Abstract
The insulin-responsive glucose transporter GLUT-4 is found in muscle and fat cells in the transGolgi reticulum (TGR) and in an intracellular tubulovesicular compartment, from where it undergoes insulindependent movement to the cell surface. To examine the relationship between these GLUT-4–containing compartments and the regulated secretory pathway we have localized GLUT-4 in atrial cardiomyocytes. This cell type secretes an antihypertensive hormone, referred to as the atrial natriuretic factor (ANF), in response to elevated blood pressure. We show that GLUT-4 is targeted in the atrial cell to the TGR and a tubulo-vesicular compartment, which is morphologically and functionally indistinguishable from the intracellular GLUT-4 compartment found in other types of myocytes and in fat cells, and in addition to the ANF secretory granules. Forming ANF granules are present throughout all Golgi cisternae but only become GLUT4 positive in the TGR. The inability of cyclohexamide treatment to effect the TGR localization of GLUT-4 indicates that GLUT-4 enters the ANF secretory granules at the TGR via the recycling pathway and not via the biosynthetic pathway. These data suggest that a large proportion of GLUT-4 must recycle via the TGR in insulin-sensitive cells. It will be important to determine if this is the pathway by which the insulin-regulatable tubulo-vesicular compartment is formed.
Glucose entry into mammalian cells occurs in most cases by facilitative transport, a process that is mediated by a family of glucose transporter proteins. The individual members of this family, GLUTs 1–7, are variably expressed in different tissues (Bell et al., 1993). GLUT-4 is expressed in cell types, such as skeletal muscle, cardiac muscle, white and brown adipose tissue, that exhibit acute changes in glucose transport (Birnbaum, 1989; James et al., 1989). Most glucose transporter isoforms constitutively reside at the cell surface to optimize exposure of the cell to the extracellular glucose (James and Piper, 1994). In contrast, GLUT-4 is found in an intracellular compartment from where it can be rapidly translocated to the cell surface in response to insulin, thus allowing the cell to transiently increase its access to extracellular glucose (Cushman and Wardzala, 1980; Suzuki and Kono, 1980). This process plays an important role in the postabsorptive removal of glucose from the bloodstream in mammals and also during enhanced energy consumption, such as exercise in the case of muscle.
Despite considerable progress in our understanding of insulin-regulated GLUT-4 movement, the nature of the intracellular GLUT-4 storage compartment, and the intracellular trafficking pathway(s) undertaken by GLUT-4, remain to be fully defined. Morphological studies have provided important clues concerning GLUT-4 trafficking in insulin-sensitive cells (Slot et al., 1991a ,b; James et al., 1994). GLUT-4, like a number of other cell surface recycling proteins, is internalized from the cell surface via clathrin-coated pits (Robinson et al., 1992). Thereafter, the transporter is sorted from the lysosomal pathway into a compartment comprising tubules and vesicles that we have referred to as tubulo-vesicular (T-V)1 elements. The relationship of this compartment to other secretory compartments is currently not known. It has been suggested that it has specialized properties that distinguish it from the endosomal/trans-Glogi reticulum (TGR) system (Martin at al., 1994; Herman et al., 1994). However, it is not clear if it arises like small synaptic vesicles from the endosomal system, or like secretory granules from the biosynthetic pathway (Rindler, 1992). One way to address this issue is to study the trafficking of GLUT-4 in a cell type that possesses one or more of these secretory systems. GLUT-4 has been expressed in PC12 cells, a neuroendocrine cell line that contains two regulated secretory systems: small synaptic vesicles, which evolve from endosomes, and dense core granules, which are formed in the biosynthetic pathway. In one study (Hudson et al., 1993), a small proportion (∼14%) of GLUT-4 was targeted to dense core granules with the remainder being found in T-V elements distinct from synaptic vesicles. In contrast, Herman et al. (1994) did not detect GLUT-4 in dense core granules, the majority being found in a small, vesicular compartment distinct from synaptic vesicles or recycling endosomes.
In an effort to further explore this question we have studied a bona fide regulated secretory cell type, atrial myocytes, in which GLUT-4 is endogenously expressed. The main secretory product of the atrial cardiomyocyte is the precursor of atrial natriuretic factor (ANF), an antihypertensive hormone (De Bold, 1985; Ruskoaho, 1992). In the present study we have shown that GLUT-4 is expressed at high levels in atrial cardiomyocytes. This presented the opportunity to study the distribution of GLUT-4 in a cell type that contains both an insulin-regulatable transport system and a regulated secretory pathway. Our data show that a large proportion of GLUT-4 (50–60%) is targeted to the ANF-containing secretory granules. GLUT-4 appears to enter this compartment as it recycles through the TGR and so we suggest that this may represent an important and unique aspect of the function of this molecule.
Materials and Methods
Materials
Polyclonal rabbit antiserum against the cytoplasmic carboxy terminus of GLUT-4 (James et al., 1989) has been described previously. Rabbit antisera against the NH2 and COOH termini of the pro-ANF polypeptide were used. Since only the prohormone of ANF could be detected in the myocytes the immunolabeling pattern for both antisera was similar (Cantin et al., 1990). This is referred to as ANF labeling. Rabbit anti–gamma adaptin antibodies were kindly provided by Margaret Robinson, Cambridge University, UK. Goat anti–rabbit IgG conjugated to CY3 was obtained from Jackson ImmunoResearch Laboratories, West Grove, PA. HRP-conjugated goat anti–rabbit IgG and enhanced chemiluminescence (ECL) detection kits were from Amersham (Aylesbury, UK). Colloidal gold was prepared by tannic acid-citrate reduction (Slot and Geuze, 1985) and coupled to protein A (Roth et al., 1978; Slot et al., 1988).
Immunocytochemistry
Male Wistar rats were fasted overnight, unless indicated otherwise. Stimulated animals were injected intraperitoneally with a mixture of insulin (8 U/kg) and d-glucose (1 g/kg) 30 min before fixation. Whole body fixation was performed on animals that were anesthetized with pentobarbitone as described (Slot et al., 1991a ), using a mixture of 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4. In some experiments glutaraldehyde was omitted or fresh atrial tissue was dissected and fixed by immersion. For cryosectioning (Slot et al., 1991a ) small fragments of the fixed tissue were prepared from the rim of the left or right atrium and immersed in 10% gelatin for ∼15 min at 37°C. The gelatin was then solidified on ice and small gelatin-embedded tissue blocks were immersed in 2.3 M sucrose at 4°C overnight. Tissue blocks from the left ventricle were processed similarly to the atrial tissue. Liver tissue blocks were prepared without gelatin. Omission of the gelatin-embedding step resulted in serious damage to the myocyte ultrastructure, probably due to overstretching of the sections when they were thawed on sucrose (Liou et al., 1996). On the other hand, this overstretching resulted in increased accessibility of the antigens as the immunolabeling efficiency was generally higher in these sections. Thus, in some experiments the gelatin embedding of heart tissue was omitted to take advantage of this increased labeling efficiency. For light microscopy (LM), ∼300-nm-thick cryosections were cut at −90°C, and for EM, 50–70-nm sections were cut at −120°C using an Ultracut S/FCS (Leica Inc., Vienna, Austria) equipped with an antistatic device (Diatome, Biel, Switzerland) and a diamond knife (Drukker, Cuyck, The Netherlands). The EM sections were immunolabeled using protein A–gold as the marker for single and double labeling (Slot et al., 1991a ). LM sections were placed on poly-l-lysine–coated glass slides and similarly immunolabeled, using CY3-conjugated goat anti–rabbit IgG as a fluorescent marker.
Immunoblotting
Different regions of the heart were dissected from rats and frozen at −80°C. Frozen tissue was thawed into ice-cold PBS containing protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin, 250 μM phenylmethane sulphonylfluoride), washed free of blood and homogenized thoroughly in the same buffer (1 ml) first using a polytron homogenizer and then by passage through a 22-gauge needle (Polytron; Brinkmann Instruments, Westbury, NY). Aliquots of the homogenate were removed and solubilized for 1 h in 1% Triton X-100 (final concentration) at 4°C. The insoluble material was pelleted for 10 min in a microfuge (Sorvall, Wilmington, DE) and the supernatant retained for immunoblotting. The protein concentration of the homogenates and the solubilized extracts was determined using the BCA reagent (Pierce Chemical Co., Rockford, IL).
Equal amounts of protein (25 μg) were separated by SDS-PAGE using a 10% acrylamide resolving gel. Proteins resolved by SDS-PAGE were electrophoretically transferred to nitrocellulose. Nitrocellulose sheets were incubated with the polyclonal antibodies diluted 1/1,000 (vol/vol) in 1% dried milk in PBS, pH 7.4. Detection of immunoreactive bands was achieved using ECL and quantitation was performed using an imaging densitometer (GS-670; Bio-Rad Laboratories, Richmond, CA) and the molecular analyst program.
[3H]-2-Deoxyglucose Uptake
Male Wistar rats were fitted with cannulae introduced into the carotid artery and jugular vein as previously described (Kraegen et al., 1985). Cardiac glucose uptake was assessed 7 d after surgery. Basal studies or euglycemic hyperinsulinemic clamps were performed after a 24-h fast as described previously (Kraegen et al., 1985). Briefly, insulin (Actrapid-HM Neutral; Novo Nordisk, Bagsvaerd, Denmark) was infused via the carotid cannula, at 0.25 U/kg/h for 2 h while the blood glucose concentration was maintained at basal fasting levels by a variable rate glucose infusion (dextrose 30 g/100 ml; Astra Pharmaceuticals, North Ryde, NSW, Australia).
At steady-state euglycemia (∼75 min after commencement of the clamp or 2 min after intravenous saline injection), an intravenous bolus of [3H]-2-deoxyglucose (80 μCi) (3H-2DG) (Amersham) was administered. Blood samples (200 μl) were obtained after administration of the bolus for estimation of plasma tracer and glucose concentration. Plasma samples for determination of tracer concentration were deproteinized immediately in 5.5% ZnSO4 and saturated Ba(OH)2. At completion of the study (45 min after 3H-2DG administration), rats were anesthetized (pentobarbitone; 60 mg/kg, intravenously) and the right and left atria and ventricles were rapidly removed and frozen. An estimate of tissue glucose uptake (the glucose metabolic index, Rg') was calculated from the tissue accumulation of phosphorylated 3H-2DG (Kraegen et al., 1985).
Plasma ANF Determinations
To assess the effect of insulin on ANF secretion, the plasma level of the NH2-terminal fragment (1–98) of pro-ANF was determined 10 min before and 10, 40, and 70 min after commencement of the euglycemic hyperinsulinemic clamp in 24-h fasted rats by radioimmunoassay as described previously (Thibault et al., 1988). The NH2-terminal fragment, being cosecreted with ANF in the blood, reflects the secretory function of the atria (Itoh et al., 1988). The major advantage of measuring the NH2-terminal fragment rather than ANF is that the former requires less plasma (0.1 ml vs 2.0 ml), allowing multiple samples to be obtained from the same animal.
Results
In the present study our primary goal was to characterize the intracellular GLUT-4 compartment. Therefore, most of the localization studies were performed in atrial myocytes of nonstimulated, overnight-fasted animals.
Immunofluorescence Microscopy
The alignment of myocytes in atrial tissue (Fig. 1, A and B) is ordered in a much more complicated fashion than in the ventricle (Fig. 1 C), and so the atrial fibers are sectioned much more randomly. Ventricular cells were viewed in longitudinally cut sections. We observed no significant difference in ANF or GLUT-4 labeling between the right and left atrium. As reported previously (Cantin et al., 1990; Thibault et al., 1989), the localization of ANF was restricted to secretory granules which appear by LM as dots scattered throughout the cells, but primarily concentrated near the nucleus (Fig. 1 A). The labeling pattern observed for GLUT-4 in the atrium was more complex (Fig. 1 B). GLUT-4 labeling, like that of ANF, occurred in large punctate structures both in the perinuclear region and elsewhere. However, beyond the perinuclear region very fine granular structures were predominantly labeled for GLUT-4. In longitudinal-sectioned myocytes these appeared arranged parallel to the cross-striation of the myofibers. In the ventricle a similar grainy labeling pattern is evident for GLUT-4, again with clear cross arrangement with a periodicity similar to the sarcomeres (Fig. 1 C). In addition, some arrangement in the longitudinal direction was apparent, the dots either following the cell surface or the spaces between the myofibrils (Fig. 1 C). Previously, we observed that this fine granular labeling is due to small T-V elements that occur predominantly at the Z-line level in cardiomyocytes (Slot et al., 1991b ). Typically, the GLUT-4 labeling pattern in the atrium, at the level of LM, resembled a composite image of atrial ANF labeling and ventricular GLUT-4 labeling.
Figure 1.
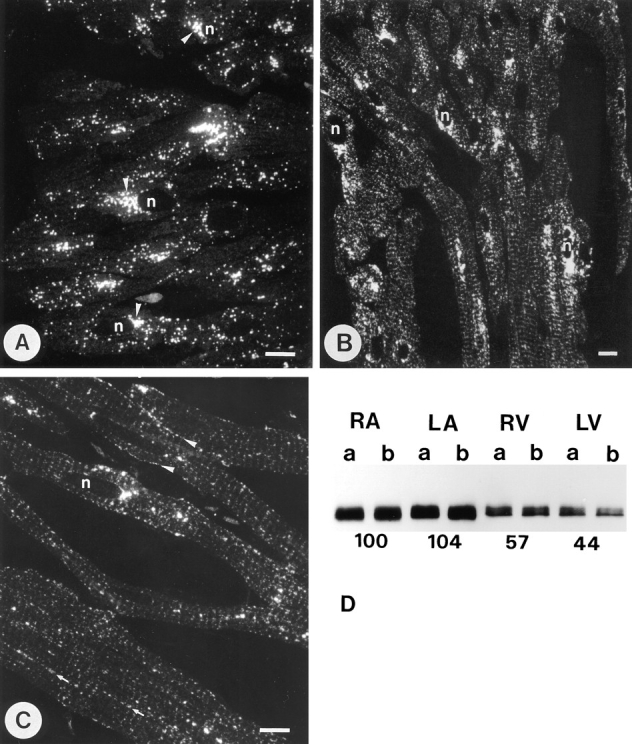
Distribution of ANF and GLUT-4 in cardiac tissues. (A) Fluorescence distribution of ANF in the right atrium. Most of the labeling was observed in small punctate dots that seemed to accumulate in a juxta-nuclear (n) position (arrowheads). (B) Distribution of GLUT-4 in right atrium. As well as positive dots that resemble the ANF labeling pattern, GLUT-4 reactivity was also found in a fine granular pattern that displays a periodicity in places where the cells are cut longitudinally. In the GLUT-4 staining pattern of the left ventricle (C), the fine granular staining is more predominant and clearly follows the sarcomeric periodicity. At some places it seemed to follow the plasma membrane (arrrowheads) or other longitudinal lines (arrows). Concentrations of GLUT-4 were occasionally observed near the nucleus (n) but these were less significant than in the atrium. Bars, 10 μm. (D) Immunoblots of GLUT-4 from tissue samples of right atrium (RA), left atrium (LA), right ventricle (RV), and left ventricle (LV). The results are from two experiments (a and b) in each of which equivalent samples from three rats were pooled. The optical density of the GLUT-4 reaction, underneath the bands of each tissue, is given as the average of the two experiments in percentages of the value measured for the right atrium.
Expression of GLUT-4 in Heart
The immunofluorescence signal for GLUT-4 was consistently stronger in atrium than in ventricle. This was confirmed by immunoblotting (Fig. 1 D), which indicated a higher expression of GLUT-4 in atrial tissue. When expressed per unit of protein, the immunoreactivity for GLUT-4 was approximately two times higher in the left and right atrium than in either ventricle.
Immuno-EM of ANF
The ANF localization observed in the present study was similar to that described previously (Thibault et al., 1989; Cantin et al., 1990). Most of the labeling occurred in secretory granules (Fig. 2 A). Forming granules in the Golgi complex were smaller than the secretory granules and were labeled with similar intensity. Besides the forming granules there was also some disperse labeling in the Golgi cisternae. In general, ANF labeling in both of these locations was more exaggerated in tissues from animals fed ad lib. However, labeling of the Golgi complex was rather variable between individual animals and cells. Diffuse labeling was often more obvious in the cisternae at the cis side of the Golgi stack (Fig. 4 E).
Figure 2.
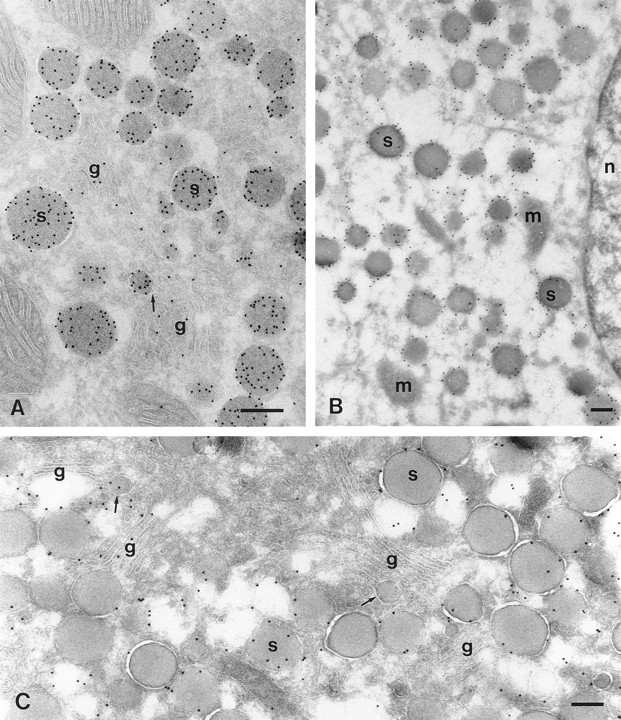
Immuno-EM images of GLUT-4 and ANF labeling in atrial myocytes. (A) Immunogold labeling of a cryosection of the right atrium for ANF (10 nm gold). Most gold is in the spherical secretory granules (s). Similar labeling is also associated with smaller structures (see center part of image), which are presumably forming granules. The profile of one of these (arrow) suggests its connection to the Golgi cisternae (g). Some disperse labeling of the Golgi cisternae is present as well. (B) GLUT-4 labeling of a section taken from right atrial tissue that was not embedded in gelatin (see Materials and Methods). Such sections became overstretched during thawing (Liou and Slot, 1996), which resulted in considerable structural damage, but also a more efficient labeling due to better penetration conditions for the immunoreagents. GLUT-4 labeling is clear around all of the secretory granules. m, mitochondria; n, nucleus. C, as B, but this section is from gelatin-embedded tissue. GLUT-4 labeling (10 nm gold) is clearly associated with the secretory granule membranes. Forming granules (arrows) attached to Golgi cisternae (g) occasionally show GLUT-4 labeling (left), but this was rare (right; see also Fig. 4). The Golgi stacks at the right are negative for GLUT-4, but GLUT-4 is clearly present at the trans side of the stacks at the left. Bars, 200 nm.
Figure 4.
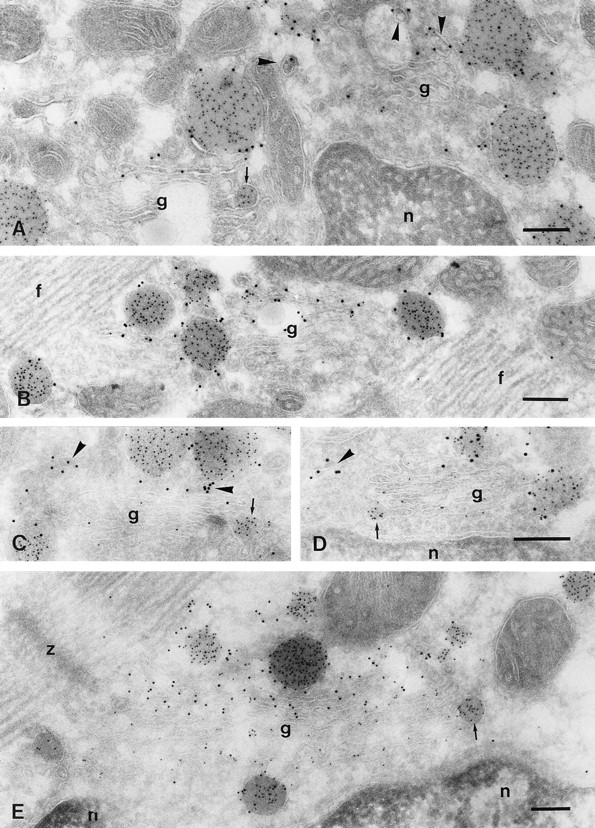
Immuno-EM of ANF and GLUT-4 labeling in the Golgi region of atrial myocytes. Cryosections are double labeled for GLUT-4 (10 nm) and ANF (5 nm). A and B are from overnight-fasted rats, whereas C–E are from rats fed ad lib. Disperse labeling of the Golgi cisternae (g) for ANF is sometimes almost absent (A and B), sometimes low (C and D), and occasionally quite abundant (E). In the latter case, disperse ANF labeling seemed to be present mainly in the cis cisternae of the Golgi. The cis side of the Golgi faces the nucleus (n). GLUT-4 labeling is present in trans-Golgi elements (arrowheads in A, C, and D). In B and more clearly in E, one or two cisternae at the trans side of the Golgi stack are labeled for GLUT-4. Secretory granules show solid ANF and peripheral GLUT-4 labeling, but ANF-positive forming granules (arrows), which are attached to the first (D), second (E), and third (A and C) cisternae from the cis side, are GLUT-4 negative. f, myofibrils; z, Z-line. Bars, 200 nm.
Immuno-EM of GLUT-4
A significant proportion of the total GLUT-4 labeling (50– 60%) was found in ANF secretory granules (Table I) in regular sections. In overstretched sections, where GLUT-4 labeling was enhanced (Fig. 2 B), virtually all secretory granules were labeled for GLUT-4, whether they were in the Golgi area or toward the cell periphery. The secretory granules of the atrial myocyte are unusual in that their membrane is often coated (Jamieson and Palade, 1964; Newman and Severs, 1992). In the cryosections these coats appeared as fuzzy layers, which were most clear in the overstretched sections of tissue fixed without glutaraldehyde (Fig. 3). The coats were present on granules in the perinuclear (Fig. 3 A) as well as in the peripheral (Fig. 3 B) regions of the cell. These coats were labeled with antibodies specific for both clathrin (data not shown) and for AP1, the gamma adaptor protein (Fig. 3 C). The density of GLUT-4 labeling was similar within coated and noncoated regions of the granules (Fig. 3, A and B). Apart from the granule labeling, the distribution of GLUT-4 within the atrial myocyte was similar to that reported previously in ventricular myocytes (Slot et al., 1991b ). Small GLUT-4– positive T-V elements were scattered throughout the cells, often associated with the Z-line zones. These probably cause the cross-striated pattern in the fluorescence observations (Fig. 1, B and C). These structures, with which ∼30% of the GLUT-4 labeling was associated (Table I), were morphologically indistinguishable from the GLUT4–containing T-V elements in the ventricle myocytes (Slot et al., 1991b). Labeling of similar vesicles and tubules was often observed at the trans side of the Golgi complex in the atrial cell (Fig. 4, A, C, and D). These we considered as part of the TGR.
Table I.
Relative Distribution of GLUT-4 Gold Particles in Myocytes of the Right Atrium of Overnight-fasted Rats
| Region | Gold labeling | |
|---|---|---|
| Plasma membrane | <1 | |
| Golgi + TGR | 13.8 ± 1.7 | |
| T-V elements | 31.0 ± 0.3 | |
| ANF granules | 54.9 ± 1.7 |
The plasma membrane includes only the cell surface membrane. Transverse tubules, where detectable, were usually unlabeled, indicating that the proportion of gold associated with these structures was negligible. TGR and Golgi cisternae have been combined because they could not always be distinguished. Each value represents the mean ± SEM of three separate experiments. For each experiment at least 500 gold particles were quantified. The labeling over the nucleus, myofilaments, mitochondria, and cytoplasm was very low (<0.3 gold/μm) and considered nonspecific. Thus, this has not been included in these analyses.
Figure 3.

Clathrin coats on ANF granules. Sections of atrial tissue fixed without glutaraldehyde and not embedded in gelatin. (A and B) Sections treated as in Fig. 2 B. Coated parts can be seen on granules (arrows), in A, in the cell center (g, Golgi complex) and, in B, near the cell surface (ex, extracellular space). GLUT-4 labeling (10 nm gold) is similar in coated segments and other parts of the granule surface. (C) Section labeled for the TGR-type adaptor protein, AP1. Coats on ANF granules are labeled (10 nm gold) for AP1 (arrows). n, nucleus. Bars, 200 nm.
The structure of the Golgi complex in the rat atrial cell has been studied in detail by Rambourg and colleagues (1984). They described the entire structure as a continuum of plate-shaped stacks of cisternae that are interconnected by tubular regions in such a way that they form a beltlike structure around the nucleus. This belt is complicated by the formation of loops at both nuclear poles. A characteristic stack comprises five cisternae. Each cisterna has specific morphological and cytochemical features. In the perinuclear stacks the first or cis-most cisternae always faces the nucleus, which facilitates the recognition of the cis– trans orientation, making the use of specific markers for either side unnecessary. The fifth cisterna, which is often partly detached from the stack, probably represents part of the TGR as defined by Griffiths and Simons (1986). Rambourg and colleagues also focused on the biogenesis of ANF-secretory granules in the atrial myocyte. They found forming granules attached to all, except the cis-most, Golgi cisternae. The attachment is preferentially via tubular extensions, which explains why such connections are rarely seen in thin sections (Fig. 2 A). We found GLUT-4 labeling in the TGR (Fig. 4, A, C, and D) and sometimes in the trans-most cisternae of the Golgi stack (Fig. 4, B and E) but not in the medial and cis cisternae. Forming secretory granules in the trans-Golgi were often labeled for GLUT-4 (Fig. 2 C), but this was not the case for ANF-positive granules that were connected to the medial or cis cisternae of the Golgi stack (Fig. 4, A, C, D, and E). In nonstimulated cells there was no detectable (<1%) GLUT-4 labeling at the cell surface (Table I). The internal plasma membrane of the transverse tubules was excluded from the counting since these structures could not always be recognized in the sections. However, distinct transverse tubule profiles were usually not labeled for GLUT-4.
Effects of Cyclohexamide Treatment
To determine if GLUT-4 within the trans-Golgi originated from the biosynthetic pathway or the recycling pathway, we performed experiments using the protein synthesis inhibitor cyclohexamide. To test the efficacy of the cyclohexamide treatment we first examined its effects on albumin labeling in liver. After 1 h of cyclohexamide treatment there was a substantial reduction in liver albumin labeling (compare Fig. 5, A and B). Longer periods of cyclohexamide treatment (2 h) did not further change this situation. Similarly, in atrial cardiomyocytes from the same animals cyclohexamide treatment completely abolished the presence of newly forming granules in the Golgi cisternae. Also the disperse labeling for ANF in the cisternae that we observed in control cells was virtually absent (Fig. 5 C). These data indicate that the cyclohexamide treatment effectively blocked protein synthesis in the atrial myocytes. Despite this, GLUT-4 labeling at the trans side of the Golgi was not affected by cyclohexamide treatment, indicating that GLUT-4 at this location is not derived from the biosynthetic pathway.
Figure 5.
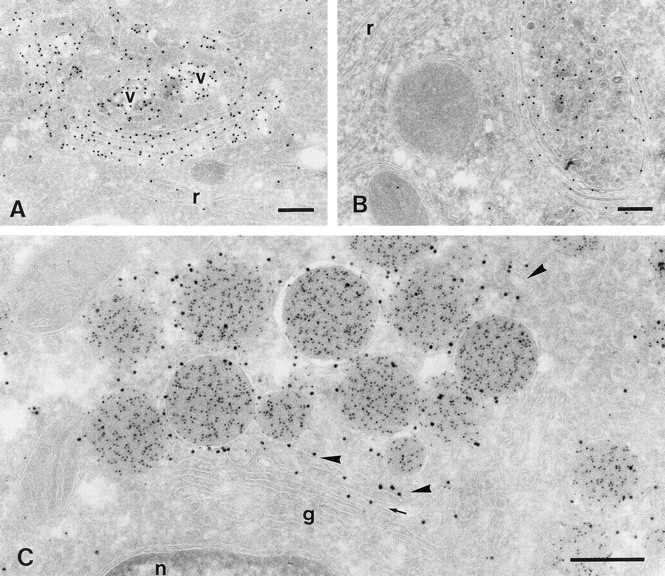
Effects of cyclohexamide on ANF and GLUT-4 labeling. Cryosections are from rats fed ad lib. (A and B) Liver sections immunolabeled for rat serum albumin (10 nm gold). (C) Right atrium, double labeled for GLUT-4 (10 nm gold) and ANF (5 nm gold). A is from a control rat; B and C are from a rat treated for 1 h with cyclohexamide. In normal rat liver, albumin marks the biosynthetic secretory route, with diffuse labeling in the RER (r) and dense labeling in Golgi cisternae and secretory vesicles (v). After cyclohexamide treatment albumin is much less present in these compartments and secretory vesicles are not seen. In atrial myocytes (C) of the same rat as in B, Golgi cisternae (g) are devoid of diffuse ANF labeling and ANF-positive forming granules are rarely seen. GLUT-4 labeling is still associated with elements at the trans side of the Golgi stack (arrowheads) and with the trans-most cisterna (arrow) like in control animals (Fig. 4). n, nucleus. Bars, 200 nm.
Insulin Stimulation
We have previously shown that insulin stimulates the movement of GLUT-4 from the T-V elements and TGR to the cell surface in other insulin-sensitive cells (Slot at al., 1991a; James et al., 1994). In the present study it was of considerable interest to examine the effects of insulin on exocytosis of the GLUT-4/ANF-containing granules in atrial myocytes. Insulin caused a significant increase in GLUT-4 labeling all along the plasma membrane in atrial cardiomyocytes. However, this effect was less than that observed in ventricular cells. In five measurements from longitudinally cut cells from left ventricle and right atrium of the same animals, we observed 0.96 ± 0.03 and 0.41 ± 0.06 gold particles per micrometer of plasma membrane, respectively. In basal myocytes of ventricle as well as atrium, GLUT-4 labeling of the plasma membrane was at undetectable low level (Table I).
To further confirm the response to insulin in atrium we next examined the effect of insulin on 2-deoxyglucose (2DG) uptake in different heart regions using the hyperinsulinemic euglycemic clamp technique in whole animals (Table II). This technique enables measurement of 2DG uptake, an estimate of glucose transport, in individual tissues of the rat in vivo after the infusion of insulin and variable amounts of glucose to prevent the onset of hypoglycemia (Kraegen et al., 1985). The basal 2DG uptake rate was very low, consistent with the predominant intracellular distribution of GLUT-4 observed by EM under these conditions (Table I; Slot et al., 1991b ). In addition, there was no significant difference in 2DG uptake among the various heart regions in basal animals. Insulin caused a substantial increase in 2DG uptake in both atrium and ventricle. However, quantitatively the effect was significantly lower in the atrium than in the ventricle, once again consistent with the EM observations. Together with the observation that GLUT-4 expression is twofold higher in atrial tissue (Fig. 1 D), this suggests that insulin-induced translocation of the transporter is less efficient there (we estimate five to eight times) than in the ventricular myocyte. Indeed, initial immunocytochemical observations indicated that the GLUT-4 labeling that could be measured at the cell surface after insulin stimulation represented no more than 2–4% of the total cellular labeling (data not shown). This seems rather low when compared with the ∼25% surface labeling that we found previously in stimulated ventricular myocytes, but that was in response to a maximal stimulation involving both insulin and exercise. Taking that into account together with the five to eight times lower response of the atrial cell to insulin, one cannot expect more than a few percent GLUT-4 labeling at the cell surface after insulin treatment. Such minor changes were too inconspicuous for establishing immunocytochemically to what extent each of the intracellular pools (T-V elements; TGR; ANF granules) contributed to the translocation of GLUT-4. For that reason we did not pursue such studies in insulinstimulated atrium.
Table II.
Influence of Insulin on [3H]-2-Deoxyglucose Uptake in Heart Regions of 24-h–fasted Rats
| Glucose metabolic index (Rg') | ||||
|---|---|---|---|---|
| Basal | Insulin | |||
| μmol/min/100 g | ||||
| Right atrium | 1.5 ± 0.1 | 33 ± 5 | ||
| Left atrium | 2.4 ± 0.5 | 46 ± 10 | ||
| Right ventricle | 2.2 ± 0.3 | 88 ± 7 | ||
| Left ventricle | 2.7 ± 0.3 | 133 ± 8 | ||
Animals with indwelling catheters in the jugular vein and carotid artery were fasted for 24 h. Animals were either infused with saline (basal) or insulin and a variable rate glucose infusion to maintain euglysemia for 75 min. A bolus of [3H]-2-deoxyglucose was then administered intravenously, and after 45 min tissues were removed and radiolabeled. 2-Deoxyglucose was quantified by scintillation counting and expressed as a glucose metabolic index (Rg') as described previously (Kraegen et al., 1995). Values represent mean ± SEM of n = 8 (basal) and n = 3 (insulin).
We also explored if insulin treatment had an effect on ANF secretion which could imply a certain participation of the secretory granule-associated GLUT-4 pool in insulin-dependent GLUT-4 translocation. However, we could not detect a significant effect of insulin on ANF secretion in the rat. There was no substantial difference in the amount of the prohormone detected by immunoblotting in homogenates of atrial tissue, obtained from nonstimulated and insulin-treated (30 min) animals (data not shown). Furthermore, the serum concentration of NH2-terminal ANF, which reflects the atrial secretory fraction (Itoh et al., 1988), was measured. Severalfold raises of serum concentration of ANF have been reported after appropriate stimuli of ANF release (Horky et al., 1985; Manning et al., 1985; Lang et al., 1985), which are mostly related to atrial stretch the major stimulator of ANF secretion (Ruskoaho, 1992). No detectable changes in serum levels of ANF could be detected after insulin stimulation (Table III).
Table III.
Effect of Insulin on ANF Secretion
| Time after insulin | Serum ANF | |
|---|---|---|
| min | ||
| −10 | 100 | |
| 10 | 93.5 ± 13.5 | |
| 40 | 101.8 ± 23.2 | |
| 70 | 94.3 ± 4.2 |
Animals with indwelling catheters in the jugular vein and carotid artery were fasted for 24 h. Animals were infused with insulin and a variable rate glucose infusion to maintain euglycemia. Blood samples were removed 10 min before commencement of the insulin infusion and at 10, 40, and 70 min during the infusion. Values represent mean ± SEM (n = 4). All values were expressed as a percentage of the preinsulin value.
Discussion
In muscle and adipose tissue, GLUT-4 is localized to an intracellular compartment comprising small tubules and vesicles clustered in the vicinity of the endosomal/TGR system (Slot et al., 1991a ,b; Rodnick et al., 1992; James et al., 1994). Despite partial overlap with recycling endosomes and the TGR (Hanpeter and James, 1995; Livingstone et al., 1996; Martin et al., 1996) a large proportion of intracellular GLUT-4 appears to be segregated into a population of vesicles that contain the neuronal v-SNARE, VAMP-2, and an amino peptidase, vp165 (Cain et al., 1992; Kandror and Pilch, 1994; Martin et al., 1996; Mastick et al., 1994). This compartment may represent a specialized intracellular storage depot possibly analogous to small synaptic vesicles (Herman et al., 1994; Martin et al., 1996; Verhey et al., 1995). How is this storage compartment formed and what is its relationship to other regulated secretory compartments? By examining the location of GLUT-4 in an insulinregulated cell type that also contains a bona fide regulated secretory system we hoped to address these questions. Using immunoelectron microscopy to localize GLUT-4 in atrial cardiac myocytes we have found that a substantial proportion (50-60%) of the total GLUT-4 found in these cells is targeted to ANF-containing secretory granules with the remainder localized to the TGR and T-V elements, similar to those identified in other insulin-sensitive cells (Slot et al., 1991a ,b).
Regulated secretory granules of the type found in atrial myocytes are synthesized along the biosynthetic route of the secretory pathway. In the atrial cell secretory granules at various stages of maturation are readily detected throughout the Golgi apparatus, in the TGR and scattered throughout the cytoplasm. GLUT-4 may enter this organelle either during the course of its own biosynthesis or via recycling, presumably through the trans-Golgi elements. The earliest point along the secretory pathway where we could detect ANF labeling was in the cis and medial Golgi cisternae. At this point ANF labeling is usually dispersed or just starting to aggregate (Fig. 4), presumably representing the early stages of granule formation, as suggested previously (Jamieson and Palade, 1964). The membranes around these forming granules were not labeled for GLUT-4, but they became GLUT-4 positive at the trans side of the Golgi stacks and in the TGR. To determine if this was newly synthesized GLUT-4 accumulating in the TGR we examined the effects of protein synthesis inhibitors on GLUT-4 targeting. Cyclohexamide caused a pronounced inhibition of protein synthesis in atrial myocytes as determined by the complete disappearance of ANF labeling throughout the Golgi apparatus. Despite this, GLUT-4 levels in the TGR remained unaffected by this treatment. It is possible that newly synthesized GLUT-4 is actively retained in the TGR for long periods. However, several observations indicate that TGR-derived GLUT-4 stems from the recycling pathway rather than the biosynthetic pathway: (a) In response to acute insulin treatment in adipocytes and cardiac myocytes (Slot et al., 1991a ,b) there is a significant decline in GLUT-4 levels in the TGR, suggesting that GLUT-4 in the TGR readily exchanges with the cell surface and presumably endosomes. (b) After expression of GLUT-4 in fibroblasts using a viral expression system we have detected GLUT-4 labeling throughout the biosynthetic pathway. In response to cyclohexamide treatment for 45 min, GLUT-4 labeling in the ER and Golgi cisternae of these cells was completely depleted whereas the level of GLUT-4 in the TGR remained constant (Piper et al., 1992). (c) Previous studies have shown that cyclohexamide does not alter the localization of other recycling proteins, such as the asialoglycoprotein receptor and the mannose 6-phosphate receptor (MPR), in the TGR (Geuze et al., 1984) consistent with the data reported here for GLUT-4. Collectively, these findings indicate that GLUT-4 traffics through the TGR and this explains its presence in this organelle in all of these cell types. Thus, it is most likely that GLUT-4 enters the ANF granules via this recycling pathway rather than the biosynthetic route.
The recycling of membrane proteins through the TGR is quite specific as most endosomal proteins appear to avoid this route. Using resialylation as an index of sorting through the TGR, Duncan and Kornfeld (1988) showed that both MPRs are resialylated with a t 1/2 of ∼3 h whereas other cell surface glycoproteins acquire sialic acid at a 10-fold slower rate. The most likely explanation for this is that a small subset of surface glycoproteins cycle through the TGR at a similar rate to the MPR while the remainder do not cycle through this compartment at all. This being the case, the fact that we observe such a large proportion of the total GLUT-4 complement within secretory granules in myocytes implies that the majority of GLUT-4 probably recycles via the TGR in these cells. Further studies using approaches similar to that used for the MPRs will be required to test this hypothesis and to determine if a similar trafficking pathway is used by GLUT-4 in other insulin-sensitive cell types.
The recycling of GLUT-4 via the TGR may be central to the unique character of this protein. GLUT-4 has been shown to have a much slower exocytic rate than other recycling proteins such as the transferrin receptor (for review see James et al., 1994) and this may be in part because it follows a completely distinct trafficking pathway through the TGR. Immuno-EM studies taught us that GLUT-4 is concentrated in the TGR in all insulin-sensitive cells (Slot et al., 1991 a,b; James et al., 1994) consistent with it possibly being retained in this organelle. Also it is conceivable that the intracellular GLUT-4 storage depot (Herman et al., 1994; Martin et al., 1996; Verhey et al., 1995) may form at the level of the TGR rather than endosomes. This may explain the significant colocalization between GLUT-4 and MPR in insulin-sensitive cell types (Hanpeter and James, 1995; Martin et al., 1996). In addition, we have recently shown that there is significant colocalization between AP1, the Golgi-specific adaptor protein, and GLUT-4 in vesicles isolated from adipocytes (Martin, S., and D. James, unpublished data). Furthermore, insulin stimulates the efflux of a number of TGR recycling proteins as well as constitutive secretory proteins such as adipsin (for review see Lienhard, 1989). It has been reported that the TGR-specific protein TGN38 does not colocalize with GLUT-4 in adipocytes (Martin et al., 1994). This may reflect the heterogeneous composition of the TGR as shown recently (Glickman et al., 1996). In our present study we could not confirm this, being unsuccessful in detecting TGN38 in the sections of the atrial cells.
Despite the targeting of GLUT-4 to the ANF-containing secretory granules there was also a significant amount in T-V elements that were morphologically indistinguishable from those found in other cell types. Hence, it is likely that if both organelles bud from the TGR they do so via different sorting mechanisms. The presence of GLUT-4 in ANF granules does not appear to reflect a default pathway in these cells because vp165, which colocalizes with GLUT-4 in adipocytes, is not targeted to the ANF granules in the atrial cells, but colocalizes there with GLUT-4 in the T-V elements (Martin et al., 1997). Furthermore, it has been reported that GLUT-4 but not GLUT-1 is targeted to secretory granules in PC12 cells (Hudson et al., 1993). Thus, this implies the presence of a specific sorting signal in GLUT-4 that targets it for entry into this organelle.
The targeting of GLUT-4 into regulated secretory granules appears to be cell specific. In PC12 cells or insulinoma cells transfected with the GLUT-4 cDNA, relatively low levels (Hudson et al., 1993), or no GLUT-4 (Herman et al., 1994; Thorens and Roth, 1996), were detected in the secretory compartment. In renal arteriolar cells of the juxta glomerular apparatus, where GLUT-4 is also expressed endogenously, there is very little GLUT-4 labeling of the renin-containing secretory granules, most being found in T-V elements (Anderson, T.J., S. Martin, D.E. James, J.W. Slot, J.L. Berka, and J. Stow, manuscript submitted for publication). However, similar differences have been reported for other secretory proteins. For instance, in the case of P-selectin, which is very efficiently targeted to α-granules in platelets (Stenberg et al., 1985), only a small proportion is found in secretory granules in AtT-20 cells (Koedam et al., 1992). Such differences likely reflect differences between individual cell types in GLUT-4 expression, the rate of granule formation and their average lifetime before secretion, or the rate of GLUT-4 TGR recycling. The atrial cell is in some respects an atypical secretory cell because granule formation begins at a very early stage of the biosynthetic pathway (Fig. 4, and Rambourg et al., 1984) and there is not much indication of further maturation of the granule after its exit from the TGR. For example, many ANF granules appear to retain the AP1-specific coat in the atrial myocyte (Fig. 3 C) until secretion (Newman and Severs, 1992). This is quite different from other endocrine cells where granules begin to form in the TGR and continue to mature after budding from the TGR (Arvan and Castle, 1992; Rindler, 1992; Tooze, 1991), during which process they tend to lose clathrin coats (Dittié et al., 1996). Thus, it is possible that GLUT-4 is efficiently targeted to the regulated secretory pathway in all cases but is transported out of the secretory granules during their postTGR maturation to different extent in individual cell types. Therefore, the proportion of GLUT-4 that is found in the secretory granule compartment in a particular cell will reflect a balance of each of these parameters which in all likelihood vary considerably from one cell to another.
Does insulin stimulate the movement of GLUT-4 from ANF-containing secretory granules to the cell surface in atrial myocytes? Answers to this question may provide insight into the physiological basis for the targeting of GLUT-4 to this organelle, which at present remains unknown. In theory GLUT-4 could move from the granules to the cell surface in two ways. First, as the granules fuse with the cell surface during secretion the granule membrane protein cargo presumably inserts into the surface membrane. However, as described above we have been unable to detect an effect of insulin on ANF secretion and therefore regulation of this route by insulin seems unlikely. The second possibility is that small GLUT-4–containing vesicles may bud from the ANF granules. This would probably result in GLUT-4 translocation without substantial amounts of ANF secretion. Such budding via coated vesicles commonly occurs from immature secretory granules in other endocrine cell types. The presence of AP1-positive clathrin coats on the ANF granules, with some decreasing frequency during their lifetime (Newman and Severs, 1992), indicates that there may be a route for GLUT-4 out of the granules by budding. It remains to be seen if this is regulated by insulin. The insulin effects on GLUT-4 distribution were too small to measure shifts of GLUT-4 labeling either from the ANF granules or from other cellular pools (T-V elements; TGR). Therefore we cannot yet determine the precise intracellular origin of the GLUT-4 that is translocated to the cell surface by insulin in this cell. However, the involvement of the ANF granules via these budding vesicles would place them functionally in line with the TGR, as is suggested for the regulated secretory compartment at an immature stage in other cell types (Arvan and Castle, 1992).
Acknowledgments
We thank Jenny Stow, Rob Parton, Hans Geuze, and Peter Peters for helpful discussions and for reading the manuscript, and Russel Wilson for his contribution to the practical part of this study.
This work was supported by grants from the National Health and Medical Research Council of Australia and the Juvenile Diabetes Foundation International. D.E. James is a Wellcome Trust Professorial Research Fellow.
Footnotes
1. Abbreviations used in this paper: ANF, atrial natriuretic factor; LM, light microscopy; MPR, mannose 6-phosphate receptor; 2DG, 2-deoxyglucose; TGR, trans-Golgi reticulum; T-V, tubulo-vesicular.
Please address all correspondence to Jan W. Slot, Department of Cell Biology, Medical School, Utrecht University, AZU Room H02.314, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. Tel.: (31) 30-2507654. Fax: (31) 30-2541797.
References
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993;268:19161–19164. [PubMed] [Google Scholar]
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transportercontaining vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- Cantin M, Thibault G, Haile H, Meskel, Ballak M, Garcia R, Jasmin G, Genet J. Immuno-electron microscopy of atrial natriuretic factor secretory pathways in atria and ventricles of control and cardiomyopathic hamsters with heart failure. Cell Tissue Res. 1990;261:313–322. doi: 10.1007/BF00318672. [DOI] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell: apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- De Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science (Wash DC) 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- Dittié AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJ, Luzio JP, Schwartz AL. A cyclohexamide-resistant pool of receptors for asialoglycoproteins and mannose 6-phosphate residues in the Golgi complex of hepatocytes. EMBO (Eur Mol Biol Organ) J. 1984;3:2677–2685. doi: 10.1002/j.1460-2075.1984.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JM, Morton PA, Slot JW, Kornfeld S, Geuze HJ. The biogenesis of the MHC class II compartment in human I-cell disease B lymphocytes. J Cell Biol. 1996;132:769–785. doi: 10.1083/jcb.132.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science (Wash DC) 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hanpeter D, James DE. Characterisation of the intracellular GLUT-4 compartment. Mol Membr Biol. 1995;12:263–269. doi: 10.3109/09687689509072426. [DOI] [PubMed] [Google Scholar]
- Herman GA, Bonzelius F, Cieutat A-M, Kelly RB. A distinct class of intracellular storage vesicles, identified by expression of the glucose transporter GLUT-4. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky K, Gutkowska J, Garcia J, Thibault G, Genest J, Cantin M. Effect of different anesthetics on immunoreactive atrial natriuretic factor concentrations in rat plasma. Biochem Biophys Res Commun. 1985;129:651–657. doi: 10.1016/0006-291x(85)91941-2. [DOI] [PubMed] [Google Scholar]
- Hudson AW, Fingar DC, Seidner GA, Griffiths G, Burke B, Birnbaum MJ. Targeting of the insulin-responsive glucose transporter (GLUT-4) to the regulated secretory pathway in PC12 cells. J Cell Biol. 1993;122:579–588. doi: 10.1083/jcb.122.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Nakao K, Sugawara A, Saito Y, Mukoyama M, Morii N, Yamada T, Shiono S, Arai H, Hosoda K, Imura H. γ-atrial natriuretic polypeptide (γANP)-derived peptides in human plasma: cosecretion of N-terminal γANP fragment and aANP. J Clin Endocrinol Metab. 1988;67:429–437. doi: 10.1210/jcem-67-3-429. [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC. Insulin resistance, diabetes, and the insulinregulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Strube M, Mueckler M. Molecular cloning and characterisation of an insulin regulatable glucose transporter. Nature (Lond) 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC, Slot JW. Insulin stimulation of GLUT-4 translocation: a model for regulated recycling. Trends Cell Biol. 1994;4:120–126. doi: 10.1016/0962-8924(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Jamieson JD, Palade GE. Specific granules in atrial muscle cells. J Cell Biol. 1964;25:151–172. doi: 10.1083/jcb.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulinactivated glucose transport. Proc Natl Acad Sci USA. 1994;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam JA, Cramer EM, Briend E, Furie B, Furie BC, Wagner DD. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose- response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248:E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- Lang RE, Tholken H, Ganten D, Luft FC, Ruskoaho H, Unger T. Atrial natriuretic factor-a circulating hormone stimulated by volume loading. Nature (Lond) 1985;314:264–266. doi: 10.1038/314264a0. [DOI] [PubMed] [Google Scholar]
- Lienhard, G. 1989. Insulin may cause translocation of proteins to the cell surface by stimulating membrane trafficking from the trans Golgi reticulum. In Genes and Gene Products in the Development of Diabetes Mellitus. J. Nerup, T. Mandrup-Poulson, and B. Hokfelt, editors. Elsevier Science Publishing Co., Inc., New York. 313–328.
- Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT-4) in 3T3-L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PT, Schwartz D, Katsube NC, Holmberg SW, Needleman P. Vasopressin-stimulated release of atriopetin: endocrine antagonists in fluid homeostasis. Science (Wash DC) 1985;229:395–397. doi: 10.1126/science.2990050. [DOI] [PubMed] [Google Scholar]
- Martin S, Reaves B, Banting G, Gould GW. Analysis of the co- localisation of the insulin-responsive glucose transporter (GLUT-4) and the trans Golgi network marker TGN38 within 3T3-L1 adipocytes. Biochem J. 1994;300:743–749. doi: 10.1042/bj3000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulinsensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., J.E. Rice, G.W. Gould, S.R. Keller, J.W. Slot, and D.E. James. 1997. The glucose transporter GLUT4 and the aminopeptide vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J. Cell Sci. In press. [DOI] [PubMed]
- Mastick CC, Aebersold R, Lienhard GE. Characterisation of a major protein in GLUT-4 vesicles. J Biol Chem. 1994;269:6089–6092. [PubMed] [Google Scholar]
- Newman TM, Severs NJ. Coated vesicles are implicated in the post-fusion retrieval of the membrane of rat atrial secretory granules. Cell Tissue Res. 1992;268:463–469. doi: 10.1007/BF00319153. [DOI] [PubMed] [Google Scholar]
- Piper RC, Tai C, Slot JW, Hahn CS, Rice CM, Huang H, James DE. The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2terminus. J Cell Biol. 1992;117:729–743. doi: 10.1083/jcb.117.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A, Segretain D, Clermont Y. Tridimensional architecture of the Golgi apparatus in the atrial muscle cell of the rat. Am J Anat. 1984;170:163–179. doi: 10.1002/aja.1001700204. [DOI] [PubMed] [Google Scholar]
- Rindler MJ. Biogenesis of storage granules and vesicles. Curr Opin Cell Biol. 1992;4:616–622. doi: 10.1016/0955-0674(92)90080-v. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT-4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP, insulin and GTPγS and localization of GLUT-4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick KJ, Slot JW, Studelska DR, Hanpeter DE, Robinson LJ, Geuze HJ, James DE. Immunocytochemical and biochemical studies of GLUT-4 in rat skeletal muscle. J Biol Chem. 1992;267:6278–6285. [PubMed] [Google Scholar]
- Ruskoaho H. Atrial natriuretic peptide: synthesis, release and metabolism. Pharmacol Rev. 1992;44:479–602. [PubMed] [Google Scholar]
- Roth J, Bendayan M, Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978;26:1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. A new method of preparing gold probes for multiple labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Weerkamp AH. Localization of macromolecular components by application of the immunogold technique on cryosectioned bacteria. Methods Microbiol. 1988;20:211–236. [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immunolocalization of the insulin-regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991a;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT-4 in cardiac myocytes of the rat. Proc Natl Acad Sci USA. 1991b;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg PE, McEver RP, Schuman MA, Jaques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes the translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault G, Murthy KK, Gutkowska J, Seidah NG, Lazure C, Chretien M, Cantin M. NH2-terminal fragment of rat pro-atrial natriuretic factor in the circulation: identification, radioimmunoassay and half-life. Peptides. 1988;9:47–53. doi: 10.1016/0196-9781(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Thibault G, Haile-Meskel H, Wrobel-Conrad E, Ballak M, Garcia R, Genest J, Cantin M. Processing of the natriuretic factor propeptide by atrial cardiocytes as revealed by immunocryoultramicrotomy. Endocrinology. 1989;124:3109–3116. doi: 10.1210/endo-124-6-3109. [DOI] [PubMed] [Google Scholar]
- Thorens B, Roth J. Intracellular targeting of GLUT-4 in transfected insulinoma cells: evidence for association with constitutively recycling vesicles distinct from synaptophysin and insulin vesicles. J Cell Science. 1996;109:1311–1323. doi: 10.1242/jcs.109.6.1311. [DOI] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules. Implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS (Fed Eur Biochem Soc) Lett. 1991;285:220–224. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Yeh J-I, Birnbaum MJ. Distinct signals in the GLUT-4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J Cell Biol. 1995;130:1071–1079. doi: 10.1083/jcb.130.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]


