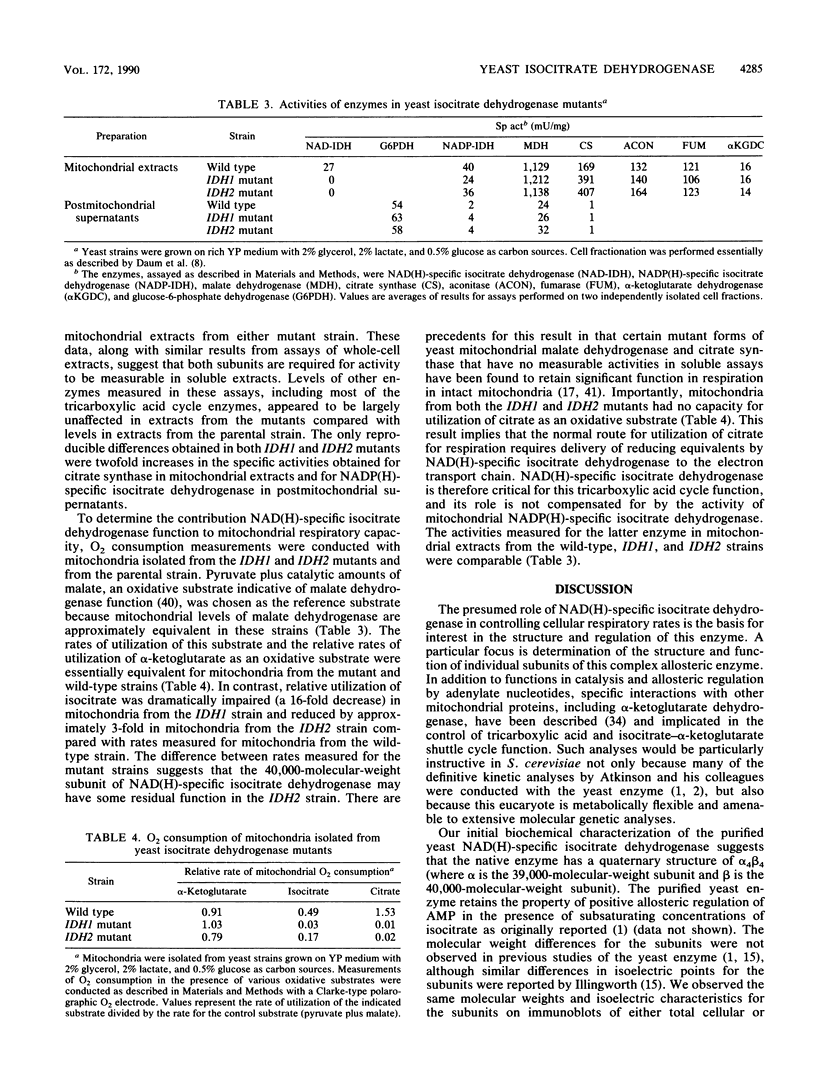
Abstract
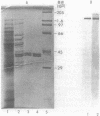
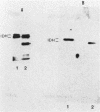
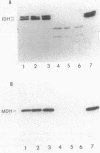
Mitochondrial NAD(H)-specific isocitrate dehydrogenase was purified from Saccharomyces cerevisiae for analyses of subunit structure and expression. Two subunits of the enzyme with different molecular weights (39,000 and 40,000) and slightly different isoelectric points were resolved by denaturing electrophoretic techniques. Sequence analysis of the purified subunits showed that the polypeptides have different amino termini. By using an antiserum to the native enzyme prepared in rabbits, subunit-specific immunoglobulin G fractions were obtained by affinity purification, indicating that the subunits are also immunochemically distinct. The levels of NAD(H)-specific isocitrate dehydrogenase activity and immunoreactivity were found to correlate closely with those of a second tricarboxylic acid cycle enzyme, malate dehydrogenase, in yeast cells grown under a variety of conditions. S. cerevisiae mutants with defects in NAD(H)-specific isocitrate dehydrogenase were identified by screening a collection of yeast mutants with acetate-negative growth phenotypes. Immunochemical assays were used to demonstrate that one mutant strain lacks the 40,000-molecular-weight subunit (IDH1) and that a second strain lacks the 39,000-molecular-weight subunit (IDH2). Mitochondria isolated from the IDH1 and IDH2 mutants exhibited a markedly reduced capacity for utilization of either isocitrate or citrate for respiratory O2 consumption. This confirms an essential role for NAD(H)-specific isocitrate dehydrogenase in oxidative functions in the tricarboxylic acid cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes L. D., Kuehn G. D., Atkinson D. E. Yeast diphosphopyridine nucleotide specific isocitrate dehydrogenase. Purification and some properties. Biochemistry. 1971 Oct 12;10(21):3939–3944. doi: 10.1021/bi00797a022. [DOI] [PubMed] [Google Scholar]
- Barnes L. D., McGuire J. J., Atkinson D. E. Yeast diphosphopyridine nucleotide specific isocitrate dehydrogenase. Regulation of activity and unidirectional catalysis. Biochemistry. 1972 Nov 7;11(23):4322–4329. doi: 10.1021/bi00773a019. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Burke W. F., Johanson R. A., Reeves H. C. NADP+-specific isocitrate dehydrogenase of Escherichia coli. II. Subunit structure. Biochim Biophys Acta. 1974 Jun 7;351(2):333–340. doi: 10.1016/0005-2795(74)90196-2. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chapman C., Bartley W. The kinetics of enzyme changes in yeast under conditions that cause the loss of mitochondria. Biochem J. 1968 Apr;107(4):455–465. doi: 10.1042/bj1070455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton D., Weinstock S. B., Fraenkel D. G. Glycolysis mutants in Saccharomyces cerevisiae. Genetics. 1978 Jan;88(1):1–11. doi: 10.1093/genetics/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Duntze W., Neumann D., Gancedo J. M., Atzpodien W., Holzer H. Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1969 Aug;10(1):83–89. doi: 10.1111/j.1432-1033.1969.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein D. B., McAlister L. alpha-Factor-mediatd modification of a 32P-labeled protein by MATa cells of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2561–2566. [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Glenney J. Antibody probing of western blots which have been stained with india ink. Anal Biochem. 1986 Aug 1;156(2):315–319. doi: 10.1016/0003-2697(86)90259-9. [DOI] [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- Illingworth J. A. Purification of yeast isocitrate dehydrogenase. Biochem J. 1972 Oct;129(5):1119–1124. doi: 10.1042/bj1291119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Rosenkrantz M. S., Guarente L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol Cell Biol. 1986 Jun;6(6):1936–1942. doi: 10.1128/mcb.6.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Evans C. T., Malloy C., Srere P. A. Metabolic studies on citrate synthase mutants of yeast. A change in phenotype following transformation with an inactive enzyme. J Biol Chem. 1989 Jul 5;264(19):11204–11210. [PubMed] [Google Scholar]
- Kispal G., Rosenkrantz M., Guarente L., Srere P. A. Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthases. J Biol Chem. 1988 Aug 15;263(23):11145–11149. [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Walsh K., LaPorte D. C. Sensitivity of metabolic fluxes to covalent control. Curr Top Cell Regul. 1985;27:13–22. doi: 10.1016/b978-0-12-152827-0.50009-8. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., Barnes L. D., Atkinson D. E. Yeast diphosphopyridine nucleotide specific isocitrate dehydrogenase. Binding of ligands. Biochemistry. 1971 Oct 12;10(21):3945–3951. doi: 10.1021/bi00797a023. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaPorte D. C., Chung T. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem. 1985 Dec 5;260(28):15291–15297. [PubMed] [Google Scholar]
- Machado A., Nuñez de Castro I., Mayor F. Isocitrate dehydrogenases and oxoglutarate dehydrogenase activities of baker's yeast grown in a variety of hypoxic conditions. Mol Cell Biochem. 1975 Feb 28;6(2):93–100. doi: 10.1007/BF01732003. [DOI] [PubMed] [Google Scholar]
- McAlister-Henn L., Thompson L. M. Isolation and expression of the gene encoding yeast mitochondrial malate dehydrogenase. J Bacteriol. 1987 Nov;169(11):5157–5166. doi: 10.1128/jb.169.11.5157-5166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1985 Dec 5;260(28):15019–15027. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpaczy Z., Sumegi B., Alkonyi I. Interaction between NAD-dependent isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase complex, and NADH:ubiquinone oxidoreductase. J Biol Chem. 1987 Jul 15;262(20):9509–9514. [PubMed] [Google Scholar]
- Ramachandran N., Colman R. F. Chemical characterization of distinct subunits of pig heart DPN-specific isocitrate dehydrogenase. J Biol Chem. 1980 Sep 25;255(18):8859–8864. [PubMed] [Google Scholar]
- Reeves H. C., Daumy G. O., Lin C. C., Houston M. NADP + -specific isocitrate dehydrogenase of Escherichia coli. I. Purification and characterization. Biochim Biophys Acta. 1972 Jan 20;258(1):27–39. doi: 10.1016/0005-2744(72)90964-3. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984 Aug;3(8):1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]