Abstract
Transport of proteins to the thylakoid lumen is accomplished by two precursor-specific pathways, the Sec and the unique Delta pH transport systems. Pathway selection is specified by transient lumen-targeting domains (LTDs) on precursor proteins. Here, chimeric and mutant LTDs were used to identify elements responsible for targeting specificity. The results showed that: (a) minimal signal peptide motifs consisting of charged N, hydrophobic H, and cleavage C domains were both necessary and sufficient for pathway-specific targeting; (b) exclusive targeting to the Delta pH pathway requires a twin arginine in the N domain and an H domain that is incompatible with the Sec pathway; (c) exclusive targeting to the Sec pathway is achieved by an N domain that lacks the twin arginine, although the twin arginine was completely compatible with the Sec system. A dual-targeting signal peptide, constructed by combining Delta pH and Sec domains, was used to simultaneously compare the transport capability of both pathways when confronted with different passenger proteins. Whereas Sec passengers were efficiently transported by both pathways, Delta pH passengers were arrested in translocation on the Sec pathway. This finding suggests that the Delta pH mechanism evolved to accommodate transport of proteins incompatible with the thylakoid Sec machinery.
Many proteins of the chloroplast thylakoid membranes are encoded in the nucleus, synthesized in the cytosol, and localized by a two-step process (for review see Cline and Henry, 1996). In the first step, cytosolic precursors are imported across the chloroplast envelope membranes into the stroma. In the second step, stromal intermediates are integrated into the thylakoid membrane or transported across it into the lumen. Precursors destined for the lumen possess bipartite aminoterminal transit peptides. The amino-proximal stroma-targeting domain (STD)1 directs import into the chloroplast and is removed by a stromal processing protease. The carboxy-proximal lumen-targeting domain (LTD) governs transport into the lumen and is cleaved by a lumen-facing thylakoidal processing protease. Thus, the stromal intermediates of luminal proteins are intermediate in size between the full precursor and the mature form.
Four precursor-specific pathways (or mechanisms) for protein transport/integration into thylakoids have been described (Cline and Henry, 1996). In particular, transport of proteins to the lumen occurs by two distinct pathways: the Sec and Delta pH translocation systems. Transport of one subset of lumen-resident proteins by the Sec pathway is stimulated by a ΔpH and requires ATP (Hulford et al., 1994; Yuan and Cline, 1994) and CPSecA (Nakai et al., 1994; Yuan et al., 1994). CPSecA is a chloroplast homologue of the bacterial SecA protein, a translocation ATPase (Wickner, 1994). Transport of a second subset of lumen-resident proteins by the Delta pH pathway takes place uniquely in the absence of nucleotides or soluble factors and is absolutely dependent on the trans-thylakoid ΔpH (for reviews see Robinson and Klösgen, 1994; Cline and Henry, 1996).
The existence of these parallel transport pathways is intriguing. Results of biochemical studies argue that the two subgroups of proteins are exclusively transported on their corresponding pathways (Cline and Henry, 1996). Studies of two chimeric proteins indicate that pathway selection is determined by the respective LTDs. A chimeric precursor composed of the transit peptide of OE23 (a Delta pH pathway substrate) and the mature sequence of plastocyanin (PC) (a Sec pathway substrate) was efficiently transported only on the Delta pH pathway (Henry et al., 1994; Robinson et al., 1994). Another chimera that combined the transit peptide of OE33 (Sec pathway) with the mature sequence of OE17 (Delta pH pathway) was exclusively transported on the Sec pathway, although with greatly reduced efficiency (Henry et al., 1994). These observations suggest that the existence of two transport systems serves a purpose beyond that of an overflow mechanism.
Two questions are immediately relevant. First, what elements in LTDs commit a precursor to a specific pathway? All LTDs have embedded motifs for signal peptides similar to those that direct transport across bacterial and ER membranes (von Heijne et al., 1989; Pugsley, 1993). Recent work by Chaddock et al. (1995) indicates that a twin arginine in the signal peptide is required for Delta pH transport. However, the role of other elements in LTDs has not been addressed. A second question: what is the underlying reason for the existence of two transport pathways? It is not related to the specific site of luminal protein function. For example, the OE33 and OE23 subunits of the photosystem II oxygen evolving complex are transported by the Sec and Delta pH pathways, respectively. Nor does it correlate with the attachment of prosthetic groups; e.g., the Sec pathway is responsible for transport of OE33 and PSI-F, which do not possess prosthetic groups, as well as the copper-binding PC and heme-binding cytochrome f (Voelker and Barkan, 1995; Nohara et al., 1996).
Here we used a biochemical approach to identify elements of LTDs and passenger proteins that determine pathway-specific transport. Our results show that the transport process can be divided into two steps: targeting and translocation. Pathway-specific targeting is mediated solely by the signal peptide motifs of LTDs. In contrast, translocation depends upon passenger protein compatibility with the respective system. Our studies show that the Sec system is not capable of efficiently translocating passenger proteins normally passed through the Delta pH system, whereas the Delta pH system transports passengers from both pathways. These results define the molecular elements that govern specific transport on these systems and further suggest that the novel Delta pH pathway may have arisen to compensate for translocation limitations of the thylakoid Sec mechanism.
Materials and Methods
Materials
All reagents, enzymes, and standards were purchased commercially. In vitro transcription plasmids for precursors to OE23 (pOE23), OE33 (pOE33), the stromal intermediate of OE33 (iOE33), plastocyanin (pPC) from pea, pPC from Arabidopsis, and the precursor for OE17 (pOE17) from maize have been described elsewhere (Cline et al., 1993). The transcription plasmid for PSII-T and the chimeric precursor between the OE23 transit peptide and the PC mature protein (23tPCsel) were as described (Henry et al., 1994). The transcription plasmid for PSI-N from Arabidopsis (accession No. U32176) was the generous gift of Dr. Paul Sehnke (University of Florida, Gainesville). Escherichia coli–expressed and purified iOE23 was as described (Cline et al., 1993).
Construction of Recombinant Precursor Proteins
Coding sequences for all recombinant precursor proteins were constructed by PCR-based methods using the above plasmids as templates, as well as templates prepared in this study and described below. Most amplifications were performed with Pfu polymerase (Stratagene, La Jolla, CA), and the remainder with Taq polymerase. PCR products containing restriction sites incorporated into the forward, reverse, or both primers were digested with the appropriate restriction enzymes as noted below and ligated into appropriately restricted pGEM 3Z (Promega, Madison, WI) or pGEM 4Z in the SP6 direction. When PCR primers lacked restriction sites, PCR reactions were conducted with Pfu, and PCR products were blunt-end cloned into the HincII or SmaI sites of pGEM 3Z or 4Z. All cloned constructs were verified by DNA sequencing. Sequencing was done with ABI Prism Dye Terminator cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, CA) and an Applied Biosystems model 373 Stretch DNA Sequencer (Perkin-Elmer Corp.).
Truncated Precursors.
The coding sequence for the intermediate form of OE23 (iOE23) was constructed as described previously (Cline et al., 1993), except that the forward primer incorporated an XbaI restriction site for ligating into the XbaI–HindIII site of pGEM 4Z. The coding sequence for tOE23, an amino-terminal truncated form of iOE23, was constructed in the same manner as iOE23 except that the initiator methionine incorporated in the forward primer was positioned so that the tOE23 translation product begins MVSRR (see Fig. 1). A further truncation of tOE23 that lacks the N domain was similarly constructed; the initiator methionine was positioned such that the translation product starts MLALSV. The presumed intermediate form of OE17 (iOE17) and an amino-terminal truncated form of iOE17 (tOE17) were amplified using the pOE17 plasmid as template. The forward primers for iOE17 and tOE17 were designed such that the translation products started with MASAE and MAGRR, respectively. The PCR products were cloned into the SmaI–XbaI site of pGEM 4Z. Amino-terminal truncated precursors of PC (tPC) from Arabidopsis and pea were amplified from the respective pPC plasmids with forward primers designed such that translation products started MASLKD. The PCR products were cloned into the HincII–SstI site of pGEM 3Z. An additional truncation of tPC from Arabidopsis to remove the N domain used a forward primer to give a translation product beginning MFGVIA.
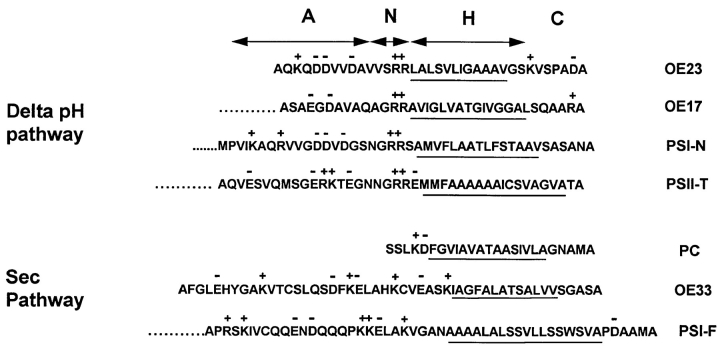
Figure 1.
LTDs of precursors targeted to the Delta pH or Sec transport systems in thylakoid membranes. The acidic (A), charged (N), and hydrophobic (H) regions are shown for LTDs of proteins transported by the Delta pH pathway or Sec pathway. OE33 (from pea), OE23 (from pea), and OE17 (from maize) are the 33-, 23-, and 17-kD subunits of the oxygen evolving complex of photosystem II. PSII-T (from cotton) is the T subunit of photosystem II. PSI-F (from spinach) and PSI-N (from Arabidopsis) are the F and N subunits of photosystem I, respectively. The PC LTD from Arabidopsis is depicted. The cleavage consensus A-X-A appears on the far right of the sequences. The hydrophobic residues of the H domain are underlined, and charged residues are indicated by (+) or (−). The amino termini for OE23, OE33, and PC correspond to those determined by Bassham et al. (1991). The precise amino termini for LTDs of OE17, PSI-N, PSII-T, and PSI-F are not known.
Chimeric Precursors.
The chimeric precursor t23-PC is an exact fusion between coding sequences for the t23 signal peptide (MVSRRLAL . . . SPADA) and the mature domain of Arabidopsis PC (MEVLL . . . LTVK). The t23-PC coding sequence was constructed using 23tPCsel as template and the same forward primer as that used to make tOE23. The PCR product was cloned into XbaI–SstI cut pGEM 3Z. PCn23h/c-PC and 23nPCh/cPC are chimeric truncated precursors in which combinations of N and H/C domains from OE23 and Arabidopsis PC were fused to the sequence for mature Arabidopsis PC. PCn23h/c-PC was constructed with t23-PC as template. The forward primer incorporated the coding sequence for the tPC N domain and the first 16 bases of the OE23 H/C region. This created an exact fusion such that the translated protein begins MASLKD-LALSV . . . SPADA followed by mature PC. The PCR product was cloned into the HincII–SstI site of pGEM 3Z. 23nPCh/c-PC, which is referred to in the text as DT-PC, was constructed with Arabidopsis pPC as template. The forward primer incorporated the coding sequence for the OE23 N domain and the first 16 bases of the PC H domain such that the translated polypeptide begins MVSRR-FGVIA . . . GNAMA followed by mature PC. The PCR product was cloned into the SmaI–PstI site of pGEM 4Z.
The DT-PC coding sequence served as template for constructing exact fusions between the coding sequence of the DT signal peptide and the mature domains of OE33 (DT-33), OE23 (DT-23), OE17 (DT-17), PSII-T (DT-T), and PSI-N (DT-N). Two stages of PCR reactions were used in the construction of these clones. In the first stage, two PCR reactions produced fragments coding for the DT signal and the appropriate mature sequence, respectively. The forward primer for mature sequence contained an overlap corresponding to last 15 bases of the DT coding sequence. The two PCR products were purified and spliced by overlap extension (SOE) in a third PCR reaction (Horton et al., 1989). The DT-33, DT-23, DT-17, DT-T, or DT-N SOE products were restricted and ligated into pGEM 4Z cut with SstI–XbaI, EcoRI–HindIII, EcoR1, EcoRI–HincII, and EcoRI– HincII, respectively.
DT-PC 23h/PCc encodes a polypeptide in which the H domain of DTPC was replaced with the OE23 H domain. This was produced by PCR/ SOE using tOE23 and pPC as templates for the first two PCR reactions. The SOE product was cloned into the HindIII–SstI site of pGEM 3Z. The encoded polypeptide begins MVSRR-LALSVLIGAAAVGS-AMA. DTPC PCh/c23c is a construct in which the OE23 C domain was inserted between the PC H/C domain and mature PC of DT-PC. The coding sequence for the OE23 C domain was inserted using PCR/SOE with DT-PC and 23tPCsel serving as templates for the first two PCR reactions. The SOE product was cloned into the SstI site of pGEM 4Z. The encoded translation product begins MVSRR-FGVIAVATAASIVLAGNAMAKVSPADA followed by mature PC.
DT-PC 17h/c and DT-PC 33h/c are constructs in which the H/C coding region of DT-PC was replaced with the OE17 and OE33 H/C regions, respectively. Both constructs were generated by first amplifying the appropriate H/C region from pOE17 and pOE33 templates. The forward primers incorporated an MVSRR coding sequence; the reverse primers contained an overlap for the 5′ end of the mature Arabidopsis PC sequence. In an SOE reaction, PCR-amplified mature PC was then spliced to the 3′ end of the OE17 or OE33 H/C coding regions and the SOE products were cloned into HincII–SstI-digested pGEM 3Z. The resulting constructs produced translated polypeptides that begin MVSRR-AVIGL . . . AARA and MVSRR-IAGFA . . . GASA, respectively, followed by mature PC.
Site-directed Mutation of DT-PC.
KK-PC is an altered form of DT-PC in which the twin arginine in the signal sequence was changed to a twin lysine. The coding region was amplified with the Arabidopsis pPC plasmid as template and a forward primer that codes for the sequence starting MVSKK-FVGIA. The PCR product was cloned into the HincII–SstI site of pGEM 3Z. DT-PC E/K codes for DT-PC in which the second amino acid (glutamate) of mature PC was changed to a lysine using PCR/SOE. The single amino acid change was made by altering the appropriate bases in the two overlapping internal primers used in the first two PCR reactions. The resulting SOE product was cloned into the SstI site of pGEM 4Z. The same method was used to change a lysine in the 23 C domain to asparagine in PCn23h/c-PC, resulting in PCn23h/c-PC K/N. The amplified SOE product was cloned into the HindIII–SstI site of pGEM 3Z. Several mutants were constructed in which aspartic acid replaced valine residues in the H domain of DT-PC, i.e., amino acids 8, 11, and 18, respectively. These mutations were made by PCR/SOE using the DT-PC as template and incorporating GTC to GAC codon changes in the forward primer for the first set of PCR reactions. The SOE product was cloned into the SstI site of pGEM 4Z.
Constructs for Expression in E. coli.
Coding sequences for t23 and DT23 were prepared for expression in E. coli by amplifying each sequence from pGEM clones (see above). The forward primer contained an NdeI site that also encoded the initiator methionine. The PCR products were digested with NdeI and HindIII and cloned into pETH3c (Cline et al., 1993).
Preparation of Precursor Proteins
Capped RNA for authentic, chimeric, and mutant precursors was produced in vitro with SP6 polymerase and uncut plasmid; RNA was translated in a wheat germ system in the presence of [3H]leucine (Cline et al., 1993). Translation products were generally diluted threefold and adjusted to import buffer (50 mM Hepes/KOH, pH 8.0, 0.33 M sorbitol), containing 30 mM unlabeled leucine.
Preparation of Chloroplasts, Lysates, Thylakoids, and Stromal Extract
Intact chloroplasts were isolated from 9–10-d-old pea seedlings (Laxton's Progress 9) and were resuspended in import buffer. Lysates and washed thylakoids were prepared from isolated chloroplasts (Cline et al., 1993). Stromal extract (SE) for transport assays was prepared from chloroplast lysate (1.0 mg/ml chlorophyll) by centrifugation for 8 min at 3,200 g to remove the thylakoids, followed by centrifugation at 40,000 g for 30 min to remove the envelope membranes. Chlorophyll was determined according to Arnon (1949).
Preparation of Purified CPSecA
CPSecA was purified from SE as described by Yuan et al. (1994), except that studies reported here used CPSecA obtained after the Mono-Q ion exchange step. The concentration of purified CPSecA was estimated by Coomassie staining of SDS polyacrylamide gels using BSA as a standard.
Assays for Thylakoid Protein Transport
Transport of radiolabeled proteins into thylakoids was conducted with chloroplast lysate or washed thylakoids as previously described (Cline et al., 1993) in 150-μl assays (unless noted otherwise in the figure legend) containing 50 μg chlorophyll and 5 mM Mg-ATP (pH 8.0). Assays were conducted in microcentrifuge tubes in a 25°C water bath illuminated with 70 μE/m2/s of incandescent light. For assays conducted in the presence of inhibitors, chloroplast lysates (50 μg chlorophyll in 100 μl) were preincubated with azide (7 mM final) or a combination of the ionophores nigericin (0.5 μM final) and valinomycin (1.0 μM final) on ice for 15 min before the addition of Mg-ATP (5 mM final) and radiolabeled precursor. For assays conducted in the absence of ATP, lysate (50 μg chlorophyll in 50 μl) and diluted translation product (25 μl) were preincubated separately for 10 min at room temperature with 1 U of apyrase. Competition assays for thylakoid transport were conducted as described previously (Cline et al., 1993). SE equivalent to 50 μg chlorophyll or purified CPSecA (80 nM final) was added to competition assays as noted in the figure legends. Recovered thylakoids were posttreated with thermolysin, which was terminated by adding an equal volume of 50 mM EDTA in import buffer, collected by centrifugation, and dissociated with SDS-PAGE sample buffer.
Analysis of Samples
Samples from the above assays were analyzed by SDS-PAGE followed by fluorography. Quantification of transport was by scintillation counting of radiolabeled proteins extracted from excised gel bands (Cline, 1986) and is reported as a percentage of radiolabeled precursor added to the assay.
Expression of Proteins in E coli
Expression plasmids for tOE23 and DT-23 were introduced into the host BL21(λDE3). 5-ml cultures in Luria-Bertani medium containing 0.1 mg/ ml ampicillin were initiated from overnight colonies and grown at 37°C to an OD ∼0.8 at 600 nm. Expression was induced with 1.0 mM isopropyl β-d-thiogalactoside. For inhibition of SecA-mediated transport, cultures were adjusted to 2 mM sodium azide at the time of induction. After an additional 2 h of culture, cells (1.4 ml) were pelleted and fractionated into periplasm and cell contents according to the Novagen (Madison, WI) protocol. Basically, the cell pellet was resuspended in residual media, and 15 μl of chloroform was added, followed after 15 min at room temperature by 75 μl of 10 mM Tris/HCl, pH 8.0. The periplasm and cell pellet were separated by centrifugation for 15 min at 12,000 g. Residual chloroform was removed from cell pellets, and pellets were resuspended in the same volume as the periplasm fraction before analysis by SDS-PAGE. For fractionation of cells into soluble and insoluble fractions, 50 ml of culture was pelleted at 3,000 g for 10 min. The pellet was resuspended in 5 ml of 10 mM Tris/HCl, pH 8.0, 2 mM EDTA, and 0.1 mg/ml lysozyme. Triton X-100 was added to 0.1%, and the suspension was allowed to sit at room temperature for 15 min. The suspension was passed twice through a Yeda Press (Cline et al., 1993). Soluble and insoluble fractions were separated by centrifugation at 12,000 g for 15 min.
Results
Domain Composition of Lumen-targeting Peptides
LTDs for precursors known to be transported on Delta pH and Sec pathways are shown in Fig. 1. Embedded within each LTD is a canonical signal peptide motif. In E. coli, the typical signal peptide possesses a five- to six-residue positively charged amino-terminal N domain, followed by an ∼12-residue hydrophobic core H domain, and a more polar cleavage C domain (Izard and Kendall, 1994). The N and H domains are important for transport; the C domain is necessary only for proteolytic processing (Izard and Kendall, 1994). In addition to this minimal signal peptide, nearly all LTDs possess extended amino-terminal regions that are notable for their content of acidic residues, which are uncommon in transit peptides. In the present study, these acidic regions are called A domains. Despite the fact that LTDs govern pathway selection, only one pathway-related consensus sequence has been identified; Delta pH precursors invariably contain a twin arginine in their N domains (Fig. 1).
Pathway-specific Transport Requires Only the Signal Peptide Motif of LTDs
Our strategy for identifying essential and pathway-specific elements was to treat each LTD domain as a module, to create deletions and swapping constructs with these modules, and then to test the recombinant precursor proteins in thylakoid transport assays. Based on previous analyses of chimeric proteins, we focused on the precursors for OE23 (Delta pH pathway) and PC (Sec pathway). Transport into isolated thylakoids was selected as an assay because it yields unambiguous assignments for transport pathway. Criteria used to assign precursors to the Sec or Delta pH pathway include: (a) energy requirements unique to each pathway; (b) the requirement for stromal extract of which CPSecA is the essential component; and (c) precursor competition for transport, which relies on the ability to saturate components unique to each transport system with chemical quantities of precursor.
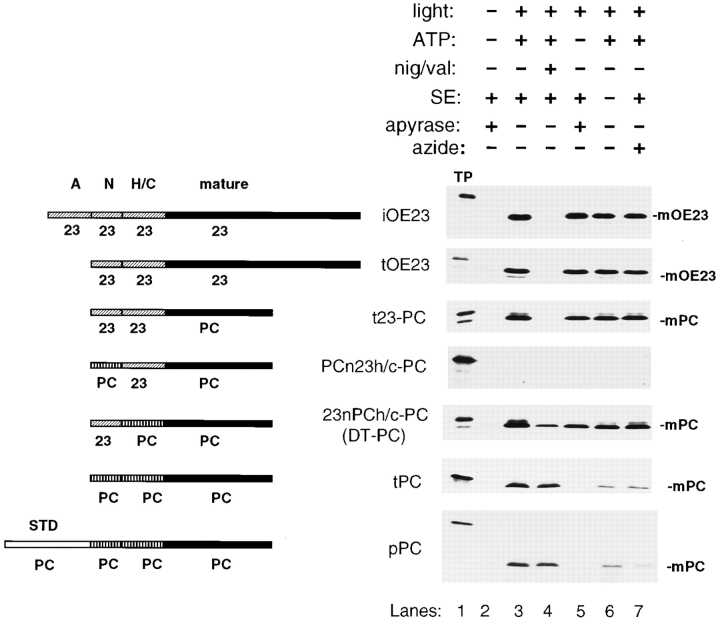
Pathway-specific energy and stroma requirements are illustrated in Fig. 2. Transport of pOE23 (not shown) and the stromal intermediate form (iOE23) was completely abolished by ionophores that dissipate the ΔpH (lane 4). As with other precursors that use the Delta pH pathway, transport was unaffected by removal of ATP (lane 5), by the absence of stromal extract (lane 6), or by sodium azide (lane 7), a SecA inhibitor (Oliver et al., 1990). In contrast, pPC transport, which is stimulated only slightly by a ΔpH (Yuan and Cline, 1994), was relatively unaffected by ionophores. Like other precursors that are localized by the Sec system, pPC transport was abolished by removing ATP (lane 5) and greatly reduced by azide (lane 7) or removing stromal extract (lane 6), the source of ∼90% of the CPSecA found in chloroplasts.
Figure 2.
The signal peptide motifs of LTDs are necessary and sufficient for targeting to the Sec or Delta pH pathway. Transport of precursors across thylakoid membranes was conducted for 30 min at 25°C with lysate to provide stromal extract (SE) or with washed thylakoids (see Materials and Methods). Assay conditions were designed to examine the requirement for SE, ATP, a ΔpH, or sensitivity to azide. Apyrase was used to eliminate residual ATP in lysate and translation products. A combination of nigericin (0.5 μM) and valinomycin (1 μM) (nig/val) was used to dissipate the ΔpH induced in the presence of light. These conditions are designated above the fluorogram panels. Recovered thylakoids were posttreated with thermolysin, and then analyzed by SDS-PAGE and fluorography. The radiolabeled precursor (TP) represents 0.8% of the amount in the assay. Lanes were loaded with equivalent amounts of recovered thylakoids representing 13% of each assay. The position of mature protein is indicated on the right of each panel. The sequence composition of each precursor is diagrammed to the left of the fluorogram; the domains of LTDs are patterned to designate their origin from OE23 (slanted lines) or PC (vertical lines). All mature protein sequences are shaded black. Exact sequences are described in Materials and Methods. pPC and tPC shown in the figure were from pea; virtually identical results were obtained with pPC and tPC from Arabidopsis.
Deletion of amino-terminal elements of the transit peptide was without effect on transport specificity. OE23 and PC constructs with minimal signal peptide motifs (tOE23 and tPC, respectively) exhibited transport characteristics identical to the full-length or intermediate precursors (Fig. 2). Measurements made during the linear phase of transport (0–10 min for the Delta pH pathway; 0–30 min for the Sec pathway) showed that tOE23 was transported at least twice as efficiently as either pOE23 or iOE23. Similarly, a truncated form of the OE17 precursor, tOE17 (see Materials and Methods for description), was exclusively transported on the Delta pH pathway with at least twice the efficiency of either pOE17 or iOE17 (data not shown). Transport efficiencies of tPC and pPC from pea were comparable (e.g., see Fig. 2). In contrast, transport of Arabidopsis tPC was only 20–50% as efficient as that of Arabidopsis pPC (data not shown). Further deletions that removed the N domain of OE23 and PC eliminated transport, implying that the N domain is at least a general requirement for transport on either pathway (data not shown). Fig. 2 also shows that a chimeric precursor protein containing the minimal OE23 signal peptide fused to mature PC (t23-PC) was transported exclusively on the Delta pH pathway (Fig. 2), which confirms and extends previous studies (Henry et al., 1994; Robinson et al., 1994).
These data show that the OE23 signal peptide motif is both necessary and sufficient for pathway-specific transport on the Delta pH pathway. The A domains of OE23 and OE17 LTDs are required neither for pathway specificity nor the efficiency of Delta pH pathway transport across isolated thylakoids. The data further show that the PC signal peptide is necessary and sufficient to direct PC across the Sec pathway. The role of amino-terminal regions of the PC transit peptide in transport efficiency is presently unclear.
A Dual-targeting Signal Peptide Directs Passenger Proteins to Both Sec and Delta pH Systems
To assess the targeting role of each signal peptide domain, precursors containing chimeric signals fused to mature PC were constructed. Energy and soluble factor requirements served as initial diagnostics for pathway use. The chimeric precursor PCn23h/c-PC was not transported by either pathway (Fig. 2). Since the efficiency of thylakoid transport is generally greater in organello (within chloroplasts), the PC STD was fused to this construct, and the assay was conducted with intact chloroplasts. The precursor was efficiently imported into chloroplasts but accumulated as an intermediate in the stroma (data not shown). Another modification to this construct was made to eliminate the possibility of incompatibility between the H/C domain and the amino-terminal region of the mature protein (Laforet et al., 1989; see Discussion); the first 23 residues of mature OE23 were inserted immediately after the signal peptide. This precursor also failed to be transported into thylakoids (data not shown).
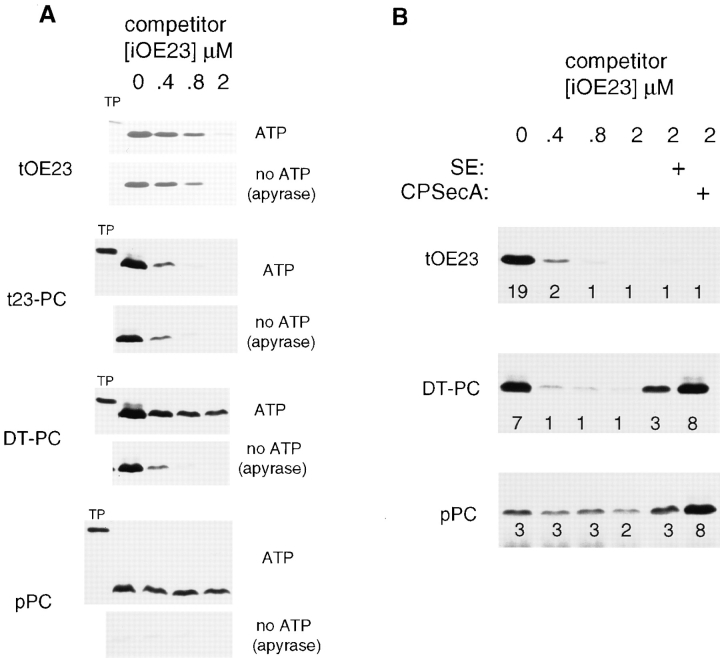
The opposite result was obtained with the reciprocal chimera 23nPCh/c-PC; this precursor was transported by both pathways and therefore is designated dual-targeting (DT)–PC. The first indication for dual targeting was that DT-PC transport requirements were intermediate between those of exclusively Sec or exclusively Delta pH transport (Fig. 2, e.g., lanes 4 and 5). To confirm transport of DT-PC on both pathways, a different set of pathway-specific criteria was used. Transport on the Delta pH pathway was assessed by precursor competition with unlabeled, E. coli– produced iOE23, and transport on the Sec pathway was assessed with purified CPSecA. If DT-PC uses both pathways, then saturating concentrations of iOE23 should shift transport to the Sec pathway, and this transport should be ATP dependent. Such an experiment is shown in Fig. 3 A. Assays were conducted with thylakoids, stromal extract, light (to generate a ΔpH), increasing amounts of competitor iOE23, and the presence or absence of 5 mM ATP. As expected, transport of the Delta pH pathway substrates, tOE23 and t23-PC, was similarly competed in the presence or absence of ATP. Transport of the Sec pathway substrate, pPC, was unaffected by iOE23 competitor but virtually eliminated by ATP removal. DT-PC transport exhibited characteristics expected for a dual-targeted substrate. Transport was reduced ∼60–70% by iOE23 competitor in the presence of ATP, demonstrating that a substantial amount of DT-PC transport was using the Delta pH mechanism. The residual transport was virtually eliminated by ATP removal, indicating that DT-PC was also transported by an ATP-dependent mechanism, presumably the CPSecA mechanism.
Figure 3.
A chimeric signal peptide targets mature PC to both the Sec and Delta pH pathways. Transport competition assays were conducted with radiolabeled precursors in the presence of increasing concentrations of unlabeled iOE23 competitor. Assays were as in Fig. 2 and Materials and Methods. The precursors used are designated to the left of the fluorograms using the same nomenclature as in Fig. 2 and Materials and Methods. The final concentration of iOE23 competitor is indicated above the fluorograms. (A) Assays were performed in the presence or absence of ATP with chloroplast lysate to provide a source of SE. Assays lacking ATP were pretreated with apyrase to eliminate residual ATP in lysate and translation products. The precursor (TP) represents 0.8% of the amount in each assay. Lanes were loaded with recovered thylakoids representing 13% of each assay. All assays derived from the same precursor (with and without ATP) were analyzed on the same gel. (B) Assays were conducted with buffer-washed thylakoids and 5 mM ATP. At the highest concentration of iOE23 competitor (2.0 μM), SE or purified CPSecA (80 nM final concentration) was added to boost Sec-mediated transport (see Materials and Methods). Numbers below each lane represent the percentage of precursor transported.
The experiment shown in Fig. 3 B confirms this presumption regarding CPSecA. Transport assays were conducted in the absence of stromal extract, and Sec pathway transport was assessed by adding purified CPSecA in the presence of 2.0 μM iOE23, which virtually eliminated transport of the Delta pH pathway substrate, tOE23. A small amount of pPC transport occurred without stromal extract, consistent with residual thylakoid-bound CPSecA (Yuan et al., 1994); this was unaffected by iOE23 competitor. Addition of stromal extract or purified CPSecA in the presence of 2.0 μM competitor boosted the transport of DT-PC as well as pPC, but it had no effect on tOE23 transport (Fig. 3 B). Together with Figs. 2 and 3 A, these results demonstrate that DT-PC is transported by both the Sec and Delta pH systems.
As can be seen from Fig. 3 B as well as similar 30-min assays (see below), under conditions allowing only Sec transport (saturating iOE23 competitor and added CPSecA), the DT signal peptide directed transport about as efficiently as the bona fide pPC targeting signal. The efficiency of the DT signal peptide for Delta pH transport was measured separately in 10-min assays with washed thylakoids lacking ATP; DT-PC was transported at least as efficiently as t23-PC. Thus, the DT signal peptide is as effective as authentic pathway-specific signal peptides in governing transport of the same passenger protein on both pathways.
Both the N and H Domains Play a Role in Exclusive Targeting
The results with both chimeric signal peptides suggested that the N and H/C domains have different and distinct roles in pathway-specific targeting. We used DT-PC as a starting point to further identify critical specificity elements and analyze similar regions from other precursors. The DT signal is advantageous in that altering a specificity element is likely to lead to a loss of transport on only one pathway rather than a total loss of transport activity. This is important for distinguishing between a pathway-specific targeting defect and loss of transport due to unrelated effects on precursor structure.
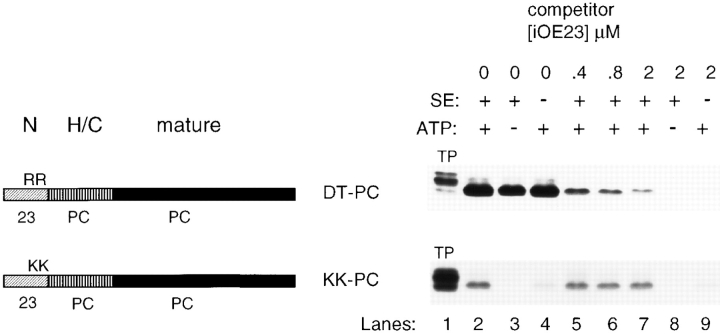
Our initial modification of DT-PC confirmed, in part, the results of Chaddock et al. (1995), i.e., that a twin arginine is necessary for transport on the Delta pH pathway. Altering the N domain of DT-PC by replacing both arginines with lysines (KK-PC) selectively eliminated transport on the Delta pH pathway. Fig. 4 shows that KK-PC transport was not inhibited by competitor iOE23 (lanes 5–7), but it was abolished by removing ATP (lanes 3 and 8) and severely inhibited by removing stromal extract (lanes 4 and 9). In addition, CPSecA was able to replace the stromal requirement for transport of KK-PC (data not shown).
Figure 4.
A dual-targeting signal peptide is converted to a Sectargeting signal by replacing a twin arginine with a twin lysine. Transport competition assays were conducted in the light with lysate as a source of SE or washed thylakoids with or without 5 mM ATP as described in Fig. 3 and shown above the fluorograms. Precursor designations are the same format as in Fig. 2. Concentrations of iOE23 competitor are indicated above each lane. The radiolabeled precursor (TP) represents 0.8% of the amount in each assay. Each lane contains recovered thylakoids equivalent to 13% of the assay.
Other changes to DT-PC suggest that exclusive targeting to the Delta pH system involves the H/C domain; i.e., the H/C domains of Delta pH precursors are incompatible with the Sec system. For example, replacing the H/C domain of DT-PC with the OE17 H/C also results in exclusive transport on the Delta pH pathway (Table I). One possibility for this result was that basic residues in the C domain of OE17 as well as OE23 inhibit translocation by the Sec pathway, similar to the effect of basic residues in this region on bacterial Sec transport (Andersson and von Heijne, 1991). However, substituting a lysine for glutamic acid two residues into the PC mature domain (MEV to MKV) of DT-PC (Table I) did not alter its ability to transport on both pathways. Similarly, replacing the lysine in the cleavage site of PCn23h/c-PC (see Fig. 2) with an asparagine did not restore transport (data not shown). Finally, adding the OE23 cleavage domain (KVSPADA) just COOH-terminal to the PC cleavage site only had the effect of reducing but not eliminating transport on the Sec pathway (Table I).
Table I.
Effect of Changes to the DT-PC Signal Peptide on Sec and Delta pH Transport
| Transport | ||||||
|---|---|---|---|---|---|---|
| Sec | Delta pH | Sequence of signal peptide | Change | |||
| + ++ − | ||||||
| + | + | MVSRRFGVIAVATAASIVLAGNAMA MEV | none (DT-PC)* ‡ | |||
| + ++ − | ||||||
| + | − | MVSKK FGVIAVATAASIVLAGNAMA MEV | RR TO KK‡ | |||
| + ++ + | ||||||
| + | + | MVSRRFGVIAVATAASIVLAGNAMA MKV | E TO K* | |||
| + ++ + − − | ||||||
| + | + | MVSRRFGVIAVATAASIVLAGNAMA KVSPADA MEV | OE23 C domain added* ‡ § | |||
| + ++ − | ||||||
| − | + | MVSRRLALSVLIGAAAVGSAMA MEV | OE23 H domain* ‡ | |||
| + ++ + − | ||||||
| − | + | MVSRRAVIGLVATGIVGGALSQAARA MEV | OE17 H/C* ‡ | |||
| + ++ − | ||||||
| − | + | MVSRRIAGFALATSALVVSGASA MEV | OE33 H/C* ‡ | |||
| + ++ − − | ||||||
| − | − | MVSRRFGD IAVATAASIVLAGNAMA MEV | V to D∥¶ | |||
| + ++ − − | ||||||
| − | − | MVSRRFGVIAD ATAASIVLAGNAMA MEV | V to D‖ | |||
| + ++ − − | ||||||
| − | − | MVSRRFGVIAVATAASI DLAGNAMA MEV | V to D∥¶ | |||
Pathway transport determined by energetics and stromal requirement.
Pathway transport determined by competition with iOE23+/− CPSecA.
Transport on Sec pathway reduced by 80% relative to DT-PC.
Total transport assayed under optimal conditions.
Trace level of transport relative to DT-PC.
On the other hand, replacing the PC H domain with the OE23 H domain (DT-PC 23hPCc) inhibited transport on the Sec pathway to below detectable levels, whereas Delta pH–mediated transport was relatively unaffected (Table I). Together, these results imply that a property of the OE23 H domain is incompatible with Sec-dependent transport. We also found that insertion of the OE33 H/C domain (DT-PC 33h/c) resulted in transport only on the Delta pH system (Table I). This was surprising given that OE33 is normally transported by the Sec mechanism. Taken together, these results imply that the Delta pH system is able to tolerate a variety of H/C regions that are incompatible with the thylakoid Sec mechanism. Nevertheless, both transport systems do share a minimal H domain hydrophobicity requirement. Substitution of aspartate residues for valine residues at several locations within the DT-PC H domain virtually eliminated transport (Table I).
Delta pH Passenger Proteins Limit Transport by the Sec-dependent Translocation System
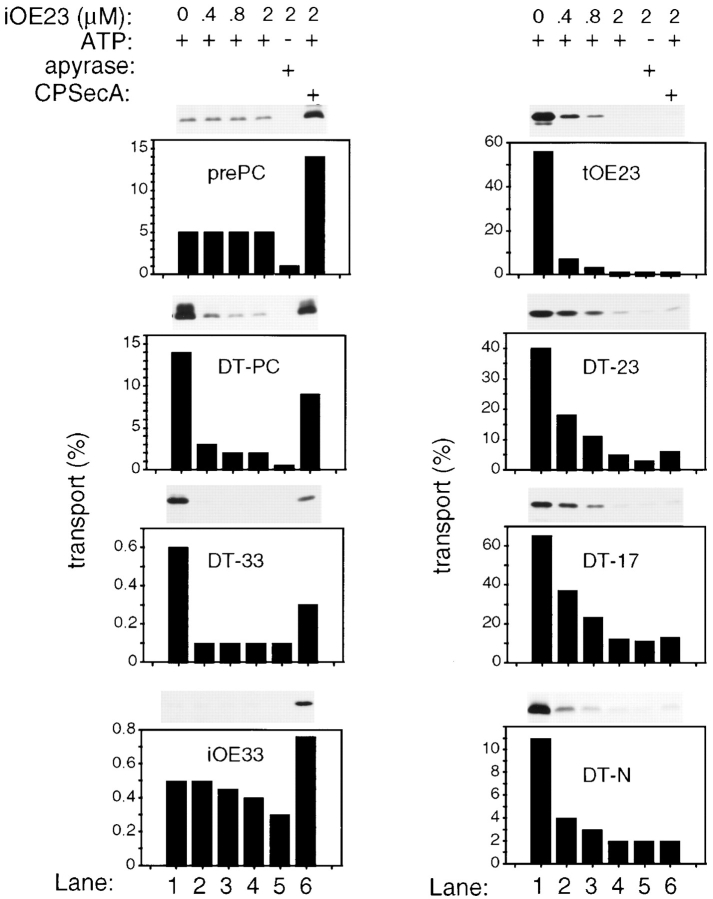
The ability of passenger proteins other than PC to use either pathway was assessed with fusions to the DT signal peptide. This included all proteins known to be normally transported by the Delta pH mechanism (OE17, OE23, PSI-N, and PSII-T; fusions designated DT-17, DT-23, DT-N, and DT-T, respectively) as well as OE33 (designated DT-33). As shown in Fig. 5, competition by iOE23 severely inhibited transport of tOE23 and all of the DT constructs across buffer-washed thylakoids (compare lanes 1 and 4), indicating that all passenger proteins could be translocated by the Delta pH mechanism. To assess Sec pathway transport, purified CPSecA was added in the presence of 2.0 μM iOE23 competitor. Transport of pPC, iOE33, and DT constructs with Sec passengers (DT-PC and DT-33) was stimulated by CPSecA (compare lane 6 to lane 4). Transport of Delta pH passengers showed virtually no enhancement by the addition of CPSecA (compare lane 6 to lane 4). This was surprising given that the mature domain of PSI-N is nearly the same size as mature PC (∼10 kD) and mature PSII-T is only ∼3 kD. Transport assays with DT-T yielded essentially the same results as those with DT constructs with other Delta pH passenger proteins (data not shown). Thus, although the DT chimeras with Delta pH passengers are competent substrates as shown by their efficient transport by the Delta pH system, the Delta pH passenger proteins apparently pose a translocation challenge that the thylakoid Sec mechanism cannot overcome.
Figure 5.
Delta pH passenger proteins are not efficiently transported by the thylakoid Sec pathway. The ability of different passenger proteins to be translocated by the Delta pH and Sec pathways was assessed by making fusions with the DT signal peptide and assaying the chimeric precursors in the presence of iOE23 competitor +/− CPSecA. Transport competition assays were conducted with buffer-washed thylakoids using increasing concentrations of unlabeled iOE23 as indicated above each lane. All assays were conducted in the light with 5 mM ATP, except the assays in lane 5, which were treated with apyrase to eliminate ATP. CPSecA (80 nM final) was included in the assays in lane 6 to boost Sec-mediated transport. The percentage of precursor transported in each assay is shown below the fluorogram. Assays were conducted as described in Materials and Methods and Fig. 3.
E. coli Recognizes Sec But Not Delta pH–targeting Determinants, but Is Capable of Transporting a Delta pH Passenger Protein on the Sec Pathway
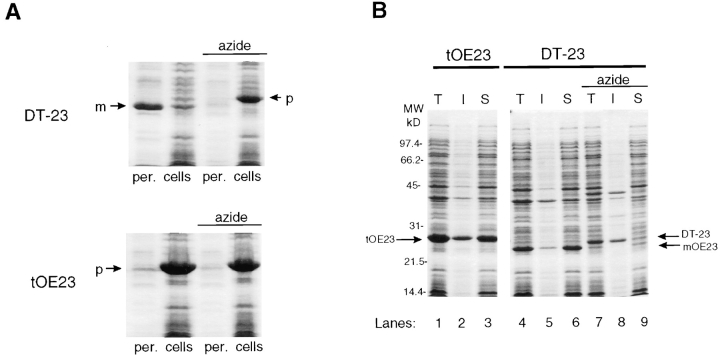
In E. coli, the SecA/SecY/SecE/SecG system is the major route for export of signal peptide–bearing proteins across the cytoplasmic membrane (Pugsley, 1993; Wickner, 1994). Identification of chloroplast homologues of SecA and SecY (Laidler et al., 1995) suggests that the thylakoid Sec system is homologous and mechanistically similar. To test this assumption, we transformed E. coli with a plasmid harboring the DT-23 coding sequence. Fig. 6 A shows that after induction with isopropyl β-d-thiogalactoside, mature OE23 accumulated to a high level and was recovered predominantly in the periplasmic fraction. To determine if DT-23 was transported by the SecA-mediated system, bacteria were induced in the presence of 2 mM sodium azide (Oliver et al., 1990). Under these conditions, only DT-23 precursor accumulated and was recovered in the cellular fraction. This indicates that DT-23 transport was mediated predominantly, if not exclusively, by the Sec system. When the bacteria expressed tOE23, which has the authentic OE23 signal peptide and differs from DT-23 only in the H/ C domain, only tOE23 precursor accumulated and was recovered in the cytoplasmic fraction. Fig. 6 B shows that the tOE23 was largely soluble and thus accessible to the translocation apparatus. This was subsequently verified by the observation that tOE23 is efficiently transported and processed in prlA suppressor strains of E. coli (McCaffery, M., and K. Cline, unpublished results). These findings argue that targeting to the thylakoid and E. coli Sec systems is similar, but that differences between the two mechanisms allow translocation of the OE23 passenger protein in E. coli.
Figure 6.
The E. coli Sec machinery efficiently transports DT-23 to the periplasm but cannot transport OE23 with a Delta pH signal peptide. E. coli strain BL21 (λDE3) harboring an expression plasmid for either DT-23 or tOE23 was induced for 2 h with 1 mM isopropyl β-d-thiogalactoside. To assess SecA-mediated transport, 2 mM sodium azide was included in the culture during induction. (A) Cells were pelleted and fractionated into periplasm (per.) or cellular contents (cells) as designated below each lane and described in Materials and Methods. A portion of a Coomassie-stained SDS polyacrylamide gel is shown. The locations of precursor (p) and mature OE23 (m) are shown with arrows. (B) Cells were lysed and the lysate (T) was fractionated into soluble (S) and insoluble (I) fractions as described in Materials and Methods. A Coomassie-stained SDS polyacrylamide gel is shown. The identities of tOE23, DT-23, and mOE23 were confirmed by immunoblotting.
Discussion
In this study we used a biochemical approach to analyze the determinants of signal and passenger proteins that specify transport by the thylakoid Delta pH or Sec pathways. Two relevant questions were addressed: what specific elements in the LTD determine pathway specificity, and what is the underlying reason for the existence of the two separate pathways? The results show that transport can be divided into two steps, targeting and translocation, and make several important points. First, targeting is mediated solely by the signal peptide motif of LTDs. Second, both the N and H domains of the signal peptide are required for pathway-exclusive targeting. Third, translocation depends upon compatibility of the passenger protein with the respective system. We found that all of the Delta pH proteins are incompatible with thylakoid Sec transport, even when assayed in the presence of added CPSecA. These results define the molecular determinants of specific transport on these systems and further suggest a basis for the existence of the Delta pH pathway.
The Targeting Step: Both the N and H Domains Are Critical for Specific and Exclusive Targeting and the A Domain Is Dispensable
Previous studies with chimeric precursors showed that the LTD determines transport pathway selection (Henry et al., 1994; Robinson et al., 1994). Here we showed that amino-terminal A domains are not required for specific transport; truncated precursors of OE23, OE17, and PC possessing only minimal signal peptides were transported exclusively on the Delta pH (OE23 and OE17) or Sec (PC) pathway (Fig. 2). These findings are consistent with observations of Ko and Cashmore (1989), wherein a chimeric precursor between the STD of a stromal precursor protein and truncated OE33 lacking the A domain was imported into intact chloroplasts and localized to the thylakoid lumen. Although pathway specificity was not addressed in these early studies, it is likely that the protein was transported on the Sec pathway.
Deletion of N domains of the signal peptides eliminated transport on both pathways, as did introducing charged residues into the H domain of DT-PC (Table I). This demonstrates a general requirement for each domain in the transport mechanism of both pathways. Pathway-specific targeting is more complex and requires the proper combination of N and H domains. A signal peptide composed of the PC N domain and the OE23 H/C domain failed to transport on either pathway. In contrast, the reciprocal signal peptide with the OE23 N domain and the PC H/C domain directed efficient transport of PC on both pathways (Figs. 2 and 3). The implication of this finding was that the Delta pH pathway has a specific requirement for the N domain, whereas the Sec pathway has a specific requirement for the H domain.
The details regarding N and H specificity were further explored by modifications of the dual-targeting construct, DT-PC. An N domain twin arginine (RR) requirement for the Delta pH pathway was previously reported by Chaddock et al. (1995). Here we confirmed that result by showing that replacing the RR of DT-PC to KK caused loss of transport only on the Delta pH pathway. Our results further argue that the RR, when combined with a nonspecific H domain, is sufficient for Delta pH targeting. Additional sequences are not required, as previously suggested by Chaddock et al. (1995). This is evidenced by the ability of the MVSRR N domain, when fused to four different H/C regions as well as one chimeric H/C, to direct Delta pH transport of PC (Fig. 2; Table I). Furthermore, since the N domain of the truncated OE17 precursor (MAGRR) differs from MVSRR in all but the RR, it is unlikely that other N domain residues play a targeting role.
The Sec pathway appears to have a nonspecific N domain requirement. Signal peptides with three different N domains, MASLKD, MVSRR, and MVSKK, were shown here to direct PC across the Sec pathway. Clearly, in our studies, the RR did not mask or repel the precursor from the Sec system as previously reported (Chaddock et al., 1995). In fact, DT-PC (containing RR) was transported by the Sec pathway as efficiently as the natural PC precursor. It occurred to us that the difference between our results and those of Chaddock et al. (1995) might reside in the fact that they used in organello assays with intact chloroplasts. However, we found that a precursor in which the PC STD was fused to DT-PC (STD–DT-PC) was readily imported into intact chloroplasts and localized to the thylakoids using both pathways. Dual pathway transport in organello was shown by the inability of a single pathwayspecific inhibitor or competitor to affect thylakoid localization of DT-PC. Inhibition was only achieved with a combination of inhibitors: in this case, ionophores, azide, and a thylakoid transport–saturating concentration of pOE33 (Carrigan, M., R. Henry, M. McCaffery, and K. Cline, unpublished results). In this regard, we note that in the Chaddock et al. (1995) experiments, pPC-RR localization was affected by nigericin as well as by azide, leaving open the possibility that some transport was occurring on the Delta pH pathway.
The Sec pathway displays a much more stringent H domain requirement than the Delta pH pathway. Four different H/C domains were tested in the DT-PC construct. All of these chimeric precursors were efficiently transported by the Delta pH pathway, but only the precursor with the PC H/C domain was transported across the Sec pathway. In fact, the incompatibility was localized to the hydrophobic core itself, as replacement of only the hydrophobic core with that from OE23 eliminated Sec transport without affecting Delta pH transport (Table I). These results suggest that it is the H domain of Delta pH pathway precursors that prevents them from being targeted to the Sec pathway. Interestingly, even the E. coli Sec machinery discriminated between a Sec-compatible H/C domain and a Delta pH pathway H/C domain (Fig. 6).
The critical H domain feature for thylakoid Sec transport is presently unclear. Mean residue hydrophobicity and the propensity to adopt an α helix in hydrophobic environments have been correlated with transport efficiency by the E. coli Sec system (for review see Izard and Kendall, 1994). Mean hydrophobicity values for thylakoid precursors are slightly lower than those for bacterial signal peptides (e.g., as assessed by Doud et al., 1993), but these values do not group precursors on the basis of transport pathway (not shown). Analysis with secondary structure predictive programs suggests that the H domains of Delta pH precursors have less of a tendency to adopt an α helix (Clausmeyer et al., 1993; unpublished results), but such putative differences in secondary structure remain to be experimentally verified with biophysical studies.
One less tangible characteristic of Sec transport in E. coli was reported by Laforet et al. (1989). In N and H swapping studies carried out with E. coli precursors, they found that one of three different H domains, that of M13 procoat, failed to functionally substitute for the alkaline phosphatase H domain in vivo. Since transport was partially restored if the procoat C domain and seven residues of the mature procoat were included, Laforet et al. suggested the potential for incompatibility between the H domain, C domain, and the amino terminus of the mature protein. If the potential for such incompatibility is a property of thylakoid Sec transport, it may explain the inability of the OE33 H/C domain to support Sec transport of PC.
The Translocation Step: Delta pH Passenger Proteins Are Incompatible with the Sec Machinery
The underlying reason for the operation of more than one transport pathway in thylakoids or any other membrane system is not known. For thylakoids, the inability of Delta pH passenger proteins to be translocated by the thylakoid Sec machinery offers one possible explanation for the existence of a novel pathway. Previous studies have noted that two Delta pH passenger proteins, OE17 and OE23, impose thylakoid translocation limitations when fused to Sec pathway transit peptides (Clausmeyer et al., 1993; Henry et al., 1994). These studies used in organello assays with intact chloroplasts, in which thylakoid transport conditions are comparable to those found in vivo. Both chimeric precursors were efficiently imported into chloroplasts but poorly localized to the thylakoid. In the case of OE17, some thylakoid localization occurred, but transport was inefficient and a considerable portion of the imported protein accumulated in the stroma (Clausmeyer et al., 1993; Henry et al., 1994). In the case of OE23, none of the imported protein was transported into the lumen (Clausmeyer et al., 1993). The present studies confirm and extend those observations. In our thylakoid transport assays, all four of the known Delta pH passengers proteins were unable to be translocated on the Sec pathway when directed by the DT signal peptide.
One possible explanation for this result is that incompatibility exists between Delta pH passenger proteins and the DT signal peptide (Laforet et al., 1989). However, we do not believe this to be the case. The fact that the DT signal concurrently directed efficient transport of all of these passenger proteins on the Delta pH pathway argues against any kind of conformational instability that would mask the signal peptide. In addition, recent studies in our laboratory have shown that transport on the Delta pH pathway proceeds via a loop mechanism (Fincher, V., and K. Cline, manuscript in preparation) similar to Sec-mediated transport (Kuhn et al., 1994), indicating that the signal peptide and the amino terminus of the mature protein need to be compatible regardless of the pathway used. Finally, the fact that the E. coli Sec system very efficiently transported DT-OE23 argues against incompatibility of the DT signal peptide and mature OE23 (Fig. 6).
In fact, the differing ability of thylakoid and E. coli Sec systems to transport OE23 implies that mechanistic differences exist between the two systems. One obvious difference is that, in E. coli, the protonmotive force consists of a Δψ in addition to a ΔpH, both of which participate in the translocation process (e.g., Driessen, 1992). At steady state, thylakoids generate only a ΔpH. Thus, it will be interesting to examine the role of a Δψ in E. coli export of OE23 as well as the ability of E. coli proteins to use the Sec or Delta pH systems in chloroplasts. Other differences may exist at the level of components that constitute the two Sec systems, as virtually nothing is known regarding the composition of the thylakoid Sec pathway translocon.
Taken together, our results suggest that the Delta pH system may exist to compensate for a Sec system that is less robust than the E. coli counterpart. These results also support the need for targeting signals that are capable of exclusive targeting to the Delta pH system, thereby avoiding deleterious effects that are likely to impede thylakoid development. The fact that classical signal peptides are required for transport by both the Sec and Delta pH pathways, as well as studies showing a loop mechanism for Delta pH transport (Fincher, V., and K. Cline, manuscript in preparation), suggest that the two systems may be related, possibly sharing common or homologous components. Unlike proteins localized by the Sec system, no obvious homologues of proteins transported by the Delta pH system are found in cyanobacteria, which are thought to be the present day representative of the endosymbiont that evolved into chloroplasts. This raises the question of where these proteins and their translocation system came from. It is tempting to speculate that Delta pH proteins as well as the translocation system were recruited to the thylakoids after the endosymbiotic event. Studies aimed at identifying components of both systems should shed light on these questions.
Acknowledgments
We thank Shan Wu for technical assistance, and Vivian Fincher and Liz Summer for critical reading of the manuscript.
This work was supported in part by National Institutes of Health grant R01 GM46951 and National Science Foundation grant MCB-9419287 to K. Cline. DNA sequencing was conducted by the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR) DNA Sequencing Core, which is supported by funds supplied by the Division of Sponsored Research and the ICBR at the University of Florida. This paper is Florida Agricultural Station Journal Series #R-05505.
Abbreviations used in this paper
- DT
dual-targeting
- LTD
lumen-targeting domain
- PC
plastocyanin
- SE
stromal extract
- SOE
splicing by overlap extension
- STD
stroma-targeting domain
Footnotes
Address all correspondence to Kenneth Cline, Horticultural Sciences Department, Fifield Hall, University of Florida, Gainsville, FL 32611. Tel.: (352) 392-4711 ext. 219. Fax: (352) 392-5653. e-mail: KCC@icbr.ifas.ufl.edu
References
- Andersson H, von Heijne G. A 30-residue-long “export initiation domain” adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. . Proc Natl Acad Sci USA. 1991;88:9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. . Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Bartling D, Mould RM, Dunbar B, Weisbeek P, Herrmann RG, Robinson C. Transport of proteins into chloroplasts: delineation of envelope “transit” and thylakoid “transfer” signals within the presequences of three imported thylakoid lumen proteins. J Biol Chem. 1991;266:23606–23610. [PubMed] [Google Scholar]
- Chaddock AM, Mant A, Karnauchov I, Brink S, Herrmann RG, Klösgen RB, Robinson C. A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the ΔpH-dependent thylakoidal protein translocase. EMBO (Eur Mol Biol Organ) J. 1995;14:2715–2722. doi: 10.1002/j.1460-2075.1995.tb07272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausmeyer S, Klösgen RB, Herrmann RG. Protein import into chloroplasts: the hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993;268:13869–13876. [PubMed] [Google Scholar]
- Cline K. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986;261:14804–14810. [PubMed] [Google Scholar]
- Cline K, Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Dev Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- Cline K, Henry R, Li CJ, Yuan J. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO (Eur Mol Biol Organ) J. 1993;12:4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AJM. Precursor protein translocation by the Escherichia colitranslocase is directed by the protonmotive force. EMBO (Eur Mol Biol Organ) J. 1992;11:847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doud SK, Chou MM, Kendall DA. Titration of protein transport activity by incremental changes in signal peptide hydrophobicity. Biochemistry. 1993;32:1251–1256. doi: 10.1021/bi00056a008. [DOI] [PubMed] [Google Scholar]
- Henry R, Kapazoglou A, McCaffery M, Cline K. Differences between lumen targeting domains of chloroplast transit peptides determine pathway specificity for thylakoid transport. J Biol Chem. 1994;269:10189–10192. [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene (Amst) 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hulford A, Hazell L, Mould RM, Robinson C. Two distinct mechanisms for the translocation of proteins across the thylakoid membrane, one requiring the presence of a stromal protein factor and nucleotide triphosphates. J Biol Chem. 1994;269:3251–3256. [PubMed] [Google Scholar]
- Izard JW, Kendall DA. Signal peptides: exquisitely designed transport promoters. Mol Microbiol. 1994;13:765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Ko K, Cashmore AR. Targeting of proteins to the thylakoid lumen by the bipartite transit peptide of the 33 kD oxygen-evolving protein. EMBO (Eur Mol Biol Organ) J. 1989;8:3187–3194. doi: 10.1002/j.1460-2075.1989.tb08477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A, Kiefer D, Kohne C, Zhu H-Y, Tschantz WR, Dalby RE. Evidence for a loop-like insertion mechanism of pro-Omp A into the inner membrane of Escherichia coli. . Eur J Biochem. 1994;226:891–897. doi: 10.1111/j.1432-1033.1994.00891.x. [DOI] [PubMed] [Google Scholar]
- Laforet GA, Kaiser ET, Kendall DA. Signal peptide subsegments are not always functionally interchangeable: M13 procoat hydrophobic core fails to transport alkaline phosphatase in Escherichia coli. . J Biol Chem. 1989;264:14478–14485. [PubMed] [Google Scholar]
- Laidler V, Chaddock AM, Knott TG, Walker D, Robinson C. A secY homolog in Arabidopsis thaliana. . J Biol Chem. 1995;270:17664–17667. doi: 10.1074/jbc.270.30.17664. [DOI] [PubMed] [Google Scholar]
- Nakai M, Goto A, Nohara T, Sugita D, Endo T. Identification of the secA protein homolog in pea chloroplasts and its possible involvement in thylakoidal protein transport. J Biol Chem. 1994;269:31338–31341. [PubMed] [Google Scholar]
- Nohara T, Asai T, Nakai M, Sugiura M, Endo T. Cytochrome f encoded by the chloroplast genome is imported into thylakoids via the SecA-dependent pathway. Biochem Biophys Res Commun. 1996;224:474–478. doi: 10.1006/bbrc.1996.1051. [DOI] [PubMed] [Google Scholar]
- Oliver DB, Cabelli RJ, Dolan KM, Jarosik GP. Azide-resistant mutants of Escherichia colialter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Klösgen RB. Targeting of proteins into and across the thylakoid membrane: a multitude of mechanisms. Plant Mol Biol. 1994;26:15–24. doi: 10.1007/BF00039516. [DOI] [PubMed] [Google Scholar]
- Robinson C, Cai D, Hulford A, Brock IW, Michl D, Hazell L, Schmidt I, Herrmann RG, Klösgen RB. The presequence of a chimeric construct dictates which of two mechanisms are utilized for translocation across the thylakoid membrane: evidence for the existence of two distinct translocation systems. EMBO (Eur Mol Biol Organ) J. 1994;13:279–285. doi: 10.1002/j.1460-2075.1994.tb06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R, Barkan A. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO (Eur Mol Biol Organ) J. 1995;14:3905–3914. doi: 10.1002/j.1460-2075.1995.tb00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Wickner WT. How ATP drives proteins across membranes. Science (Wash DC) 1994;266:1197–1198. doi: 10.1126/science.7973701. [DOI] [PubMed] [Google Scholar]
- Yuan J, Cline K. Plastocyanin and the 33-kDa subunit of the oxygen-evolving complex are transported into thylakoids with similar requirements as predicted from pathway specificity. J Biol Chem. 1994;269:18463–18467. [PubMed] [Google Scholar]
- Yuan J, Henry R, McCaffery M, Cline K. SecA homolog in protein transport within chloroplasts: evidence for endosymbiont-derived sorting. Science (Wash DC) 1994;266:796–798. doi: 10.1126/science.7973633. [DOI] [PubMed] [Google Scholar]