont New England Nuclear, Boston, MA) with peroxidase-conjugated goat anti–rabbit or rabbit anti–mouse Ig (Jackson ImmunoResearch, West Grove, PA). Protein bands were analyzed by densitometry (Personal Densitometer SI; Molecular Dynamics Inc., Sunnyvale, CA) and quantified using the ImageQuaNT software, version 4.2, Microsoft for Windows.
Cell Fractionation
Cell fractionation and analysis of the different fractions was carried out by differential centrifugation as described by Evans (1992). HER14 fibroblasts were washed once in PBS and once in homogenation buffer (250 mM sucrose, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM PMSF, 1 mM benzamidine). Cells were collected in homogenation buffer and homogenized by 10 passages through a 24-G needle. Whole cells and nuclei were removed by centrifugation for 5 min at 1,000 g at 4°C. To clear the supernatant from the remaining nuclei, the cell lysate was pelleted through a 2-M sucrose layer. The supernatant was subsequently centrifuged for 12 min at 10,000 g at 4°C to give a pellet that was washed two times with homogenation buffer. The supernatant of the 10,000-g spin was subsequently centrifuged at 150,000 g for 30 min at 4°C. The remaining supernatant was considered as the cytosol. The separate fractions were assayed for various enzyme markers. The highest activity of lactate dehydrogenase was found in the cytosol. The highest activities of 5′ nucleotidase and acid phosphatase were found in the 10,000-g pellet, demonstrating that plasma membrane vesicles and lysosomes are enriched in this fraction. NADH cytochrome c reductase and anti–α-adaptin antibodies were used as markers for the ER and coated vesicles, respectively, and were both enriched in the 150,000-g pellet.
Immunofluorescence Microscopy
Cells were grown for 3 d in 12-well plates on glass coverslips in DME7.5% vol/vol FCS. Cells were fixed in 3% formaldehyde (Polysciences, Warrington, PA) in PBS for a minimum of 30 min. Cells were washed twice with PBS and permeabilized with 0.2% Triton X-100 in PBS for 5 min. The cells were subsequently washed twice with PBS and incubated in 50 mM glycine in PBS for 10 min. Cells were washed twice in 0.2% gelatin (Merck, Darmstadt, Germany) in PBS and incubated with either antiEps15 serum, anti–clathrin heavy chain (HC), anti–α-adaptin, anti-NH (Bottger et al., 1996), or anti-G antibodies (Kreis and Lodish, 1986) diluted in 0.2% gelatin/PBS for 45 min at room temperature. Cells were washed in 0.2% gelatin/PBS and incubated with either goat anti–rabbit conjugated to FITC or goat anti–mouse conjugated to Texas red for 30 min at room temperature, followed by four washes in 0.2% gelatin/PBS. Finally, cells were embedded in 10% Mowiol 4-88, 25% glycerol, 100 mM Tris, pH 8.5, containing 1 mg/ml p-phenylene-diamine (PPD; Sigma, St. Louis, MO) and examined in a CSLM (Lasersharp mrc-500; Bio Rad, Hemel Hempsted, UK) or in a Leitz Orthoplan microscope equipped with epi-illumination.
Image processing and analysis was performed following the method of van Steensel et al. (1996). In short, a cross-correlation analysis of the red and green images was calculated by shifting the red image over a distance of Δx pixels in the x direction with respect to the green image (with −20⩽Δx⩽20). For each value of Δx, the Pearson's correlation coefficient rP was calculated by a computer program (written in BASIC), according to the following formula:
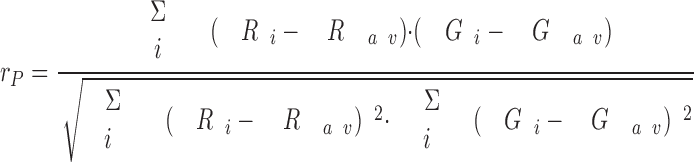
where R i (red) and G i (green) are the values of pixel i and R av and G av are the average values of R i and G i, respectively. The cross-correlation function was obtained by plotting rP against Δx.
Stripping of Coat Proteins from Plasma Membrane Fragments
HER14 cells were grown on glass coverslips coated with poly-l-lysine (1 mg/ml). Membrane fragments were prepared by rapid freeze-thaw rupturing of cells (Chang et al., 1993a ). Clathrin coat proteins were removed by four washing steps in ice-cold 20 mM N-Tris[hydroxymethyl]methyl-3amino-propanesulfonic acid buffer (TAPS), pH 9.2, incubation in TAPS buffer for 20 min at 4°C, followed by four washes in the same buffer. AP-2 coat proteins were removed by four washing steps in ice-cold Tris buffer (0.5 M Tris, pH 7.0, 1 mM DTT), incubation for 20 min in Tris buffer at 4°C, followed by four washes in the same buffer. The membranes were subsequently washed seven times at 4°C with an ice-cold buffer containing 36.4 mM Hepes, pH 7.0, 68.2 mM KCl, 4.1 mM Mg-acetate, 1 mM DTT, 1 mM PMSF, and 1 mM benzamidine, were fixed in 3% formaldehyde in 20 mM Hepes, pH 6.8, 3 mM EGTA, 5 mM MgCl2; and 100 mM KCl, and were subsequently incubated for 1 h at 37°C in PBS supplemented with 0.2% gelatin, 1% BSA, and 50 mM glycine. Membranes were stained with either anti-Eps15, anti–α-adaptin, or anti-clathrin antibodies, and further processed for immunofluorescence microscopy, as described above.
Results
Eps15 Is Phosphorylated after Stimulation with EGF and TGF-α, but Not after Stimulation with PDGF or Insulin
Eps15 has been described as a substrate of the EGF receptor (Fazioli et al., 1993). After stimulation of the EGF receptor, ∼30% of the total pool of Eps15 was tyrosine phosphorylated (Fazioli et al., 1993). To determine whether Eps15 is a specific substrate of the EGF receptor, Eps15 was immunoprecipitated from cells treated with either EGF, insulin, or PDGF, and the phosphorylated Eps15 was detected using an antibody against phosphotyrosine residues. For these experiments, antibodies that were raised against either Eps15 or Eps15R were used (Schumacher et al., 1995). In HER14 cells, very low levels of Eps15R protein were detected, indicating that Eps15R is not highly expressed in mouse fibroblasts (data not shown). This is in agreement with previous studies using HeLa cells (Schumacher et al., 1995). Therefore, in this study, we have concentrated our efforts on the detection of the much more abundant Eps15 protein.
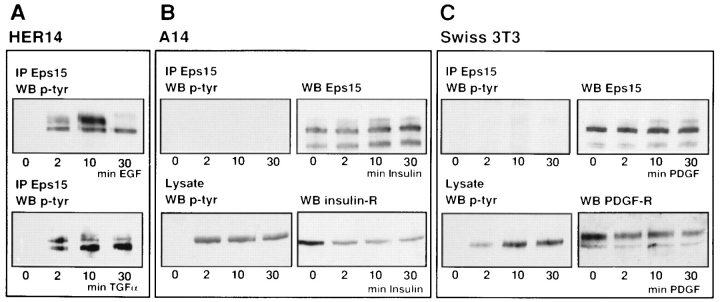
Three different cell types were used: HER14 cells containing ∼4 × 105 EGF receptors per cell (Rotin et al., 1992), A14 cells containing ∼05 × 105 insulin receptors (Burgering et al., 1991), and Swiss 3T3 cells with ∼4 × 105 PDGF receptors per cell (Bowen-Pope and Russell, 1982). These fibroblast cell lines were chosen because of their similar amounts of the respective cell surface receptors. Cells were serum starved overnight and were either left unstimulated or stimulated for 2, 10, or 30 min with 50 ng/ml EGF, 20 ng/ml PDGF, or 1 μg/ml insulin. Eps15 immunoprecipitates were Western blotted and probed with an antiphosphotyrosine antibody. A clear phosphorylation of Eps15 was seen in HER14 cells after stimulation with EGF (Fig. 1 A). Furthermore, EGF stimulation induced a transient mobility shift of phosphorylated Eps15, as seen on the Western blot by a shift in molecular mass from 142 to 150 kD. After 10 min of EGF stimulation, almost 50% of the Eps15 was shifted to the 150-kD form. At this time, it is unknown whether this shift is caused by hyperphosphorylation or to another type of posttranslational modification. No phosphorylation of Eps15 was observed in A14 cells that were stimulated with insulin (Fig. 1 B) nor in Swiss 3T3 cells stimulated with PDGF (Fig. 1 C). Control experiments showed that Eps15 was present in both cell types (Fig. 1, B and C), and that stimulation of the cells with EGF induced Eps15 phosphorylation (data not shown). Furthermore, Western blots of cell lysates from the same samples indicated the presence and phosphorylation of both the insulin receptor and PDGF receptor (Fig. 1, B and C). A14 cells and Swiss 3T3 cells both contained a substantial amount of the 122-kD protein next to the 142-kD Eps15 band (Fazioli et al., 1993), in contrast to HER14 cells, where a 122-kD band was not detected. This 122-kD band may reflect a degradation product of Eps15 (Fazioli et al., 1993).
Figure 1.
Eps15 is phosphorylated after stimulation with EGF and TGF-α, but not after stimulation with PDGF or insulin. (A) Eps15 immunoprecipitates from HER14 cells that were either mock treated or stimulated for 2, 10, and 30 min with 50 ng/ml EGF or 50 ng/ml TGF-α. The proteins were separated on 8% SDS-PAGE, and the Western blot was probed with antiphosphotyrosine antibody. (B) Eps15 immunoprecipitates and lysates from A14 cells that were either mock treated or stimulated for 2, 10, and 30 min with 1 μg/ml insulin. Western blots were probed with an antiphosphotyrosine antibody, an anti-Eps15 antibody, and an anti–insulin receptor antibody. (C) Eps15 immunoprecipitates and lysates from Swiss 3T3 cells that were left unstimulated or stimulated for 2, 10, and 30 min with 20 ng/ml PDGF. Western blots were probed with an antiphosphotyrosine antibody, an anti-Eps15 antibody, and an anti–PDGF receptor antibody.
These data show that Eps15 is phosphorylated specifically by activated EGF receptors, and not by insulin receptors or PDGF receptors. To obtain further indication for the EGF receptor specificity of Eps15 phosphorylation, an EGF-related peptide was used: TGF-α. Like EGF, TGF-α binds and activates the EGF receptor (Winkler et al., 1989). HER14 cells were either left unstimulated or stimulated with 50 ng/ml TGF-α, Eps15 immunoprecipitates were separated by SDS-PAGE, and Western blots were probed with an antiphosphotyrosine antibody. Stimulation of these cells with TGF-α showed a clear phosphorylation of Eps15 (Fig. 1 A). However, TGF-α–induced phosphorylation kinetics differed from EGF-stimulated phosphorylation. TGF-α–induced phosphorylation peaks at 2 min, whereas EGF-induced phosphorylation peaks at 10 min. Together, these data show that Eps15 is a specific substrate of the EGF receptor tyrosine kinase, but not of the PDGF or insulin receptor tyrosine kinase.
Association of Eps15 with the EGF Receptor, α-Adaptin, and Clathrin LC
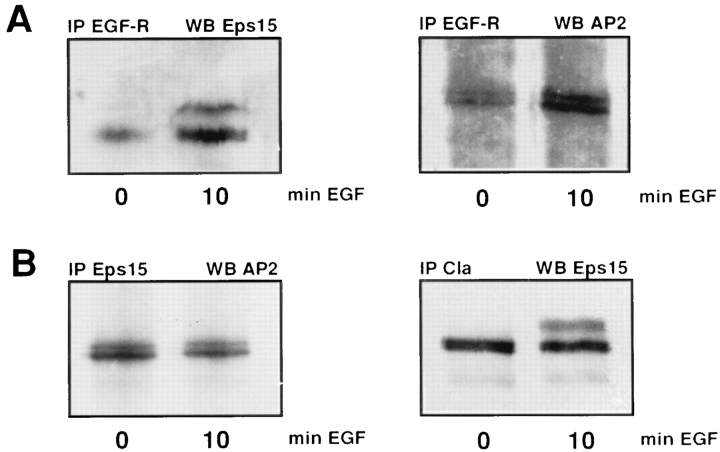
Eps15 has homology with the yeast protein End3, a protein involved in α factor endocytosis (Benedetti et al., 1994). Moreover, it has recently been shown that Eps15 is constitutively associated with α-adaptin (Benmerah et al., 1995). Therefore, we wanted to determine whether Eps15 is associated with proteins other than α-adaptin that are involved in the receptor-mediated endocytosis of EGF receptors. HER14 cells were serum starved and either left unstimulated or treated with 50 ng/ml EGF. Subsequently, EGF receptors were immunoprecipitated from cell lysates and the samples were separated by SDS-PAGE. Western blots were analyzed for the presence of Eps15 and α-adaptin. A modest association between the EGF receptor and Eps15 was observed in unstimulated cells (Fig. 2 A, lane 1). However, stimulation of the cells with EGF resulted in a dramatic increase in the binding of Eps15 to the EGF receptor. Also, the tyrosine-phosphorylated and the posttranslational modified form of Eps15 was observed in the EGF receptor immunoprecipitates (Fig. 2 A, lane 2). Similarly, an EGF receptor immunoprecipitate probed with anti–α-adaptin showed an association of the EGF receptor with AP-2, which increased upon EGF stimulation (Fig. 2 A, lanes 3 and 4). The double band that is visible on the Western blot probed with anti–α-adaptin represents the αa and the αc form of this protein (Boll et al., 1995).
Figure 2.
Association of Eps15 with the EGF receptor, α-adaptin, and clathrin LC. (A) EGF receptor immunoprecipitates from HER14 cells that were either mock treated or stimulated for 10 min with 50 ng/ml EGF. The proteins were separated on 8% SDS-PAGE, and the Western blots were probed with anti-Eps15 or anti–α-adaptin (AP-2) antibody. (B) Eps15 and clathrin LC (Cla) immunoprecipitates from HER14 cells that were left unstimulated or stimulated for 10 min with 50 ng/ml EGF. Western blots were probed with an anti–α-adaptin or an anti-Eps15 antibody.
The effect of EGF on the association of Eps15 and AP-2 was further investigated. Eps15 was immunoprecipitated from unstimulated and EGF-treated cells, and Western blots were probed with anti–α-adaptin antibody (Fig. 2 B, lanes 1 and 2). EGF treatment did not affect the interaction between Eps15 and AP-2, which suggests that Eps15– AP-2 binding is EGF independent.
The adaptor proteins have been shown to bind to the clathrin coat of the coated vesicle (Pearse and Crowther, 1987). To check the association of Eps15 with clathrin, we investigated whether clathrin could be coimmunoprecipitated with Eps15 in an EGF-dependent manner. HER14 cells were treated with EGF or left untreated, and clathrin was immunoprecipitated using a specific anti–clathrin LC antibody. The Western blot was subsequently probed with an anti-Eps15 antibody and revealed an association of Eps15 with the clathrin LC. This association was not altered by EGF stimulation. Furthermore, the posttranslational modified form of Eps15 was present in the clathrin immunoprecipitates, indicating that both Eps15 forms associate to clathrin (Fig. 2 B, lanes 3 and 4). In control experiments, no Eps15 was found when the immunoprecipitation was performed with a nonspecific antibody (data not shown). In conclusion, these experiments show that Eps15 is associated with both α-adaptin and clathrin LC and that these interactions are not altered by EGF treatment. In contrast, EGF induces a strong increase in the binding of the EGF receptor to both Eps15 and α-adaptin.
The Regulatory Domain of the EGF Receptor Is Required for Association with Eps15
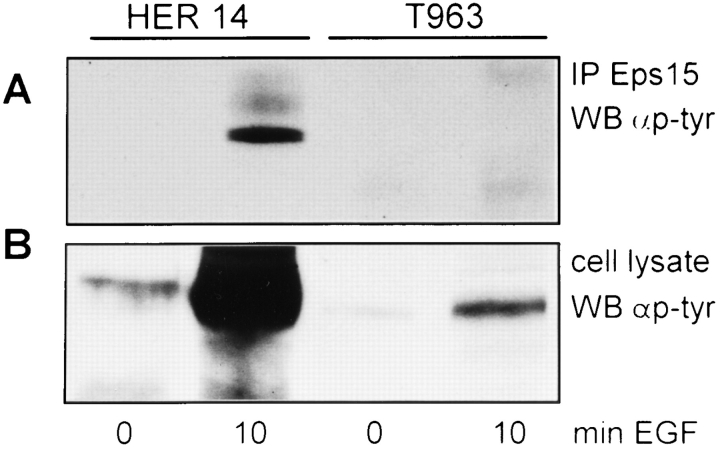
EGF stimulation of HER14 fibroblasts resulted in a strong increase in the binding of Eps15 to the EGF receptor. This interaction between Eps15 and the EGF receptor has never been described before, and thus the binding site for Eps15 on the EGF receptor is unknown. Fazioli and coworkers (1993) have shown that the juxtamembrane region of the EGF receptor and an EGF/erbB-2 receptor chimera is important for phosphorylation of Eps15. However, others have shown that a region in the regulatory domain in the COOH-terminal tail of the receptor, encompassing residues 991–1021, is critical for Eps15 phosphorylation (Alvarez et al., 1995). This particular region of the EGF receptor is a part of the region that mediates endocytosis (Chen et al., 1989). We investigated whether the regulatory domain of the EGF receptor is involved in the binding of Eps15. A mutant EGF receptor that was truncated at amino acid 963 was transfected into NIH 3T3 cells containing no detectable EGF receptors (T963 cells). This EGF receptor mutant still contains the juxtamembrane region, but lacks the regulatory domain including amino acids 991–1021. Control experiments showed that EGF-stimulated endocytosis of EGF receptors was impaired in the T963 cells (data not shown), which is in agreement with previous studies (Chen et al., 1989; Chang et al., 1993b ). To check EGF-induced phosphorylation of Eps15, both serum-starved HER14 and T963 fibroblasts were either left unstimulated or stimulated with 50 ng/ml EGF for 10 min. Eps15 was subsequently immunoprecipitated, and the samples were Western blotted and probed with an antiphosphotyrosine antibody. A clear EGF-induced phosphorylation of Eps15 is seen in HER14 cells that contain the wild-type receptor (Fig. 3 A, lanes 1 and 2). However, the truncated T963 receptor is no longer able to phosphorylate Eps15 (Fig. 3 A, lanes 3 and 4). As a control, autophosphorylation of the intact EGF receptor and the T963 receptor is shown (Fig. 3 B). Although similar amounts of EGF receptors were used in this assay, phosphorylation of the wild-type EGF receptor is much stronger than that of the truncated form because the main phosphorylation sites are deleted in the T963 mutant in contrast to the wild-type receptor. Furthermore, to demonstrate that indeed the T963 receptor contains an active tyrosine kinase, we analyzed angiotensin II phosphorylation, a specific substrate of the EGF receptor. Both the intact EGF receptor, as well as the truncated T963 receptor, showed a twofold increase in kinase activity after EGF stimulation (data not shown).
Figure 3.
The active T963 EGF receptor mutant does not phosphorylate Eps15. Eps15 immunoprecipitates from HER14 cells and NIH 3T3 cells stably transfected with a mutant EGF receptor truncated at amino acid 963 (T963). Cells were either mock treated or stimulated for 10 min with 50 ng/ml EGF. Immunoprecipitates (A) and lysates (B) were separated on 10% SDS-PAGE, and the Western blots were probed with an antiphosphotyrosine antibody.
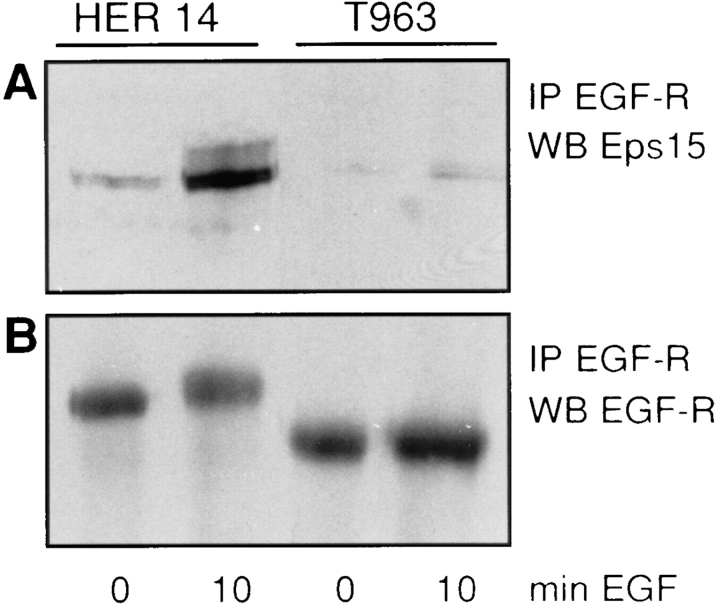
To investigate the binding of Eps15 to the truncated EGF receptor, cells were treated with EGF, and the EGF receptor was immunoprecipitated using an anti–EGF receptor antibody directed against the extracellular domain of the EGF receptor. These experiments revealed an EGFdependent coimmunoprecipitation of Eps15 with the wildtype receptor (Fig. 4 A, lanes 1 and 2), but no coimmunoprecipitation of Eps15 was seen with the T963 EGF receptor mutant (Fig. 4 A, lanes 3 and 4). For both cell lines, similar amounts of EGF receptors were immunoprecipitated, as shown by Western blotting of the wild-type and mutant receptors (Fig. 4 B). As expected, the truncated receptor migrated with a lower molecular weight, and the mobility shift, caused by hyperphosphorylation, was not visible because of the deletion of the major autophosphorylation sites. In conclusion, these experiments demonstrate that the regulatory domain in the intracellular part of the EGF receptor, previously shown to be important for receptor endocytosis, is not only required for Eps15 phosphorylation, but also for Eps15 binding to the EGF receptor.
Figure 4.
Eps15 recruitment by the EGF receptor requires the regulatory domain of the receptor. EGF receptor (EGF-R) immunoprecipitates from HER14 cells and NIH 3T3 cells stably transfected with a mutant EGF receptor truncated at amino acid 963 (T963). Cells were either mock treated or stimulated for 10 min with 50 ng/ml EGF. Proteins were separated on 8% SDSPAGE, and the Western blot was probed with anti-Eps15 antibody (A), stripped, and subsequently probed with anti–EGF receptor antibody (B).
Subcellular Localization of Eps15
To analyze the possible role of Eps15 in endocytosis of the EGF receptor further, we analyzed the subcellular localization of Eps15 by biochemical fractionation. For these experiments, HER14 cells were fractionated into a 1,000-g pellet (1k), a 10,000-g pellet (10k), a 150,000-g pellet (150k), and a cytosolic fraction, as described in Materials and Methods. These fractions were analyzed for the presence of specific marker proteins. The 1k pellet contained nuclei and the cytoskeleton and was not used for further analysis. The 10k pellet was enriched in markers for plasma membranes and lysosomes, while the 150k pellet was enriched in markers for ER and coated vesicles. HER14 cells were either left unstimulated or stimulated for 10 min with 50 ng/ml EGF. The cells were subsequently fractionated, and the different fractions were separated on SDS-PAGE. The Western blots were probed with antibodies against Eps15, α-adaptin, and the EGF receptor, and were analyzed by densitometry, as described in Materials and Methods. As expected, α-adaptin was mainly present in the 150k fraction (Fig. 5 B), and a small amount was found in the 10k fraction, probably associated to plasma membrane vesicles, whereas the EGF receptor was detectable both in the 10k and 150k fractions (Fig. 5 C). Both α-adaptin and the EGF receptor were present in the total cell lysate, but the concentration of these proteins in this fraction was much lower than in the enriched fractions. Eps15 was present in all fractions (Fig. 5 A). Interestingly, EGF treatment reduced the amount of Eps15 in both the 10k and the 150k fractions, while the amount of Eps15 in the total cell lysate and the cytosol fraction remained constant. The drastic decrease in the amount of Eps15 in the 150k fraction after EGF stimulation was ∼60%. Remarkably, the ratio between the 142- and 150-kD band of Eps15 was 1:1 in the cytosol and 10k fraction, but 3:1 in the 150k fraction. In conclusion, Eps15 is present in the cytosol, but also in the fractions enriched in plasma membrane vesicles and clathrin-coated vesicles. Furthermore, EGF stimulation of cells leads to the reduction in the amount of Eps15 present in the fraction enriched in clathrin-coated vesicles.
Figure 5.
Eps15 is present in the microsomal fraction after cell fractionation. HER14 fibroblasts were used for cell fractionation as described in Materials and Methods. Fractions were prepared from cells that were either left unstimulated or stimulated with 50 ng/ml EGF for 10 min. The different fractions (25 μg per sample) were separated by SDS-PAGE, and Western blots were probed with an anti-Eps15 antibody (A), an anti–α-adaptin antibody (B), and an anti–EGF receptor antibody (C).
Colocalization of Eps15 with α-Adaptin and Clathrin
Next, we analyzed the subcellular localization of Eps15, α-adaptin, and clathrin LC using immunofluorescence microscopy. HER14 fibroblasts were fixed, permeabilized, and stained with the relevant antibodies, and were subsequently analyzed in an immunofluorescence light microscope. Staining the cells with anti-Eps15 antibodies obtains a picture that reflects a clear punctate staining pattern throughout the whole cell (Fig. 6, A and C). Staining of the same cells with an anti–α-adaptin antibody revealed a similar punctate staining pattern representing coated pits and coated vesicles (Fig. 6 B; Guagliardi et al., 1990). Staining with an anti–clathrin HC antibody led to a punctated pattern throughout the cytoplasm representing endocytotic vesicles, and to a perinuclear staining representing exocytotic vesicles from the TGN (Fig. 6 C). Comparison of these images strongly suggests a colocalization of Eps15 with AP-2. The colocalization of Eps15 and clathrin HC is only apparent in the endocytotic vesicles, but is absent in the clathrin-coated vesicles in the perinuclear region (Fig. 6, A–D).
Figure 6.
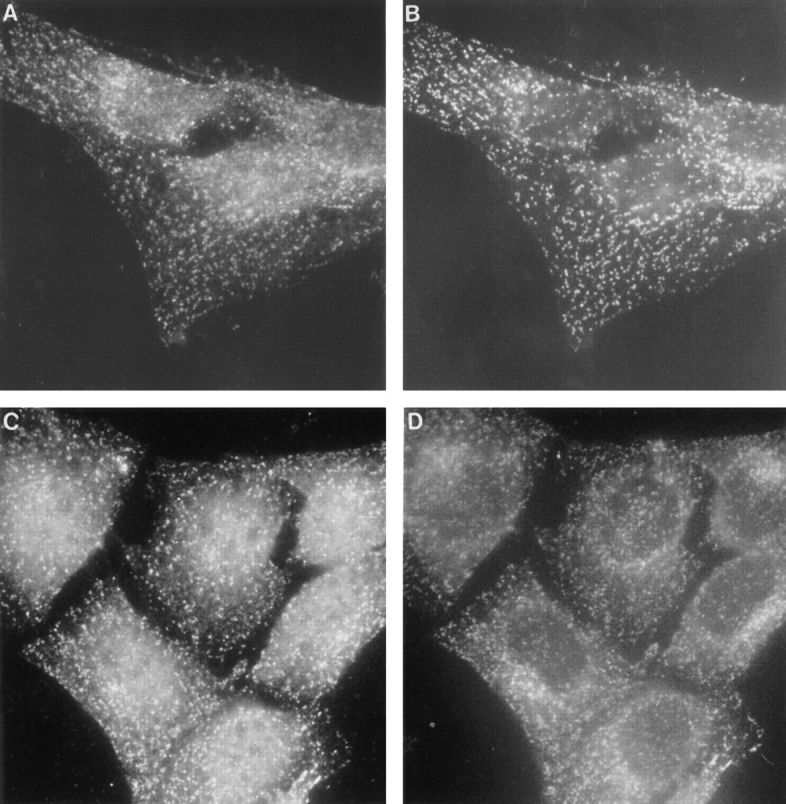
Immunolocalization of Eps15, α-adaptin, and clathrin HC. HER14 cells were grown in DME supplemented with 7.5% FCS, fixed and permeabilized as described in Materials and Methods, and stained with anti-Eps15 antibody (A and C), anti–α-adaptin antibody (B) or anti-clathrin HC antibody (D).
To verify these colocalization experiments, cells were analyzed using confocal scanning laser microscopy. HER14 cells were labeled with anti-Eps15 antibody, which is seen as the green stain (Fig. 7 A , column 1), and with anti–α-adaptin antibody, which is seen as the red stain (Fig. 7 A, column 2). A clear colocalization between Eps15 and α-adaptin is demonstrated by the yellow stain of the endocytic vesicles (Fig. 7 A, columns 3 and 4). Labeling of cells with antiEps15 (Fig. 7 B, column 1), together with anti–clathrin HC (Fig. 7 A, column 2) revealed a punctate pattern for each protein; however, only a partial colocalization was found, visible as the yellow stain (Fig. 7 B, column 3 and 4). In contrast to Eps15, clathrin showed additional staining surrounding the nucleus. Both AP-2 and clathrin were present in coated pits and coated vesicles, but not in early endosomes. To determine whether Eps15 was located in early endosomes, colocalization studies were performed with the specific early endosome markers rab4 and rab5, two members of the rab family of small ras-like, GTPbinding proteins (Chavrier et al., 1990; van der Sluijs et al., 1992). CHO cells stably transfected with cDNA of NHtagged rab4 and G-tagged rab5 (van der Sluijs, P., manuscript submitted for publication) were stained with an Eps15 antibody (Fig. 7, C and D, column 1), and with antiNH and anti-G antibodies (Fig. 7, C and D, column 2). Although both stainings show a punctate labeling, no colocalization was found between Eps15 and rab4 or rab5, suggesting that Eps15 is not present in early endosomes (Fig. 7, C and D, columns 3 and 4).
Figure 7.
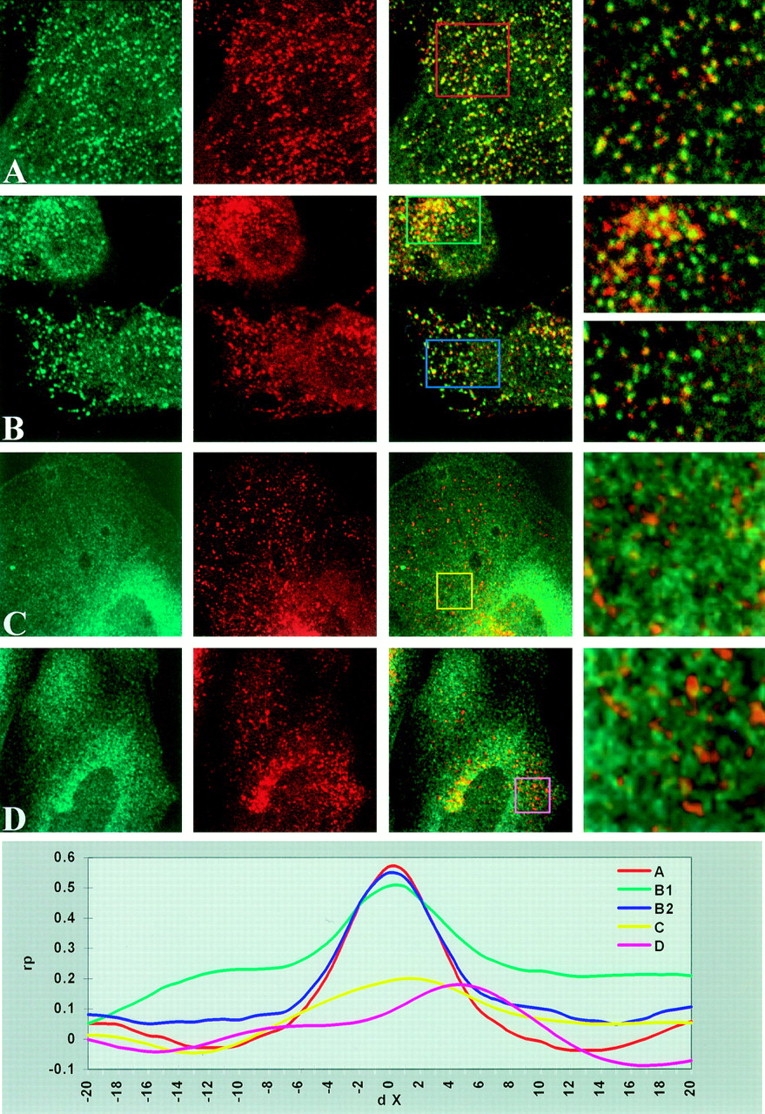
Colocalization studies of Eps15, α-adaptin and clathrin HC. HER14 cells were grown in DME supplemented with 7.5% FCS, fixed and permeabilized as described in Materials and Methods, and stained with anti-Eps15 antibody (A and B, column 1) and anti–αadaptin antibody (A, column 2) or anti–clathrin HC antibody (B, column 2). Superimposed images of Eps15 and α-adaptin or Eps15 and clathrin HC staining are shown in columns 3 and 4 (A and B). Likewise, CHO cells stably transfected with rab4 and rab5 cDNA were stained with anti-Eps15 antibody (C and D, column 1) and anti-NH antibody (rab4; C, column 2) or anti-G antibody (rab5; D, column 2). Superimposed images of Eps15 and rab4 or rab5 staining are shown in columns 3 and 4 (C and D). The graph represents the cross-correlation function of the three-dimensional images indicated by the colored squares. For all cross-correlation functions, rP was determined at Δx intervals of 0.4 μm.
To rule out that the colocalization between Eps15 and AP-2 or clathrin resulted from occasional overlap of randomly distributed vesicles and to determine that the spatial distribution of Eps15 and AP-2 or clathrin are positively correlated, a cross-correlation function was calculated, as developed by van Steensel and co-workers (1996). In a graph showing the Δx over which the image has been shifted on the x-axis and the Pearson's correlation coefficient rP on the y-axis, a colocalization will show as a peak with the maximum at Δx = 0, exclusion will result in a dip at Δx = 0, while a random distribution will result in neither a dip nor a peak. The rP is calculated as a measure of colocalization, and an rP of 1.0 indicates a 100% colocalization. This cross-correlation analysis is depicted as a graph in Fig. 6. This results for Eps15 and α-adaptin in a clear peak at Δx = 0 (red line) with a colocalization of ∼57%. For Eps15 and clathrin, two different areas of the cell were analyzed. Near the plasma membrane (blue line), a clear colocalization of 55% was found, and the area around the Golgi network (green line) showed a much lower colocalization of 30% (the baseline of this graph starts at 0.2, and therefore this value has to be deducted from the rP). In contrast, the labeling of Eps15 together with either rab4 or rab5 did not show any correlation (yellow and pink lines).
In conclusion, these colocalization experiments show that Eps15 is present in clathrin-coated pits and vesicles together with AP-2, whereas Eps15 is completely absent in early endosomes.
Eps15 Is Present in AP-2 and Clathrin-stripped Membranes
The experiments described above demonstrate the association of Eps15 with the EGF receptor, α-adaptin and clathrin, which was confirmed by colocalization studies. Furthermore, we have shown that Eps15 is located to clathrin-coated pits and vesicles. It is not clear, however, whether α-adaptin and clathrin are required for the localization of Eps15 in coated vesicles. To investigate this, HER14 cell membranes containing coated pits were isolated according to the method of Chang et al. (1993a). These membranes allow for the removal of clathrin from coated pits by an incubation with a high pH buffer and the AP-2 coat can be subsequently extracted by incubation of the membranes with a 0.5-M Tris buffer (Unanue et al., 1981). Under these conditions, the presence of Eps15 was determined by immunolabeling. In untreated membranes, labeling with anti-clathrin or anti–α-adaptin antibody showed a clear punctate pattern similar to our previous results (Fig. 8, A and D). After treatment of the membranes with a high pH buffer, clathrin HC labeling was absent (Fig. 8 B), but AP-2 and Eps15 labeling was still present (Fig. 8 E). Subsequent treatment with an 0.5 M Tris buffer abolished AP-2 labeling (Fig. 8, C and F), which is in agreement with previous results (Unanue et al., 1981; Chang et al., 1993a ). However, Eps15 staining of untreated membrane fragments showed still a punctate pattern, similar to the AP-2 and clathrin staining (Fig. 8 G). Interestingly, extraction of both AP-2 and clathrin did not change this pattern (Fig. 8, H and I). These data suggest that the presence of Eps15 in coated pits is independent of α-adaptin or clathrin.
Figure 8.
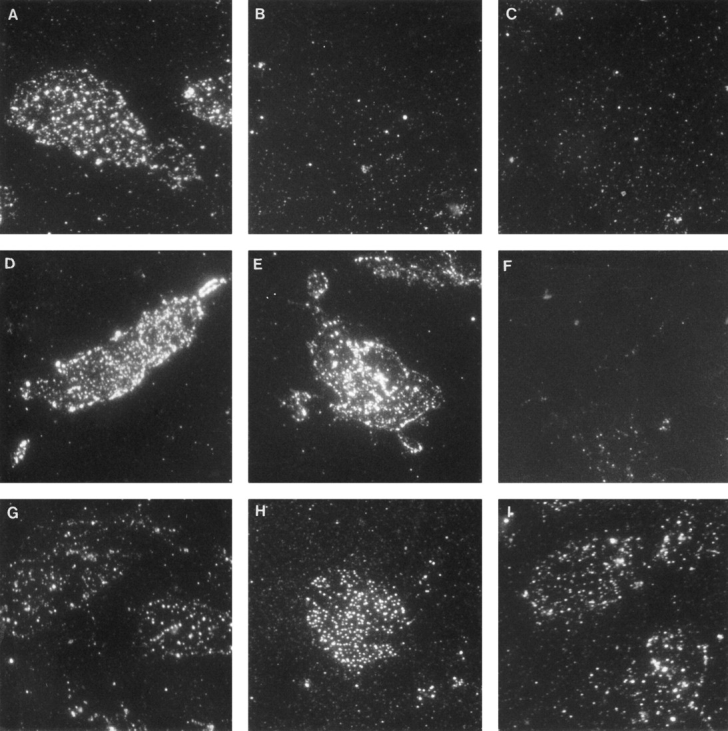
Punctated Eps15 labeling on membranes after AP-2 and clathrin extraction. Membranes from HER14 cells were prepared by rapid freeze-thaw rupturing of the cells. Membranes were left untreated (A, D, and G), treated with 20 mM TAPS buffer, pH 9.2 (B, E, and H), or treated with 0.5 M Tris, pH 7.0 (C, F, and I). Subsequently, membranes were stained with anti–clathrin HC antibody (A–C), anti–α-adaptin antibody (D–F) or anti-Eps15 antibody (G–I).
Discussion
Eps15 is a substrate of the EGF receptor, and exhibits homology with the yeast protein End3, a protein involved in the endocytosis of the α factor in S. cerevisiae (Fazioli et al., 1993; Benedetti et al., 1994). Recently, Eps15 has been shown to bind directly to the ear of α-adaptin, a component of the AP-2 complex (Benmerah et al., 1995). In this paper, we have investigated the possible role of Eps15 in EGF receptor endocytosis. We have shown that Eps15 phosphorylation is mediated by the EGF receptor, and not by PDGF or insulin receptors, which suggest a possible function of Eps15 specifically in the endocytosis of EGF receptors. Immunoprecipitation studies revealed that Eps15 can be co-immunoprecipitated with the EGF receptor. Moreover, the association of Eps15 with the EGF receptor increased dramatically after activation of the receptor, while the association of Eps15 with AP-2 or clathrin LC appeared to occur independently of EGF. The association of Eps15 to the EGF receptor has not been reported before. In previous studies, Fazioli and co-workers did not detect Eps15 in EGF receptor immunoprecipitates. This difference is probably caused by differences in receptor numbers of the cell lines used.
A possible role for Eps15 in the endocytosis of EGF receptors was further investigated by subcellular localization studies. Using a cell fractionation assay, we found that Eps15 was present in the fractions enriched in plasma membrane vesicles and in the fraction enriched in coated vesicles. Furthermore, we have shown that the localization of Eps15 resembled a staining pattern that is similar to coated pits and coated vesicles. In localization experiments using CLSM, it was shown that Eps15 colocalized with AP-2, indicating that the association between Eps15 and AP-2 is localized to coated pits and coated vesicles. A localization experiment with clathrin HC showed that Eps15 partially colocalized with this protein. Besides staining of the endocytotic pits and vesicles, clathrin labeling showed an additional staining in the perinuclear region, representing the exocytotic vesicles from the TGN (Robinson, 1987). These data suggest an involvement of Eps15 in endocytosis from the plasma membrane, but excludes an involvement in exocytosis from the TGN. This is in agreement with the fact that the function of Eps15 is EGF dependent. Additional localization experiments showed no colocalization between Eps15 and rab4 or rab5. Since both rab4 and rab5 are specific early endosome markers, we conclude that Eps15 is not present in early endosomes, but may have a functional role in coated pits and coated vesicles.
Using light microscopy, we were not able to detect an effect of EGF on Eps15 distribution. Obviously, coated vesicles do not redistribute dramatically upon growth factor treatment. A drastic decrease upon EGF stimulation was observed in the amount of Eps15 in the fraction enriched in clathrin-coated vesicles. Interestingly, the ratio of the 140-kD and the modified form of Eps15 was reduced in this fraction. This implies that clathrin-coated vesicles contain more native Eps15 than the modified form of Eps15. This can be explained in two ways. First, Eps15 modification may reduce the binding of Eps15 to the clathrin coat, and second, Eps15 modification may stimulate the uncoating of the coated vesicle. Further experiments are needed to determine the role of Eps15 modification in EGF receptor endocytosis.
An interesting question is where the binding site for Eps15 is located on the EGF receptor. Alvarez and coworkers (1995) have identified a domain of the EGF receptor that is essential for tyrosine phosphorylation of Eps15. Possibly, this domain, encompassing residues 991– 1021 in the COOH-terminal part of the EGF receptor, is not only required for Eps15 phosphorylation, but also for Eps15 association to the EGF receptor. This result, however, was in contrast to the work of Fazioli and co-workers (1993), who suggested that the juxtamembrane region is required for Eps15 phosphorylation (Fazioli et al., 1993). In this paper, we have shown that a mutant EGF receptor, truncated at amino acid 963, can neither phosphorylate nor bind Eps15, suggesting that the Eps15-bindings site is deleted or not functional in this mutant. All EGF receptor mutants that have been described in this region (T973, T957, and T963) have been shown to possess EGF-inducible tyrosine kinase activity, but they can no longer be internalized (Chen et al., 1989). These data suggest that the regulatory domain in the COOH-terminal tail of the EGF receptor is not only involved in endocytosis of the receptor, but also in phosphorylation and binding of Eps15. Interesting in this respect is the difference between the EGF receptor and the oncogenic family member ErbB2. Eps15 is a poor substrate for the ErbB2 receptor (Fazioli et al., 1993), and the endocytosis of the ErbB2 receptor is impaired (Sorkin et al., 1993), supporting the suggestion of Eps15 involvement in endocytosis of EGF receptors.
At the moment, it is also unclear which domain of Eps15 is involved in the association to the EGF receptor. Analysis of Eps15-binding proteins by Far-Western blotting revealed that protein association predominantly occurs with the COOH-terminal part of the protein, domain I (Wong et al., 1995). This domain contains three EH domains (Eps15 homology domain) that may be responsible for these interactions (Wong et al., 1995). Domain II of Eps15 contains an α-helical coiled-coil structure (Fazioli et al., 1993). Coiled-coil structures have been shown to represent common protein-binding motifs, e.g., in subunits from intermediate filaments. Domain III has been shown to bind directly to the ear of α-adaptin and; furthermore, this domain contains a proline-rich motif that binds directly to the oncogenic adaptor protein v-Crk (Benmerah et al., 1995; Schumacher et al., 1995). v-Crk has been shown to bind directly in vitro to the activated EGF receptor (Schumacher et al., 1995). At residue 992, the EGF receptor contains a possible Crk-SH2 phosphotyrosine binding motif, YLIP (Songyang et al., 1993). It is tempting to speculate that the interaction between the EGF receptor and Eps15 might be mediated by Crk. In this model, the SH2 domain of v-Crk may mediate the association to the tyrosine-phosphorylated EGF receptor, whereas the SH3 domain of v-Crk may be involved in the binding to the proline-rich motif of Eps15 (Schumacher et al., 1995). Although Crk binding to the EGF receptor has been determined for v-Crk, it is possible that c-Crk will act in a similar manner. Support for this hypothesis comes from the T963 EGF receptor mutant, which lacks the putative Crk– SH2 binding motif and neither binds nor phosphorylates Eps15. Clearly, more research is required to determine the domains of the EGF receptor and of Eps15, which are involved in this interaction.
As shown in this study, Eps15 and α-adaptin are associated to each other in an EGF-independent manner. Both proteins, however, bind the EGF receptor in an EGF-dependent manner. This may suggest that either some receptors will bind AP-2 and others will bind Eps15, or that the binding of an Eps15–AP-2 complex to the EGF-receptor is increased. Moreover, the clathrin triskelions might also be components of the Eps15–AP-2 complex, since Eps15 is present in a clathrin LC immunoprecipitate. However, the clathrin LC could not be detected in an EGF receptor immunoprecipitate, which is in agreement with published data (Boll et al., 1995; Sorkin and Carpenter, 1993). An important question is whether AP-2 or clathrin are required for the presence of Eps15 in coated vesicles. To address this question, we have demonstrated that in plasma membranes stripped from AP-2 and clathrin, Eps15 was still detectable in a staining pattern resembling that of AP-2 and clathrin. This observation suggests that neither AP-2 nor clathrin are required for the presence of Eps15 in coated vesicles.
In conclusion, our observations all point to the hypothesis that Eps15 is involved in the internalization of EGF receptors via the coated vesicle pathway. Eps15 may mediate the interaction between activated EGF receptors and the coated pit. The increase of Eps15 binding to activated EGF receptors suggests that Eps15 selectively recruits activated EGF receptors to coated pits. The absence of Eps15 in the early endosome indicates that Eps15 functioning is restricted to the early stages of EGF receptor internalization.
Acknowledgments
We wish to thank Willem Stoorvogel and Peter van der Sluijs (Department of Cell Biology, Utrecht University, School of Medicine) for stimulating discussions, cell lines, and antibodies; Marcel van der Heijden for the T963 fibroblasts; Lisette Verspui and Theo van der Krift for photographic reproductions; and Eva Ludérus and Paul Coffer for critical reading of the manuscript.
This work was supported by the Life Sciences Foundation (Levenswetenschappen, grant 17.182), which is subsidized by the Netherlands Organization for Scientific Research (Nederlandse Wetenschaps Organisatie).
Abbreviations used in this paper
- AP
adaptor protein
- CSLM
confocal laser scanning microscope
- G
vesicular stomatitis virus G protein
- HC
heavy chain
- LC
light chain
- NH
hemagglutinin
- TAPS
N-Tris[hydroxymethyl]methyl-3-amino-propanesulfonic acid buffer
Footnotes
Please address all correspondence to Sanne van Delft, Department of Molecular Cell Biology, Institute of Biomembranes, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands. Tel.: 31-30-253-3349; Fax: 31-30-251-3655; E-mail: sanne@emsaserv.biol.ruu.nl
References
- Alvarez CV, Shon KJ, Miloso M, Bequinot L. Structural requirements of the epidermal growth factor receptor for tyrosine phosphorylation of Eps8 and Eps15, substrates lacking Src SH2 homology domains. J Biol Chem. 1995;270:16271–16276. doi: 10.1074/jbc.270.27.16271. [DOI] [PubMed] [Google Scholar]
- Benedetti H, Raths S, Crausaz F, Riezman H. The END3gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Gagnon J, Begue B, Megarbane B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate Eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Beque J, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Boll W, Gallusser A, Kirchhausen T. Role of the regulatory domain of the EGF-receptor cytoplasmic tail in selective binding of the clathrin-associated complex AP-2. Curr Biol. 1995;5:1168–1178. doi: 10.1016/s0960-9822(95)00233-8. [DOI] [PubMed] [Google Scholar]
- Bottger G, Nagelkerken B, van der Sluijs P. Rab4 and Rab7 define distinct nonoverlaping endosomal compartments. J Biol Chem. 1996;271:29191–29197. doi: 10.1074/jbc.271.46.29191. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope DF, Russell R. Platelet-derived growth factor. J Biol Chem. 1982;257:5161–5171. [PubMed] [Google Scholar]
- Burgering BMTh, Medema RH, Maassen JA, van de Wetering ML, van der Eb AJ, McCormick F, Bos JL. Insulin stimulation of gene expression mediated by p21ras activation. EMBO (Eur Mol Biol Organ) J. 1991;10:1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MP, Mallet WG, Mostov KE, Brodsky F. Adaptor self aggregation, adaptor-receptor recognition and binding of α-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP-2) components of clathrin-coated pits. EMBO (Eur Mol Biol Organ) J. 1993a;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Lazar CS, Walsh BJ, Komuro M, Collawn JF, Kuhn LA, Tainer JA, Trowbridge IS, Farquhar MG, Rosenfeld MG, et al. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytotic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993b;268:19312–19320. [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen WS, Lazar CS, Lund KA, Welsh JB, Chang CP, Walton GM, Der CJ, Wiley HS, Gill GN, Rosenfeld MG. Functional independence of the epidermal growth factor from a domain required for ligand- induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Evans, W.H. 1992. Isolation and characterization of membranes and cell organelles. In Preparative Centrifugation: A Practical Approach. D. Rickwood, editor. Oxford University Press, Oxford. 233–270.
- Faure R, Baquiran G, Bergeron JJM, Posner BI. Dephosphorylation of insulin and epidermal growth factor receptors. J Biol Chem. 1992;267:11215–11221. [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. Eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardi L, Koppelman B, Blum JS, Marks MS, Cresswell P, Brodsky F. Colocalization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature (Lond) 1990;343:133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Kreis TE, Lodish HF. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986;46:929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H, Wells A, Lazar CS, Rosenfeld MG, Gill GN. Enhanced tumorigenesis of NR6 cells that express non-down-regulating epidermal growth factor receptors. Cancer Res. 1991;51:6170–6175. [PubMed] [Google Scholar]
- Northwood IC, Davis RJ. Signal transduction by the epidermal growth factor receptor after functional desensitization of the receptor tyrosine kinase activity. Proc Natl Acad Sci USA. 1990;87:6107–6111. doi: 10.1073/pnas.87.16.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF, Crowther RA. Structure and assembly of coated vesicles. Ann Rev Biophys Biochem Commun. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- Robinson MS. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Margolis B, Mohammadi M, Daly RJ, Daum G, Li N, Fischer EH, Burgess WHM, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor at Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C. EMBO (Eur Mol Biol Organ) J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988;13:443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Schmid SL. The mechanism of receptor-mediated-endocytosis: more questions than answers. BioEssays. 1992;14:589–596. doi: 10.1002/bies.950140903. [DOI] [PubMed] [Google Scholar]
- Schumacher C, Knudsen BS, Ohuchi T, Di Fiori PP, Glassman RH, Hanafusa H. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J Biol Chem. 1995;270:15341–15347. doi: 10.1074/jbc.270.25.15341. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish GG, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science (Wash DC) 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Di Fiore PP, Carpenter G. The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene. 1993;8:3021–3028. [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. BioEssays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Unanue ER, Ungewickell E, Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981;26:439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Goud B, Mellmann I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- van Steensel B, Van Binnendijk EP, Hornsby D, Van Der Voort HTM, Krozowski ZS, de Kloet R, Van Driel R. Partial colocalization of glucocorticoid and mineralcorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci. 1996;109:787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a non-internalizing epidermal growth factor receptor. Science (Wash DC) 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Winkler ME, O'Connor L, Winget M, Fendly B. Epidermal growth factor and transforming growth factor alpha bind differently to the epidermal growth factor receptor. Biochemistry. 1989;28:6373–6378. doi: 10.1021/bi00441a033. [DOI] [PubMed] [Google Scholar]
- Wong WT, Schumacher C, Salcini AE, Romano A, Castagnino P, Pelicci PG, Di Fiore PP. A protein-binding domain, EH, identified in the receptor tyrosine kinase substrate Eps15 and conserved in evolution. Proc Natl Acad Sci USA. 1995;92:9530–9534. doi: 10.1073/pnas.92.21.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]