Abstract
Integrin-mediated cell adhesion causes activation of MAP kinases and increased tyrosine phosphorylation of focal adhesion kinase (FAK). Autophosphorylation of FAK leads to the binding of SH2-domain proteins including Src-family kinases and the Grb2–Sos complex. Since Grb2–Sos is a key regulator of the Ras signal transduction pathway, one plausible hypothesis has been that integrin-mediated tyrosine phosphorylation of FAK leads to activation of the Ras cascade and ultimately to mitogen activated protein (MAP) kinase activation. Thus, in this scenario FAK would serve as an upstream regulator of MAP kinase activity. However, in this report we present several lines of evidence showing that integrin-mediated MAP kinase activity in fibroblasts is independent of FAK. First, a β1 integrin subunit deletion mutant affecting the putative FAK binding site supports activation of MAP kinase in adhering fibroblasts but not tyrosine phosphorylation of FAK. Second, fibroblast adhesion to bacterially expressed fragments of fibronectin demonstrates that robust activation of MAP kinase can precede tyrosine phosphorylation of FAK. Finally, we have used FRNK, the noncatalytic COOH-terminal domain of FAK, as a dominant negative inhibitor of FAK autophosphorylation and of tyrosine phosphorylation of focal contacts. Using retroviral infection, we demonstrate that levels of FRNK expression sufficient to completely block FAK tyrosine phosphorylation were without effect on integrin-mediated activation of MAP kinase. These results strongly suggest that integrin-mediated activation of MAP kinase is independent of FAK and indicate the probable existence of at least two distinct integrin signaling pathways in fibroblasts.
The integrins are a major class of receptors used by cells to interact with other cells and with the extracellular matrix (Ruoslahti, 1991; Hynes, 1992). Integrins are comprised of noncovalently linked α and β chains that can associate in various combinations and thus determine the ligand-binding specificities of the intact heterodimer (Hynes, 1992; Loftus et al., 1994). A number of ECM proteins including fibronectin, collagens, laminins, and vitronectin are ligands for various integrins. The binding of integrins to insoluble arrays of these proteins serves to anchor cells at specialized sites of cell–matrix adhesion termed focal contacts (Burridge et al., 1988). Interactions between integrins and the extracellular matrix help regulate a variety of fundamental biological processes including cell growth (Clarke et al., 1995; Meredith et al., 1995; Fang et al., 1996; Zhu et al., 1996), cell death (Frisch and Francis, 1994; Ruoslahti and Reed, 1994; O'Brien et al., 1996), differentiation (Yurochko et al., 1992; Huhtala et al., 1995; Riikonen et al., 1995; Streuli et al., 1995), cell morphology and motility (Chan et al., 1992; Bauer et al., 1993), and tumor growth and metastasis (Chan et al., 1991; Agrez et al., 1994; Brooks et al., 1994; Varner et al., 1995).
Recent evidence has indicated that integrins act not only as simple mediators of cell adhesion but can also transduce biochemical signals across the cell membrane (Juliano and Haskill, 1993; Clark and Brugge, 1995; Rosales et al., 1995). Attachment of integrins to their ligands leads to a profound increase in tyrosine phosphorylation of several cellular proteins, including most prominently a molecule termed focal adhesion kinase, pp125FAK (FAK)1 (Hanks et al., 1992; Schaller et al., 1992). FAK is a cytoplasmic protein tyrosine kinase that is tyrosine phosphorylated and colocalized at focal adhesion sites upon engagement and clustering of integrins (Burridge et al., 1992; Kornberg et al., 1992; Romer et al., 1994). FAK can also be tyrosine phosphorylated in response to a variety of other stimuli (Zachary et al., 1993; Seckl et al., 1995). The precise mechanism that links integrin clustering to FAK activation remains to be determined; however it is clear that integrinmediated adhesion induces autophosphorylation of FAK predominantly at Y397 (Schaller et al., 1994, 1995). In addition to clustered integrins and FAK, focal contacts contain a number of specialized cytoplasmic proteins, including talin, vinculin, α-actinin, paxillin, and others, that help to bridge between integrins and actin filaments of the cytoskeleton (Burridge et al., 1988; Parsons, 1996). Focal contacts are also enriched in a variety of signal transducing molecules including Src-family kinases, guanine nucleotide exchange factors, Ras-family proteins, and mitogen activated protein (MAP) kinases, as can be observed by immunostaining for these proteins (Miyamoto et al., 1995; Plopper et al., 1995). This suggests that focal contacts, and possibly other sites of integrin-mediated adhesive interactions, may be important parts of the cellular signal transduction machinery. Tyrosine phosphorylation during integrin-mediated adhesion seems to be prerequisite for focal adhesion formation and actin stress fiber assembly (Burridge et al., 1992; Romer et al., 1994). Recent data indicate, however, that the regulation of cytoskeletal organization and focal adhesion formation is independent of FAK-mediated tyrosine phosphorylation in focal adhesions (Wilson et al., 1995; Gilmore and Romer, 1996). Instead, FAK may be implicated in adhesion-dependent responses to growth factors (Gilmore and Romer, 1996).
The observation that integrin receptor clustering leads to autophosphorylation of a tyrosine kinase has suggested that integrin signaling may resemble the signal transduction pathway initiated by peptide mitogens. In that case, mitogen binding triggers dimerization and autophosphorylation of a receptor tyrosine kinase, binding of adaptor proteins such as Grb2 or Shc, recruitment of guanine nucleotide exchange proteins such as Sos, and subsequent activation of the Ras/Raf/MAP kinase kinase (MEK)/MAP kinase signal transduction cascade (Egan and Weinberg, 1993; Khosravi-Far and Der, 1994). In a somewhat parallel set of events, following FAK autophosphorylation, additional tyrosines are phosphorylated through the action of Src-family kinases that bind to FAK at Y397 via their SH2 domains (Schaller et al., 1994; Calab et al., 1995). This leads to the binding of other SH2 domain proteins such as PI-3kinase (Bachelot et al., 1996) and the Grb2/Sos complex (Schlaepfer et al., 1994). The binding of Grb2/Sos at Y925 of FAK raises the possibility that FAK activation may be able to trigger the Ras signal transduction cascade (Schlaepfer et al., 1994). Further, several groups have shown that MAP kinases are activated in response to integrinmediated adhesion (Chen et al., 1994; Schlaepfer et al., 1994; Zhu and Assoian, 1995), and we have recently shown that MEK and Raf-1 are also activated in this way (Chen et al., 1996). Other workers have suggested a role for Ras in integrin signaling (Kapron-Bras et al., 1993; Clark and Hynes, 1996). All of the data above suggest a link between integrins, FAK, and the consensus Ras/MAP kinase pathway. However, when we directly tested the relationship of integrin-mediated MAP kinase activation to the Ras pathway, using transfection with dominant negative constructs for that pathway, we found no evidence for Ras involvement (Chen et al., 1996). Thus, we find at least one notable difference in the integrin- and mitogen-mediated pathways leading to MAP kinase activation, with a lack of Ras involvement in the integrin-mediated events.
We have now explored possible relationships between integrin-mediated activation of FAK and of MAP kinase. Our data indicate that integrin-mediated activation of these two kinases represents distinct sets of events, and that MAP kinase activation is not dependent on FAK activation. This conclusion is based on several different types of observations. First, in studies with cells transfected with wild-type or mutated integrin β1 subunits, we found that adhesion mediated by WT β1 resulted in activation of both MAP kinase and FAK, but adhesion mediated by a β1 mutant resulted in activation of MAP kinase but not FAK. Second, we have examined cells adhering to recombinant fragments of fibronectin. Some of these fragments sustained cell adhesion that resulted in robust activation of MAP kinase but barely perceptible activation of FAK. Third, under some conditions, the time course of MAP kinase activation and FAK activation are completely distinct. Fourth, we have used constructs that express FAK-related nonkinase (FRNK), the COOH-terminal domain of FAK, as a dominant negative modulator of FAK function. Transfection with FRNK constructs interfered with integrin-mediated tyrosine phosphorylation of FAK and reduced the amount of phosphotyrosine in focal contacts but had essentially no effect on the integrin-mediated activation of MEK and MAP kinase. These findings strongly suggest that integrin-mediated activation of MEK and MAP kinase are independent of FAK; this idea has a number of important implications for current models of integrin- mediated signal transduction.
Materials and Methods
Materials
Chicken β1 cDNAs and W1B10 anti–chicken β1 mouse monoclonal were from A.R. Horwitz (University of Illinois, Urbana, IL). Anti–mouse α5 (MFR5) and β1 (9EG7) rat monoclonals were obtained from Pharmingen (San Diego, CA). Antibodies to FAK (clone 77) and MEK were from Transduction Laboratories (Lexington, KY); antibodies to MAP kinase (Sc-93, 94) were from Santa Cruz Biotechnology (Santa Cruz, CA), while antiphosphotyrosine antibodies were obtained from ICN Biochemicals (PY20, Costa Mesa, CA) or Upstate Biotechnologies Inc. (4G10, Lake Placid, NY). Fluorophore conjugated second antibodies were from Chemicon International, Inc. (Temecula, CA). Antibody 5158 was raised (L. Romer) against a GST fusion protein containing the COOH terminus of FAK.
Preparation of Ligand-coated Dishes or Flasks
Anti–mouse IgG-precoated MicroCellector flasks (Applied Immune Sciences, Inc., Santa Clara, CA) were incubated with the 20 μg/ml anti– chicken β1 integrin antibody W1B10 or 20 μg/ml anti–mouse CD44 antibody (MCA1014; Serotec Ltd., Washington, DC) in PBS at 4°C overnight. Tissue culture dishes were incubated with fibronectin or with appropriate concentrations of fibronectin fragments in PBS at 4°C overnight. The coated flasks and dishes were blocked with 2% BSA in Dulbecco's minimal essential medium (DMEM) for 1 h at room temperature and washed twice with PBS before use. The fibronectin fragments used were comprised of a series of fibronectin type III domains from domain 7 to 12, either including or excluding the two alternatively spliced domains, EIIIB and EIIIA; these were cloned and expressed using the prokaryotic expression vector pET15b (Novagen Inc., Madison, WI) as described elsewhere (Aukhil et al., 1993).
Cell Culture, Cell Adherence, and Preparation of Cell Lysate
NIH 3T3 cells were maintained in DMEM containing 10% bovine calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Confluent cells were serum starved for 4 h before detachment with 0.05% trypsin and 0.33 mM EDTA; trypsin activity was neutralized by soybean trypsin inhibitor (1 mg/ml). Cells were suspended in DMEM with 2% BSA and incubated nonadherently at 37°C for 45 min in a rotator to allow kinases to become quiescent. Cells were then plated onto dishes coated with fibronectin (Fn), Fn fragments, or antibodies and incubated at 37°C for the indicated times. After incubations, cells were washed twice with cold PBS and then lysed in a modified RIPA buffer containing 50 mM Tris, pH 7.5, 1% NP-40, 0.1% sodium deoxycholate, 150 mM NaCl, 50 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM nitrophenolphosphate, 5 mM benzamidine, 0.2 μM calyculin A, 2 mM PMSF, and 10 μg/ml aprotinin. Total cell lysates were cleared by centrifugation at 16,000 g for 5 min at 4°C. Protein concentration in the lysates was determined using the bicinchonic acid assay (Pierce, Rockford, IL).
Plasmid Transfections
Lipofectamine (GIBCO BRL, Gaithersberg, MD) was used for transfection of NIH 3T3 cells according to the manufacturer's instructions. NIH 3T3 cells were transfected with vectors expressing the wild-type chicken β1 integrin subunit or a mutant chicken β1 integrin subunit with a deletion at residues 759–771 (Reszka et al., 1992). Cells expressing chicken β1 integrins were selected using magnetic beads (Dynal, Inc., Great Neck, NY) coated with the anti–chicken β1 integrin antibody W1B10 (Reszka et al., 1992). The selected cells were allowed to grow in DMEM with 10% bovine calf serum and 250 μg/ml of G418. Cells were further selected twice using MicroCELLector flasks (Applied Immune Sciences, Inc.) coated with W1B10 and then expanded before use. In some cases cells were transfected with constructs expressing an epitope-tagged MEK (EE-MEK; Chen et al., 1996) and/or the noncatalytic domain of FAK, FRNK (Schaller et al., 1993). For the latter, chicken FRNK cDNA was ligated into the EcoRI site under the control of the cytomegalovirus promoter in the mammalian expression vector pcDNA3 (Invitrogen Corp., San Diego, CA).
Retroviral Infections
Chicken embryo (CE) cells were prepared as described (Reynolds et al., 1989). The FRNK cDNA was inserted into the RCAS-A replication competent avian retroviral vector (Hughes et al., 1987) and the plasmid transfected into CE cells. 7–10 d later FRNK expression was maximal, and virtually all of the cells were transfected. Cell adhesion experiments were performed as above.
Flow Cytometry
Cells (1 × 106) were detached by trypsin/EDTA and pretreated with normal goat IgG (2 mg/ml) for 15 min on ice. Cells were then incubated with primary antibodies (2 μg/ml) for 30 min on ice, washed, and treated with goat anti–mouse or anti–rat IgG coupled to fluorescein (20 μg/ml; Cappel Laboratories, Malvern, PA) for 30 min on ice. After three washes, cells were resuspended in PBS and analyzed for fluorescence using a Becton Dickinson (Bedford, MA) flow cytometer. Background staining was assessed by using an irrelevant monoclonal antibody (anti-macrophage NO synthetase, clone 6; Transduction Laboratories) as the primary antibody.
Immunofluorescence Microscopy
Cells were fixed in 3.7% formaldehyde in Dulbecco's PBS for 10 min, rinsed in 150 mM NaCl, 0.1% NaN3, 50 mM Tris HCl, pH 7.6 (TBS), and permeabilized for 5 min in TBS containing 0.5% Triton X-100 before staining. Coverslips were then incubated for 30 min with ICN's (Irvine, CA) PY20 anti-phosphotyrosine antibody and anti-FAK polyclonal antiserum 5158 in TBS. The coverslips were rinsed extensively in TBS and then stained with combined fluorophore-conjugated, affinity-purified donkey anti–mouse and anti–rabbit IgG antibodies in TBS for 30 min at 37°C. After the antibody incubations, the coverslips were washed in TBS, rinsed in deionized water, and mounted in gelvatol or mowiol. Coverslips were viewed on a Zeiss (Thornwood, NY) Axiophot microscope equipped for epifluorescence. Fluorescence micrographs were taken on T-max 100 film (Kodak Co., Rochester, NY).
Immunoprecipitation, Western Blot, and Immune Complex Kinase Assays
Cell lysates were first incubated with antibody recognizing FAK, MAP kinase, or EE-tagged MEK for 2 h at 4°C, followed by the addition of protein G–Sepharose, and then incubated for an additional 2 h at 4°C. For Western analysis, the precipitates were washed three times with cold RIPA buffer, and boiled with SDS-PAGE sample buffer to dissociate the proteins. For immune complex kinase assays, the precipitates were washed three times with cold washing buffer (0.25 M Tris, pH 7.5, 0.1 M NaCl). The immunocomplexes were resuspended in 40 μl of kinase assay buffer containing 10 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM DTT, 10 μM ATP, 5 μCi [32P]ATP, and 2 μg of kinase-dead MAP kinase (K−MAPK; for EE-MEK), or 10 μg of myelin basic protein (for MAP kinase), and incubated for 30 min at room temperature (Chen et al., 1996). Reactions were stopped by adding 40 μl of 2× sample buffer and boiling 3 min. The samples were subjected to SDS-PAGE, and the gels were dried. The dried gels were exposed to X-ray films, or the 32P-labeled substrate bands were quantitated using a Storm 840 Phosphorimager with Image-QuaNT software (Molecular Dynamics, Inc., Sunnyvale, CA).
Total cell lysates or immunoprecipitated proteins were separated by SDS-PAGE under reducing conditions. The proteins were transferred eletrophoretically onto polyvinylidene fluoride membranes (Immobilon P; Millipore Corp.). The membranes were blocked with 1% BSA and 0.1% Tween 20 in PBS for 1 h and subsequently incubated with primary antibody (1 μg/ml) in PBS containing 1% BSA and 0.1% Tween 20 for 1 h. The membranes were washed briefly and incubated with goat anti–mouse or anti–rabbit IgG peroxidase conjugates for 1 h. Immunoreactivity was detected on Hyperfilm using enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL). Bands from Western blots were quantitated using a GS-670 model densitometer (BioRad Laboratories, Richmond, CA).
Results
We had previously noticed that the kinetics of MAP kinase activation and of tyrosine phosphorylation of FAK were different following fibroblast adhesion to fibronectin. MAP kinase activation seemed to occur somewhat more rapidly than FAK activation, suggesting that FAK might not be responsible for MAP kinase activation. However, early results did not permit firm conclusions to be drawn. Since fibronectin-coated substrata rapidly induced both cell adhesion and extensive cell spreading, we decided it might be informative to examine FAK and MAP kinase under conditions where integrin-mediated adhesive interactions could be partially uncoupled from cell spreading and cytoskeletal organization.
Cell Adhesion Via a Mutated β1 Subunit Activates MAP Kinase but Not FAK
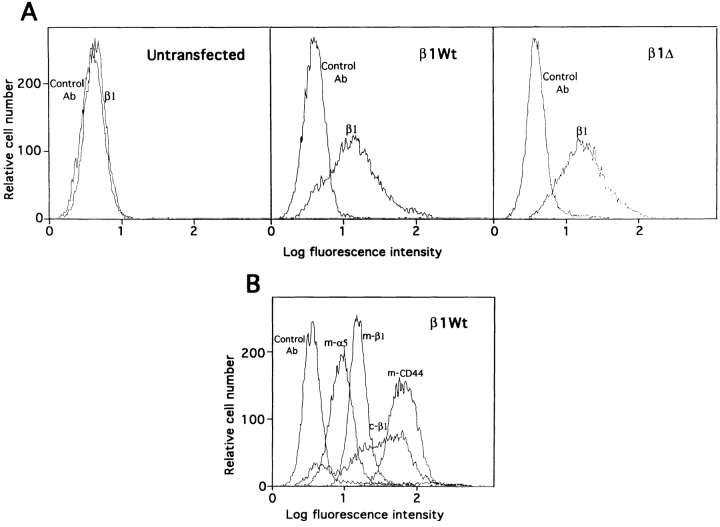
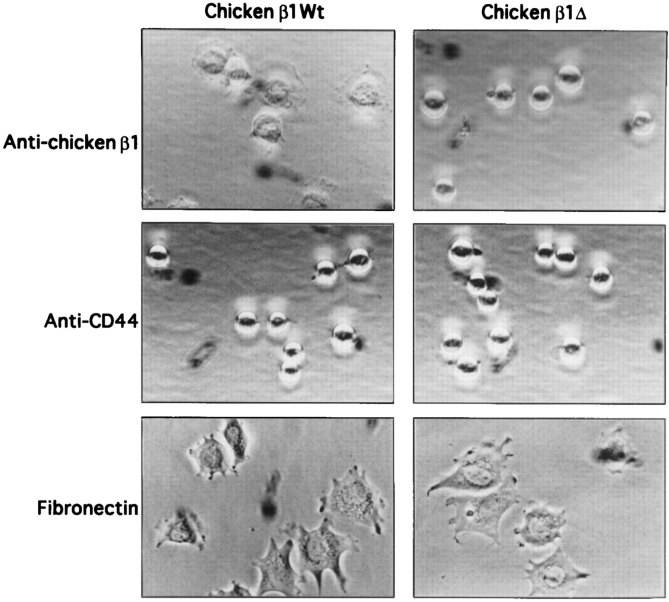
In one set of studies we used NIH 3T3 cells that had been transfected with constructs expressing wild-type (WT) or mutated versions of the chicken β1 subunit (Reszka et al., 1992). These cells were then allowed to adhere to substrata coated with an antibody directed against the β1 subunit. Adhesion to substrata coated with antibodies to the abundant nonintegrin surface protein CD44 served as a negative control, while adhesion to fibronectin was used as a positive control. One mutated β1 construct (here termed β1Δ) proved particularly interesting; this construct gives rise to a subunit with a membrane proximal deletion in the cytoplasmic domain (amino acids 759–771) that overlaps with the putative binding site for FAK (Reszka et al., 1992; Schaller et al., 1995). Fig. 1 depicts flow cytometry profiles for expression of endogenous and transfected integrin subunits, and expression of endogenous CD44, in NIH 3T3 cells. Similar levels of chicken β1 integrins were expressed on the cell surface in both β1WT and β1Δ transfected cells (Fig. 1 A). The expression of the exogenous chicken β1 integrin subunit was not as homogeneous as that of the endogenous integrin subunits (Fig. 1 B). CD44, a nonintegrin cell surface protein (Aruffo et al., 1990), was abundantly expressed on the cell surface (Fig. 1 B). As seen in Fig. 2, cells expressing WT β1 showed substantial spreading on an anti-β1 antibody–coated surface, while cells expressing β1Δ remained completely round, although firmly attached. This result is consistent with previous observations that deletion of amino acid 759–771 resulted in inhibition of cell spreading (Reszka et al., 1992). Cells adhering on fibronectin spread extensively, while cells firmly attached on anti-CD44 remained round. In these experiments, all of the cells were adherent to the various substrata and could resist vigorous washing of the plates, even though differences in appearance were obvious.
Figure 1.
Expression of endogenous and transfected integrin subunits, and expression of endogenous CD44 in NIH 3T3 cells. NIH 3T3 cells were transfected with constructs expressing chicken β1WT or β1Δ integrin subunit. Cells expressing chicken β1 integrin subunits were selected and expanded as described in Materials and Methods. (A) Untransfected cells or β1WT or β1Δ transfected cells were incubated with the anti–chicken β1 integrin antibody W1B10 or with anti-macrophage nitric oxide synthase antibody as a control antibody. (B) β1WT transfected cells were incubated with W1B10, with antibodies recognizing mouse β1 or α5 integrin subunits or CD44, or the irrelevant antibody to macrophage nitric oxide synthase. Antibodies to mouse integrins are marked with the prefix m. In both A and B, cells were washed, and incubated with goat anti–mouse IgG or goat anti–rat IgG coupled to fluorescein. After further washing, cells were resuspended and analyzed using a flow cytometer.
Figure 2.
Cell morphology: cell adherence on anti–chicken β1 antibody, anti-CD44 antibody, or Fn-coated surfaces. β1WT or β1Δ transfected cells were plated onto MicroCELLector flasks coated with anti–chicken β1 integrin antibody or anti-CD44 antibody, or onto tissue culture dishes coated with Fn (10 μg/ml), in each case for 30 min at 37°C. Cells were washed with PBS, fixed with 2% formaldehyde, and photographed at 300× magnification.
The cells expressing WTβ1 or β1Δ were tested for FAK tyrosine phosphorylation and for MAP kinase activation. As shown in Fig. 3, A and B, adherence of WT β1 cells or β1Δ cells to anti-β1–coated surfaces caused similar degrees of activation of MAP kinase (twofold). However, while adherence of WT cells resulted in a modest but clear increase in FAK tyrosine phosphorylation, no increase was detected in the β1Δ cells. Cell adhesion to an Fn substratum triggered a strong increase in MAP kinase activity (fourfold) and strong tyrosine phosphorylation of FAK in both β1WT and β1Δ transfected cells. This result ruled out the possibility that the machinery to trigger FAK tyrosine phosphorylation was defective in β1Δ transfected cells. The activation of FAK and MAP kinases is clearly mediated by integrins, since attachment of cells to an antiCD44 antibody-coated surface did not induce FAK or MAP kinase activities. These results suggested that FAK and MAP kinase activations might be uncoupled in the cells expressing β1Δ. It is interesting to note that, while at the level of light microscope morphology, the cells attaching on anti-CD44 and the β1Δ cells attaching on anti-β1 were similarly rounded in appearance, they were clearly different in terms of their biochemical capabilities.
Figure 3.

Effect of integrin ligation on FAK tyrosine phosphorylation and MAP kinase activity in β1Wt and β1Δ transfected cells. β1WT or β1Δ transfected cells were detached by trypsin and incubated in suspension for 45 min at 37°C. Cells were plated onto MicroCELLector flasks coated with anti–chicken β1 integrin antibody or anti-CD44 antibody, onto tissue culture dishes coated with Fn (10 μg/ml), or held in suspension (NAD), in each case for 30 min at 37°C. After incubating, cells were washed and lysed in modified RIPA buffer. MAP kinase activity was analyzed by a band shift assay (A) and by an immune complex kinase assay with quantitation using a Phosphorimager (B). FAK was immunoprecipitated and analyzed for tyrosine phosphorylation by anti-phosphotyrosine immunoblotting and blotting with anti– FAK (C).
Cell Adhesion to Recombinant Fibronectin Fragments Strongly Activates MAP Kinase but Only Weakly Activates FAK
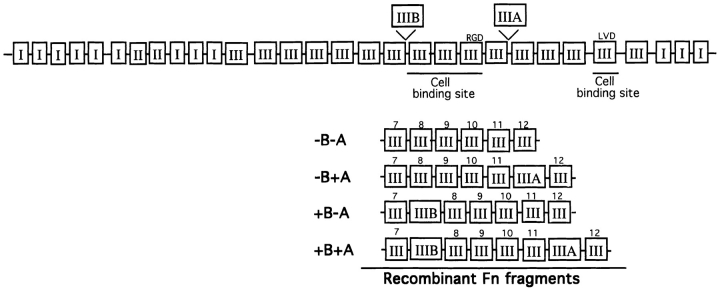
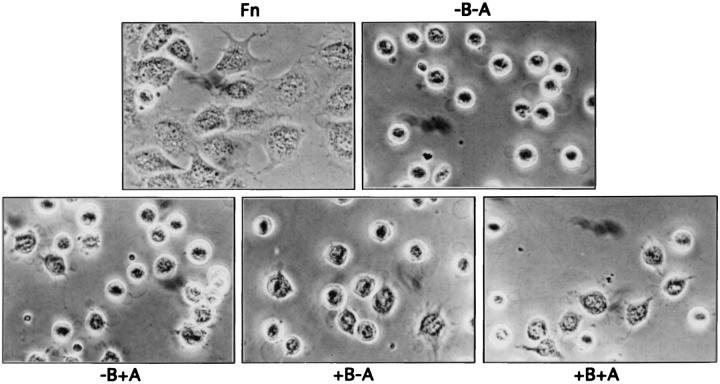
Recombinant fragments of fibronectin and of other ECM proteins such as tenascin have proven to be useful in understanding the structural and biological aspects of cell matrix interactions (Aukhil et al., 1993; Leahy et al., 1996). To further pursue the relationships between cell spreading, FAK tyrosine phosphorylation, and MAP kinase activation, we made use of several recombinant fragments of fibronectin that had been expressed in prokaryotic systems (Fig. 4). A series of fibronectin type III domains from domain 7 to 12, either including or excluding the two alternatively spliced domains EIIIB and EIIIA were cloned and expressed as described in Materials and Methods. As shown in Fig. 4, each of the recombinant fibronectin fragments contained the cell-adhesive RGD site in type III domain 10, as well as the recently identified PHSRN sequence in the “synergy” domain 9 (Aota et al., 1994; Bodwitch et al., 1994). As seen in Fig. 5, NIH 3T3 cells rapidly adhered and spread on fibronectin-coated substrata. The cells also adhered rapidly but spread less well on substrata coated with equimolar amounts of the recombinant fibronectin fragments. In particular, cells adhering to the −B−A fragment displayed very little spreading during the first 30 min; cells on the −B+A or +B−A fragments also showed modest spreading, while the +B+A fragment supported the greatest degree of spreading, although still far less than for intact fibronectin. Adhesion of cells to the fibronectin fragments was almost completely blocked by 1 mM RGD peptide but not by RGE peptide (data not shown). Thus, adhesion to the recombinant fibronectin fragments seems to be primarily an integrin-mediated process.
Figure 4.
Linear structure of Fn and recombinant Fn fragments. Fn consists of three types of repeating motifs: types I, II, and III. Two alternatively spliced variants with additional type III repeats, IIIA and IIIB, are indicated at the top. The underlines represent two putative cell binding sites. The central cell binding site contains the RGD sequence in the 10th type III repeat. The recombinant Fn fragments used in these studies contain the 7th to 12th type III repeats, with one or two or none of the alternative splicing motifs.
Figure 5.
Cell morphology: cell adherence on tissue culture dishes coated with Fn or recombinant Fn fragments. Tissue culture dishes were coated with 5 nM of intact Fn or recombinant Fn fragments overnight at 4°C. NIH 3T3 cells were plated onto these intact Fn or Fn fragment substrata for 30 min at 37°C. Cells were washed with PBS, fixed with 2% formaldehyde, and photographed at 300×.
When cells attaching on fibronectin fragments were analyzed for MAP kinase activity and FAK tyrosine phosphorylation, some very striking results were observed (Fig. 6). All of the fragments caused a rapid and robust activation of MAP kinase, measured either by a mobility shift assay (Fig. 6 A) or by an in vitro kinase assay (Fig. 6 B). We could not discern any quantitiative differences in MAP kinase activation for cells adhering to intact fibronectin or to the various fragments. By contrast, although adhesion and spreading on intact fibronectin resulted in a strong increase in tyrosine phosphorylation of FAK, only modest increases were observed in cells adhering to the −B+A, +B−A, and +B+A fragments, while there was no detectable increase in cells adhering to the −B−A fragment (Fig. 6 C). This result suggests that it is possible to obtain quite strong activation of MAP kinase in the absence of significant tyrosine phosphorylation of FAK.
Figure 6.
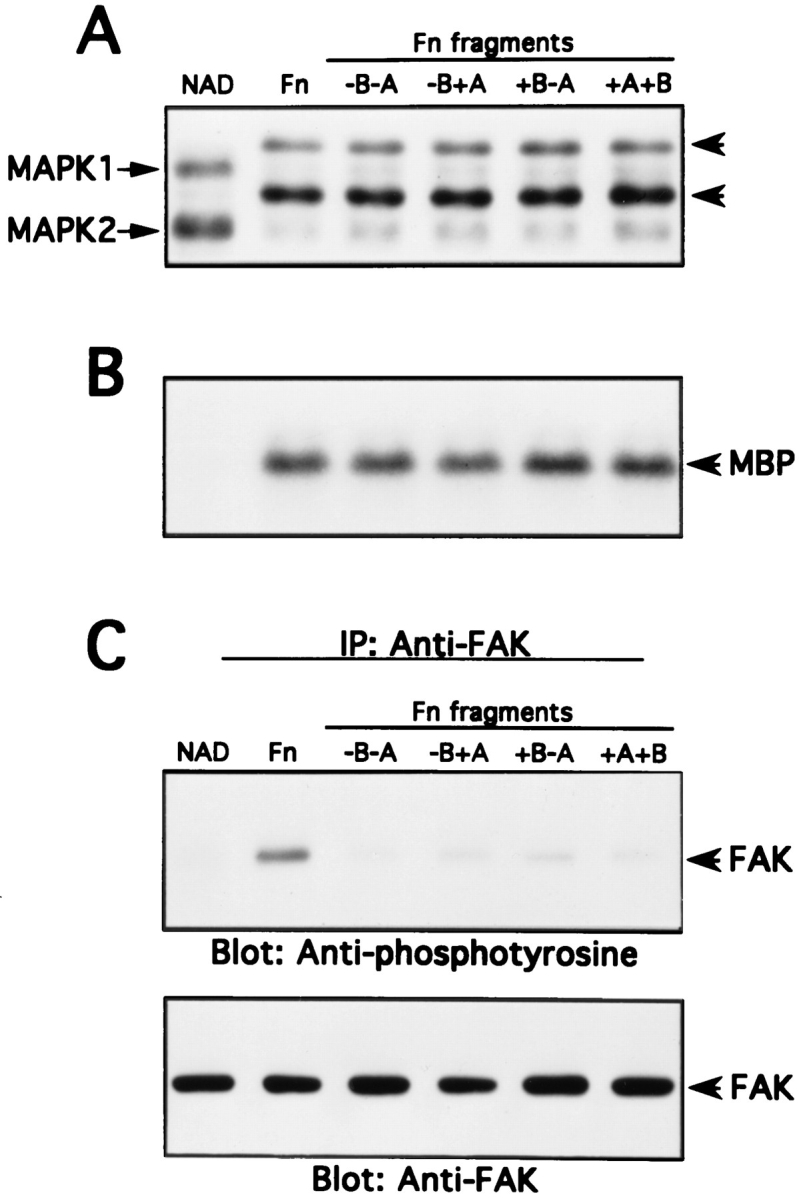
Differential activation of FAK and MAP kinase upon cell adhesion to intact Fn or recombinant Fn fragment–coated dishes. NIH 3T3 cells were detached and incubated nonadherently for 45 min at 37°C (NAD). Cells were then allowed to adhere to intact Fn or Fn fragment–coated dishes for 30 min at 37°C. MAP kinase activity and FAK tyrosine phosphorylation were determined as described in Materials and Methods. (A) MAP kinase band shift assay. (B) MAP kinase immune complex kinase assay. (C) FAK tyrosine phosphorylation and anti-FAK Western blotting.
To confirm that the activation of MAP kinase observed in cells adhering to fibronectin was primarily an integrinmediated event, we compared cells attaching to substrata coated with intact fibronectin or the −B−A fragment to cells attaching to substrata coated with the nonspecific adhesive substance poly-l-lysine. Preliminary observations showed a weak MAP kinase activation in 3T3 cells adhering to poly-l-lysine and a more robust activation in cells adhering to −B−A or to intact fibronectin (data not shown). This suggests that integrins play an important role in the MAP kinase activation events studied here, consistent with our previous observations (Chen et al., 1994).
It is possible that when cells adhere to the fibronectin fragments FAK might be specifically phosphorylated at Y925, the Grb2 binding site (Schlaepfer et al., 1994), thus leading to activation of the MAP kinase pathway even though overall tyrosine phosphorylation of FAK is low. However, when we used a Grb2–GST fusion protein to bind tyrosine phosphorylated FAK, we found that the reduced FAK tyrosine phosphorylation observed in cells adhering to the fibronectin fragments was accompanied by reduced binding of FAK to Grb 2 (data not shown). Thus, it seems likely that the tyrosine phosphorylation status of FAK is not related to adhesion-mediated MAP kinase activation. It is also striking that robust MAP kinase activation occurs in adhering cells that are completely round by light microscopic criteria (cells on −B−A), suggesting that extensive actin stress fiber organization is not required for integrin-mediated MAP kinase activation.
Activation of MAP Kinase Can Precede Activation of FAK
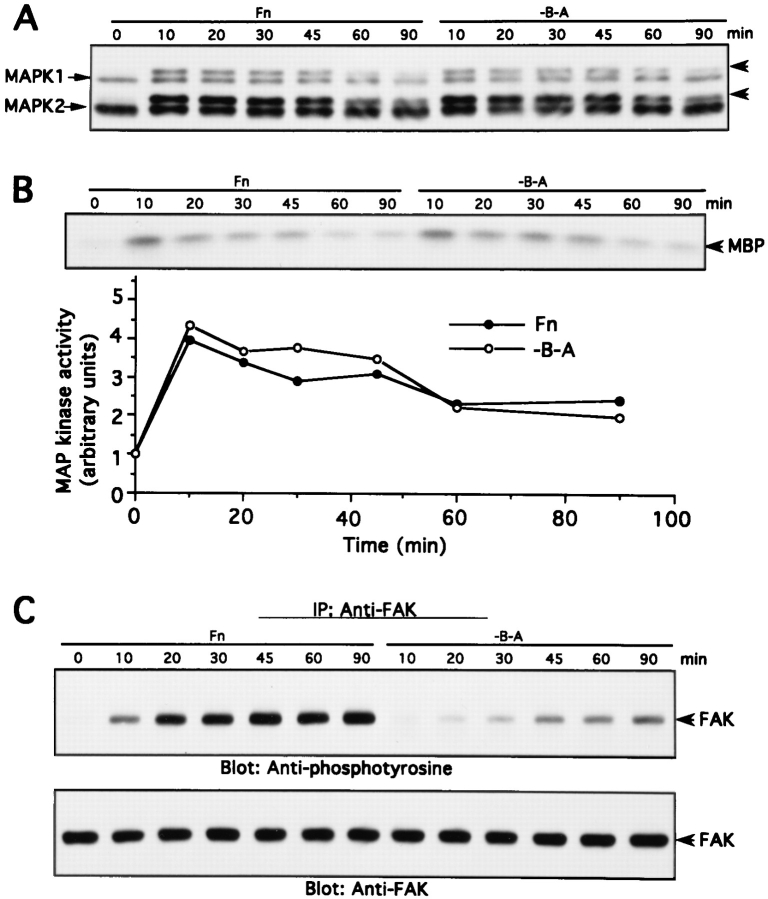
We noticed that prolonged incubation of 3T3 cells on the fibronectin fragments gradually led to increased cell spreading. We used this to examine the detailed kinetics of MAK activation and FAK tyrosine phosphorylation in cells as they spread. As seen in Fig. 7, A and B, the MAP kinase kinetic profiles were essentially identical in cells attaching to intact fibronectin or to the −B−A fragment. There was a rapid activation to ∼4× basal levels that reached a maximum between 10 and 20 min and then a gradual decline between 30 and 90 min. In contrast, the kinetic profiles of FAK activation were very different on the two substrata. In cells attaching to fibronectin, a distinct increase in FAK tyrosine phosphorylation was observed by 10 min; this continued to increase up to 45 min and then remained constant. In cells attaching on −B−A, tyrosine phosphorylation of FAK was barely above background until 30 min; it then progressively increased during the remainder of the experiment, thus paralleling spreading behavior (not shown). These results indicate that MAP kinase activation can kinetically precede FAK activation, and that MAP kinase activation can decline even as FAK activation is increasing.
Figure 7.
Time course of MAP kinase activation and FAK tyrosine phosphorylation upon cell adhesion to intact Fn or −B−A Fn fragment–coated dishes. NIH 3T3 cells were trypsinized and kept in suspension for 45 min at 37°C. Cells were then plated onto intact Fn or the −B−A Fn fragment–coated dishes at 37°C for the time indicated. After incubation, cell lysates were analyzed for MAP kinase activity and FAK tyrosine phosphorylation as described in Materials and Methods. (A) MAP kinase band shift assay. (B) MAP kinase immune complex kinase assay. (C) FAK tyrosine phosphorylation and anti-FAK Western blotting.
Expression of FRNK, the COOH-terminal Domain of FAK, by Retroviral Infection Blocks Adhesion-dependent FAK Tyrosine Phosphorylation but Not MAP Kinase Activation
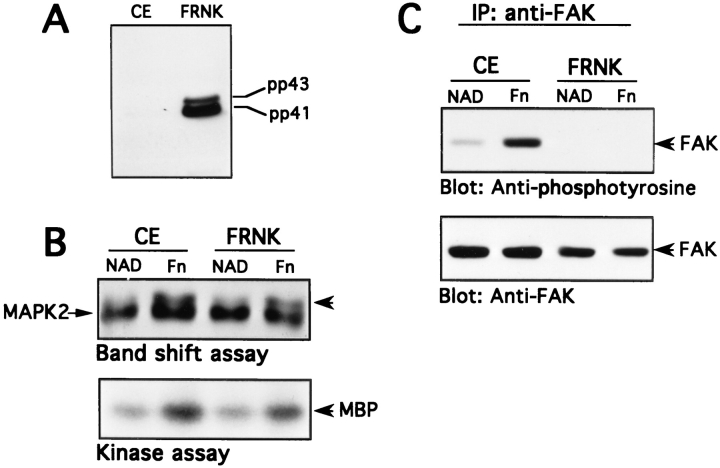
To further explore the relationship between FAK and MAP kinase we made use of constructs that could express the COOH-terminal domain of FAK, FRNK. The FRNK region of FAK contains the site responsible for targeting intact FAK to focal contacts (Hildebrand et al., 1993). Thus, it seemed likely that overexpressed FRNK might interfere with FAK function in a dominant inhibitory manner (Richardson and Parsons, 1996). As described in Materials and Methods, chicken fibroblasts were infected with a replication competent retrovirus that expresses FRNK from the LTR promoter. These cells, as well as control transfectants, were removed from the substratum and either maintained in suspension or replated on fibronectin. The cells were then lysed and tested for adhesion-induced MAP kinase activity and for tyrosine phosphorylation of endogenous FAK. As seen in Fig. 8, the cells transfected with the FRNK construct expressed substantially higher levels of FRNK than control transfectants. FRNK-expressing cells displayed dramatically reduced levels of tyrosine phosphorylation of FAK. By contrast, FRNK expression had little effect on the activation of MAP kinase by cell adhesion to fibronectin. Thus, levels of FRNK expression sufficient to almost completely inhibit FAK tyrosine phosphorylation were not able to significantly inhibit integrin-mediated MAP kinase activation.
Figure 8.
Effect of overexpression of FRNK on MAP kinase activity and FAK tyrosine phosphorylation in chicken fibroblasts. Chicken embryonic fibroblasts were infected with a retroviral expression vector for FRNK as described in Materials and Methods. Expression of FRNK in untransfected cells (CE) or in the FRNK transfected cells (FRNK) was examined by anti-FRNK immunoblotting (A). Untransfected or FRNK-transfected cells were trypsinized and incubated nonadherently for 45 min at 37°C (NAD). Cells were then plated onto Fn-coated dishes for 30 min at 37°C. After incubating, cells were washed and lysed in modified RIPA buffer. MAP kinase activity was analyzed by a band shift assay and by a immune complex kinase assay (B); it should be noted that chicken fibroblasts contain only one form of MAP kinase (MAPK2). FAK was immunoprecipitated and analyzed for tyrosine phosphorylation by anti-phosphotyrosine immunoblotting and by anti-FAK Western blot (C).
Transient Transfection with FRNK Does Not Block Activation of an Epitope-tagged MEK
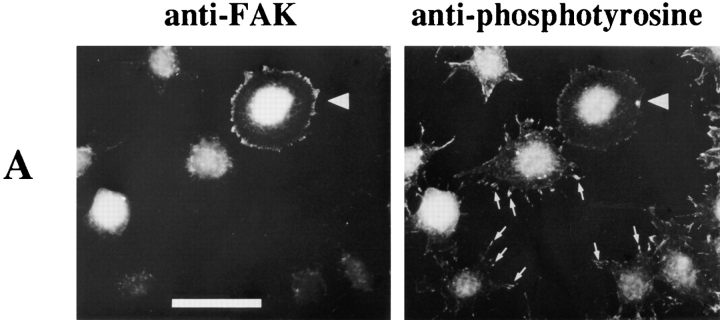
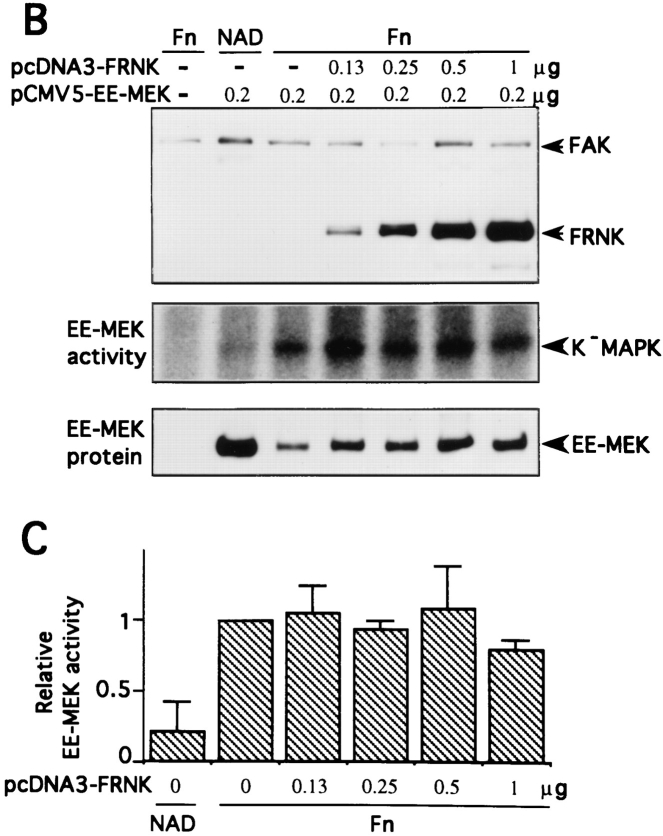
To extend our observations, the effect of FRNK expression was analyzed in NIH 3T3 cells under transient transfection conditions. Cell populations transfected with FRNK alone and plated on fibronectin were analyzed by doublelabel epifluorescence microscopy. The FRNK-expressing cells were identified by very bright and prominent focal adhesion staining using antibody 5158 (Fig. 9 A). Nontransfected cells demonstrated numerous lamellae and filopodia with focal adhesions that stained brightly for phosphotyrosine. However, FRNK transfectants exhibited greatly diminished phosphotyrosine content in focal adhesions as well as an altered morphology. Cells were cotransfected with EE-MEK and increasing amounts of FRNK or with empty vector. FRNK expression was detected by Western blotting of cell lysates (Fig. 9 B) as a 41–43-kD doublet in agreement with previously published observations (Richardson and Parsons, 1996). EE-MEK immunoprecipitated from transfected cell populations replated on fibronectin showed an increase in kinase activity compared to EEMEK isolated from suspension cells, as measured by the ability to phosphorylate K−MAPK. Even at the higher levels of FRNK expression, EE-MEK activity was robustly activated in response to adhesion to fibronectin (Fig. 9 B). Quantitation of EE-MEK activity normalized to the protein level of EE-MEK showed no significant decrease of the integrin-induced activation of EE-MEK due to FRNK cotransfection (Fig. 9 C). These data are consistent with the previous experiments in showing that integrin-induced activation of MAP kinase cascade activity and increased tyrosine phosphorylation of FAK are independent.
Figure 9.
Effect of overexpression of FRNK on MAP kinase activity and tyrosine phosphorylation in 3T3 fibroblasts. NIH 3T3 cells were transiently transfected either with different amounts of pcDNA3-FRNK or empty vector and EE-MEK. Cells were serum starved and replated on Fn or kept in suspension (NAD) as described in Materials and Methods. Transfection DNA levels were made up to 2 μg in each condition with empty vector (pCMV5 or pcDNA3). (A) After adherence to Fn-coated coverslips, transfected cell populations were fixed and coimmunostained with an anti-FAK/FRNK polyclonal antibody and for phosphotyrosine. The large arrow indicates a cell transfected with FRNK; small arrows indicate presumed focal contact sites in nontransfected cells (B) Detergent soluble lysates were analyzed for exogenous FRNK expression by Western blotting and for EE-MEK activity by immunopreciptation and in vitro kinase assay. Incorporation of 32P into K−MAPK was detected by autoradiography. The level of expressed EE-MEK was determined by Western blotting of EEimmunoprecipitates with anti-MEK antibody. (C) Quantitation of EE-MEK activity from kinase assays relative to EE-MEK protein levels. The average and standard deviation from the mean is represented for three separate transfection experiments. Bar, 60 μm.
Discussion
The fact that integrin-mediated cell adhesion is clearly involved in anchorage-dependent control of the cell cycle (Fang et al., 1996; Zhu et al., 1996), control of apoptosis (Ruoslahti and Reed, 1994; O'Brien et al., 1996), and the regulation of cell differentiation (Juliano and Haskill, 1993) has led to substantial interest in detailed mechanisms of integrin-mediated signal transduction. Initial observations on integrin signaling emphasized the importance of tyrosine phosphorylation (Guan et al., 1991; Kornberg et al., 1991) and particularly the autophosphorylation of FAK, a seemingly unique integrin-regulated cytoplasmic tyrosine kinase (Hanks et al., 1992; Schaller et al., 1992). Although FAK homologues have now been identified (Avraham et al., 1995; Lev et al., 1995; Sasaki et al., 1995) and other tyrosine kinases such as Syk have been shown to be integrin-responsive (Clark et al., 1994; Lin et al., 1995), FAK is still considered to be a key element in integrin signaling pathway(s) in many cell types. Clearly FAK is implicated in pathways that regulate cell motility and cytoskeletal organization and may possibly be involved in cell growth control as well (Romer et al., 1994; Illc et al., 1995; Frisch et al., 1996; Gilmore and Romer, 1996; Richardson and Parson, 1996). Tyrosine phosphorylated FAK can bind to a number of interesting signaling molecules via their SH2 domains, including Src family kinases, PI-3-K, and Grb2/ Sos (Chen and Guan, 1994; Schlaepfer et al., 1994; Bachelot et al., 1996). In addition, FAK can bind other structural and signal transduction proteins through SH3 domain interactions or other interactions; this includes talin, paxillin, p130Cas, and a GAP for Rho and CDC42 (Polte and Hanks, 1995; Vuori and Ruoslahti, 1995; Chen et al., 1995; Turner and Miller, 1994; Hildebrand et al., 1995, 1996). Finally, FAK can help to organize complex networks of structural and signal transduction proteins at focal contacts, since many of the binding partners of FAK bind in turn to other proteins (Birge et al., 1993; Parsons, 1996). Despite all of these interesting possibilities, the precise role of FAK in integrin signaling remains rather poorly defined.
Another set of events that has attracted considerable attention lately relates to integrin-mediated activation of MAP kinase and Jun kinase (Chen et al., 1994; Miyamoto et al., 1995). While these events are clearly integrin-mediated (Chen et al., 1996), they differ strikingly from FAK responses in that they are quite transient, with the peak of activation coming immediately after cell attachment and then rapidly returning to basal levels. The kinetics of the MAP kinase response to integrin-mediated adhesion is somewhat similar to MAP kinase activation in response to mitogen treatment. In that case, the attenuation of MAP kinase activity is due, in part, to the induction of specific phosphatases that dephosphorylate critical resides on MAP kinase (Sun et al., 1994; Ward et al., 1994); it is unclear whether this also occurs in connection with integrin signaling to MAP kinase.
The fact that integrin-mediated adhesion (or clustering) can activate both the FAK tyrosine kinase and MAP kinase, raises the possibility that FAK might be “upstream” of MAP kinase, and that Grb2/Sos and Ras might be intermediates in the integrin signaling pathway (Schlaepfer et al., 1994). However, previous data from our laboratory indicate that Ras is not essential for integrin-mediated MAP kinase activation in 3T3 cells (Chen et al., 1996). Further, our current data strongly support the concept that FAK is not involved in integrin-mediated activation of MAP kinase. Several independent lines of investigation including the use of mutant β1 subunits, the use of recombinant fibronectin fragments, and the use of the FRNK domain of FAK as a dominant negative mutant, all indicate that FAK activation can be quantitatively and kinetically uncoupled from MAP kinase activation. This set of observations is difficult to reconcile with the simple and appealing model that integrin-mediated FAK activation leads to recruitment of Grb2/Sos and subsequent activation of the Ras/Raf/ MEK/MAP kinase pathway. Rather, current work is consistent with the possibility that FAK activation and MAP kinase activation represent distinct integrin-mediated signaling pathways. Another possibility is that MAP kinase activation is upstream of FAK activation; this interesting alternative is currently being investigated. However, even if this were so, it seems unlikely that the only role for integrinmediated MAP kinase activation would be to regulate FAK, since during cell adhesion MAP kinase migrates to the nucleus where it potentially may act on key transcription factors (Chen et al., 1994). The finding that integrin-mediated MAP kinase activation is largely independent of both Ras (Chen et al., 1996) and FAK suggests the existence of a novel pathway for MAP kinase stimulation, but the components of this pathway remain to be defined.
It is apparent that the control of cell growth involves a complex interplay between signaling pathways triggered by mitogens and integrin-mediated events including assembly of focal contacts and the actin cytoskeleton (Plopper et al., 1995; Fang et al., 1996; Zhu et al., 1996). It seems likely that small GTPases may be critically important in this interplay. The function of the Rho family members CDC42, Rac, and RhoA can be regulated by a variety of mechanisms, including activation of the Ras proto-oncogene. Thus, the Ras signaling pathway bifurcates to act both on activation of Rho family members and upon the ERK subfamily of MAP kinases (Khosravi-Far et al., 1995; Qiu et al., 1995). CDC42, Rac, and Rho have been implicated in actin filament formation in filopodia, ruffled membranes, and stress fibers, respectively (Nobes and Hall, 1995). Rac and Rho also cooperate with integrins in the assembly of focal contacts, and both elements are required for the process (Hotchin and Hall, 1995). In addition, CDC42 and Rac can regulate the signaling pathway leading to activation of the JUN kinase subfamily of MAP kinases (Vojtek and Cooper, 1995), while all three Rho-family proteins regulate transcriptional events associated with cell cycle traverse (Hill et al., 1995; Olson et al., 1995). Although the relationships between the cytoskeletal and signaling activities of the Rho proteins are not fully understood, they may well be essential for growth control. One possible intersection concerns the generation of inositol lipids. A Rhodependent PI-5-kinase may control the availability of PIP2 in cells (Chong et al., 1994). Levels of PIP2 play a key role both in mitogenic signal transduction (Liscovitch and Cantley, 1995) and in assembly of cytoskeletal structures (Gilmore and Burridge, 1996), thus potentially linking these two important processes. Further, recent findings have demonstrated a role for Rho in integrin-mediated activation of MAP kinase (Renshaw et al., 1996), possibly by promoting the assembly of cytoskeletal complexes. Despite these important advances, a detailed mechanistic delineation of connections between adhesion-induced cytoskeletal events and signaling events involved in mitogenesis remains unknown.
As a further step in that direction, we have carefully examined the relationship between two important integrin- dependent events, namely MAP kinase activation, which may play a role in mitogenesis, and FAK tyrosine phosphorylation, which is clearly involved in cytoskeletal organization. Some degree of cytoskeletal assembly is necessary for both FAK tyrosine phosphorylation and MAP kinase activation. Thus, disruption of focal structures and actin filaments with cytochalasin D competely prevents integrinmediated activation of both FAK and MAP kinase (Bockholt and Burridge, 1993; Chen et al., 1994). However, current findings suggest that MAP kinase and FAK may respond to different levels or degrees of cytoskeletal assembly. Integrin-mediated MAP kinase activation seems to be an early, transient event that occurs before cell spreading, while FAK activation occurs concurrently with spreading and is persistent. Our observations on FAK and MAP kinase are consistent with the concept that integrinmediated cell adhesion creates a hierarchy of structural and signaling events (Miyamoto et al., 1995) that can be kinetically and topologically resolved. However, the precise biological and biochemical roles of the individual components of the integrin-regulated structural/signaling hierarchy remain to be determined.
Acknowledgments
This work was supported by National Institutes of Health grants GM26065 and HL45100 to R.L. Juliano and HL03299 to L. Romer.
Abbreviations used in this paper
- FAK
pp125 focal adhesion kinase
- Fn
fibronectin
- FRNK
FAK-related nonkinase
- MAP
mitogen activated protein
- MEK
MAP kinase kinase
- WT
wild type
Footnotes
The EEEEYMPME epitope–tagged rat MEK-1 construct (EE-MEK) was kindly provided by Dennis J. Templeton (Case Western Reserve University, Cleveland, OH). A.R. Horwitz (University of Illinois, Urbana, IL) generously provided the chicken β1 constructs as well as the W1B10 monoclonal antibody.
Please address all correspondence to R.L. Juliano, Department of Pharmacology, School of Medicine, University of North Carolina, Chapel Hill, NC 27599. Ph.: (919) 966-4383; Fax: (919) 966-5640.
References
- Agrez M, Chen A, Cone RI, Pytela R, Sheppard D. The αvβ6 integrin promotes proliferation of colon carcinoma cells through a unique region of the β6 cytoplasmic domain. J Cell Biol. 1994;127:547–556. doi: 10.1083/jcb.127.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Aukhil I, Joshi P, Yan Y, Erickson HP. Cell and heparin binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993;268:2542–2553. [PubMed] [Google Scholar]
- Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from magakaryoctes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- Bachelot C, Rameh L, Parsons T, Cantley LC. Association of phosphatidylinositol 3-kinase, via SH2 domains of p85, with focal adhesion kinase in polyoma middle t-transformed fibroblasts. Biochim Biophys Acta. 1996;1311:45–52. doi: 10.1016/0167-4889(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Bauer, J., J. Varner, C. Schreiner, L. Kornberg, R. Nicholas, and R.L. Juliano. 1993. Functional role of the cytoplasmic domain of the integrin alpha 5 subunit. J. Cell Biol. 122:209–221. [DOI] [PMC free article] [PubMed]
- Birge RB, Fajardo JE, Reichman C, Shoelson SE, Songyang Z, Cantley LC, Hanafusa H. Identification and characterization of a highaffinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockholt SB, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphylation of tensin. J Biol Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- Bodwitch RD, Hariharan M, Tominna EF, Smith JW, Yamada KM, Getzoff ED, Ginsberg MH. Identification of a novel integrin binding site in fibronectin: differential utilization by β3 integrins. J Biol Chem. 1994;269:10856–10863. [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonist promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAKaccompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calab MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for src family kinase. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BM, Matsuura N, Takada Y, Zetter BR, Hemler ME. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science (Wash DC) 1991;251:1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Chan BM, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Guan J-L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin mediated cell adhesion activates MAP kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chen H-C, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan J-L. Interaction of FAK with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lin TH, Der CJ, Juliano RL. Integrin-mediated activation of MEK and MAP kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science (Wash DC) 1995;286:233–235. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hynes RO. Ras activation is necessary for integrinmediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alphaIIbbeta3 . J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- Clarke AS, Lotz MM, Chao C, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the β4integrin cytoplasmic domain. J Biol Chem. 1995;270:22673–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- Egan SE, Weinberg RA. The pathway to signal achievement. Science (Wash DC) 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin-E-CDK2 kinase activity on cell anchorage. Science (Wash DC) 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A, Romer LH. Inhibition of FAK signaling in focal adhesion decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding talin and actin by phosphatidylinositol-4-5-bisphosphate. Nature (Lond) 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Guan J-L, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell spreading on fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxy terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Taylor JM, Parsons JT. An SH3 domain-containing GTPase-activating protein for rho and cdc42 associates with focal adhesion kinase. Mol Cell Biol. 1996;16:3169–3178. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes HS, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by α5β1 integrins and α4β1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Illc D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature (Lond) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapron-Bras C, Fitz-Gibbon L, Jeevaratnam P, Wilkins J, Dedhar S. Stimulation of tyrosine phosphorylation and accumulatin of GTPbound p21ras upon antibody-mediated alpha 2 beta 1 integrin activation in T-lymphoblastic cells. J Biol Chem. 1993;268:20701–20704. [PubMed] [Google Scholar]
- Khosravi-Far R, Der CJ. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994;13:67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac 1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg LJ, Earp SE, Turner CE, Procktop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of β1integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 crystal structure of a fourdomain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Masacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature (Lond) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated phosphorylation and cytokine message induction in monocytic cells: a possible signaling role for the Syk tyrosine kinase. J Biol Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Cantley LC. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- Loftus JC, Smith JW, Ginsberg MH. Integrin-mediated cell adhesion: the extracellular face. J Biol Chem. 1994;269:25235–25238. [PubMed] [Google Scholar]
- Meredith JJ, Takada Y, Fornaro M, Languino LR, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin beta IC. Science (Wash DC) 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso O, Silvio G, Burbelo P, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- O'Brien V, Frisch SM, Juliano RL. Expression of the integrin α5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res. 1996;224:208–213. doi: 10.1006/excr.1996.0130. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science (Wash DC) 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas . Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature (Lond) 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase Rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Parsons JJ. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK . Nature (Lond) 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari V-M. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Romer, L.H., N. McLean, C.E. Turner, and K., B. 1994. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol. Biol. Cell. 5:349–361. [DOI] [PMC free article] [PubMed]
- Rosales C, O'Brien V, Kornberg L, Juliano RL. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995;1242:77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasai T. Cloning and characterization of cell adhesion kinase beta, a novel proteintyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesion. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a non-catalytic domain of the focal adhesion associated protein tyrosine kinase pp 125FAK . Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, M.D., J.D. Hildebrand, J.D. Shannon, J.W. Fox, R.R. Vines, and J.T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase pp 125FAK, directs SH2-dependent binding of pp60src Mol. Cell. Biol. 1680–1688. [DOI] [PMC free article] [PubMed]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks S, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature (Lond) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Seckl MJ, Morii N, Narumiya S, Rozengurt E. Guanosine 5′-3-O- (Thio)triphosphate stimulates tyrosine phosphorylation of p125FAKand paxillin in permeabilized Swiss 3T3 cells. J Biol Chem. 1995;270:6984–6990. doi: 10.1074/jbc.270.12.6984. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CBA, Burdon TG, Schindler C, Watson CJ. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- Sun H, Tonks NK, Bar-Sagi D. Inhibition of Ras-induced DNA synthesis by expression of the phosphatase MKP-1. Science (Wash DC) 1994;266:285–288. doi: 10.1126/science.7939666. [DOI] [PubMed] [Google Scholar]
- Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 binding motifs and multiple LIM domains: identification of a vinculin and pp125FAK-binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Varner, J.A., D.A. Emerson, and R.L. Juliano. 1995. Integrin α5β1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol. Biol. Cell. 6:725–740. [DOI] [PMC free article] [PubMed]
- Vojtek AB, Cooper JA. Rho family members: activators of MAP kinase cascades. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Casand cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- Ward Y, Gupta S, Jensen P, Wartmann M, Davis RJ, Kelly K. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature (Lond) 1994;367:651–654. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- Wilson L, Carrier MJ, Kellie S. pp125FAK tyrosine kinase activity is not required for the assembly of F-actin stress fibers and focal adhesion in cultured mouse aortic smooth muscle cells. J Cell Sci. 1995;108:2381–2391. doi: 10.1242/jcs.108.6.2381. [DOI] [PubMed] [Google Scholar]
- Yurochko AD, Liu DY, Eierman D, Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci USA. 1992;89:9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I, Sinnett-Smith J, Turner CE, Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation of the focal adhesion-associated protein paxillin in Swiss 3T3 cells. J Biol Chem. 1993;268:22060–22065. [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]