Abstract
In polarized Madin-Darby canine kidney (MDCK) cells, the transferrin receptor (TR) is selectively delivered to the basolateral surface, where it internalizes transferrin via clathrin-coated pits and recycles back to the basolateral border. Mutant tailless receptors are sorted randomly in both the biosynthetic and endocytic pathways, indicating that the basolateral sorting of TR is dependent upon a signal located within the 61–amino acid cytoplasmic domain. To identify the basolateral sorting signal of TR, we have analyzed a series of mutant human TR expressed in MDCK cells. We find that residues 19–41 are sufficient for basolateral sorting from both the biosynthetic and endocytic pathways and that this is the only region of the TR cytoplasmic tail containing basolateral sorting information. The basolateral sorting signal is distinct from the YTRF internalization signal contained within this region and is not tyrosine based. Detailed functional analyses of the mutant TR indicate that residues 29–35 are the most important for basolateral sorting from the biosynthetic pathway. The structural requirements for basolateral sorting of internalized receptors from the endocytic pathway are not identical. The most striking difference is that alteration of G31DNS34 to YTRF impairs basolateral sorting of newly synthesized receptors from the biosynthetic pathway but not internalized receptors from the endocytic pathway. Also, mutations have been identified that selectively impair basolateral sorting of internalized TRs from the endocytic pathway without affecting basolateral sorting of newly synthesized receptors. These results imply that there are subtle differences in the recognition of the TR basolateral sorting signal by separate sorting machinery located within the biosynthetic and endocytic pathways.
The unique features of polarized epithelial cells rely upon the differentiation of the plasma membrane into structurally and functionally distinct domains (for review see Simons and Fuller, 1985; Nelson, 1992). The apical membrane, which faces the external environment and is the site for most of the specialized functions that characterize this cell type, is separated by tight junctions from the basolateral membrane, which contacts adjacent cells and the underlying tissue and performs common cellular functions. To generate and maintain cell surface polarity, epithelial cells must contain mechanisms that ensure the proper localization of the proteins that are characteristic of each surface.
In Madin-Darby canine kidney (MDCK)1 cells, newly synthesized apical and basolateral membrane proteins are generally thought to be sorted at the TGN into distinct transport vesicles, which then fuse with the appropriate cell surface domain (Wandinger-Ness et al., 1990). Polarized sorting of apical and basolateral membrane proteins in MDCK cells was previously postulated to consist of a signal-dependent pathway to the apical surface that operated in conjunction with a signal-independent pathway to the basolateral surface (Simons and Wandinger-Ness, 1990). Indeed, the first polarized sorting signal to be identified was the glycosyl-phosphatidylinositol anchor, which targets proteins attached to the exoplasmic leaflet of the lipid bilayer to the apical surface (Brown et al., 1989). However, no sorting signal that mediates apical delivery of an integral membrane protein has been identified.
In contrast, studies have established that basolateral sorting of most membrane proteins requires signals located within their cytoplasmic domains, demonstrating that basolateral transport is a signal-dependent process (Brewer and Roth, 1991; Casanova et al., 1991; Hunziker et al., 1991). The localization of distinct cytoplasmic determinants for basolateral sorting suggests that these signals are recognized by cytosolic proteins analogous to the adaptor complexes involved in receptor-mediated endocytosis. In fact, several basolateral sorting signals have been identified that are colinear with internalization signals located within the cytoplasmic domains of these proteins (Brewer and Roth, 1991; Hunziker et al., 1991; Matter et al., 1992; Geffen et al., 1993; Prill et al., 1993; Monlauzeur et al., 1995). In most cases, these basolateral sorting signals are dependent on a common tyrosine residue that is also important for endocytosis. One exception is the Fc receptor, which requires a di-leucine sequence for both basolateral sorting and rapid internalization (Hunziker and Fumey, 1994; Matter et al., 1994). However, in some cases, the structural requirements for basolateral sorting and internalization can be distinguished (Matter et al., 1992; Prill et al., 1993), indicating that the sorting machinery that recognizes this class of basolateral sorting signal is distinct from the sorting machinery in plasma membrane clathrincoated pits.
So far, three basolateral sorting signals have been identified that do not overlap with endocytic motifs. Both the membrane-distal basolateral sorting signal of the low-density lipoprotein receptor (LDLR) and the basolateral sorting signal of vesicular stomatitis virus glycoprotein G are tyrosine dependent, yet neither signal promotes rapid internalization (Matter et al., 1992; Thomas et al., 1993). However, aside from their common requirement for tyrosine, the structural relationship between these two basolateral sorting signals is not clear. The other basolateral sorting signal that does not overlap with an internalization signal is located within the 17-residue juxtamembrane region of the polymeric immunoglobulin receptor (pIgR) cytoplasmic tail (Casanova et al., 1991). Three residues within this region (His-656, Arg-657, and Val-660) were found to be relatively important for sorting newly synthesized pIgR to the basolateral surface (Aroeti et al., 1993). In addition, two-dimensional nuclear magnetic resonance (NMR) analysis of synthetic peptides corresponding to this 17-residue region of the pIgR suggested that Val-660 is part of a β-turn secondary structure (Aroeti et al., 1993).
Recent studies suggest that mutations that impair the polarized basolateral delivery of newly synthesized LDLR and pIgR also lead to an increase in basolateral-to-apical transcytosis of these proteins, leading to the proposal that the same basolateral sorting signal in each protein is recognized by separate sorting machinery located in the TGN and in endosomes (Matter et al., 1993; Aroeti and Mostov, 1994). However, a feasible alternative possibility is that newly synthesized membrane proteins en route to the cell surface are delivered first to endosomes and that a single set of basolateral sorting machinery within this compartment is responsible for polarized sorting of both newly synthesized and recycling basolateral membrane proteins (Aroeti and Mostov, 1994). In nonpolarized cells, evidence for an indirect pathway to the cell surface via endosomes for newly synthesized membrane proteins has been obtained from studies of the transferrin receptor (TR) and asialoglycoprotein receptor (Futter et al., 1995; Leitinger, 1995).
We have recently demonstrated that a signal-dependent sorting mechanism operates in the endocytic pathway of MDCK cells that selectively delivers TR to the basolateral border regardless of the surface from which it is internalized (Odorizzi et al., 1996). TR internalized from either the apical or the basolateral surface enter an extensively interconnected endosome compartment and are then sorted into 60-nm-diam recycling vesicles that deliver them to the basolateral surface (Odorizzi et al., 1996). Thus, for membrane proteins, such as TR, which are repeatedly internalized, basolateral sorting in the endocytic pathway is the dominant mechanism that maintains polarity.
To identify the basolateral sorting signal in the cytoplasmic domain of TR, we have examined the trafficking of a series of mutant human TR constructs expressed in MDCK cells. We find that residues 19–41 in the TR cytoplasmic tail are sufficient for the polarized basolateral delivery of newly synthesized receptors and the polarized basolateral recycling of internalized TR. The four-residue tyrosinebased internalization signal of TR, Y20TRF23, is located within this region (Collawn et al., 1990), but the basolateral sorting signal is distinct from the internalization signal and is not tyrosine based. Analysis of additional mutant TR indicates that residues 29–35 are the most important for selective delivery of newly synthesized receptors to the basolateral surface. However, the structural requirements for basolateral sorting of newly synthesized and internalized TR are not identical. This finding indicates that there must be significant differences in how TR are sorted in the biosynthetic and endocytic pathways. Finally, our functional analyses suggest that the structural properties of basolateral sorting signals may be sufficiently disparate from tyrosine-based internalization signals that the extent to which data about one type of signal can be extrapolated to the other is limited.
Materials and Methods
Construction of Mutant Human TR
Mutant human TR (Δ3–18, Δ42–59; Δ19–41; Δ3–18, Δ36–59; 29–41 transplant; V29 → A, Δ3–18, Δ42–59; and V36 → A, Δ3–18, Δ42–59) were constructed by oligonucleotide site-directed mutagenesis by the method of Kunkel (1985) using a pBluescript SK phagemid template (Stratagene, La Jolla, CA) containing a wild-type human TR insert (Jing et al., 1990). Mutants were identified by restriction mapping, and ClaI fragments encoding mutant receptors were cloned into RCAS-BP(A), a retroviral expression vector derived from Rous sarcoma virus subtype A (RSV[A]) (Hughes et al., 1990; Odorizzi and Trowbridge, 1994). The mutations were verified by dideoxynucleotide sequencing (Sanger et al., 1977; Tabor and Richardson, 1987) of the RCAS-BP(A) constructs using the Sequenase kit (United States Biochemical Corp., Cleveland, OH). All other mutant human TR in RCAS-BP(A) that were examined in this study have been previously described (Jing et al., 1990; Collawn et al., 1990, 1993).
Expression of Mutant Human TR in MDCK Cells
Chick embryo fibroblasts (SPAFAS, Norwich, CT) were transfected with RCAS-BP(A) constructs encoding mutant human TR as previously described (Odorizzi and Trowbridge, 1994). Recombinant virus produced by transfected chick embryo fibroblasts was concentrated by centrifugation of 10 ml of tissue culture supernatant at 23,000 rpm for 2.5 h at 4°C in a rotor (model SW40 Ti; Beckman Instruments, Fullerton, CA). The pelleted virus was resuspended in 1 ml of DME and then passed through a 0.45-μm filter. The receptor for RSV(A) (Bates et al., 1993) has been stably expressed in MDCK II cells, rendering them susceptible to infection by RSV(A) (Odorizzi et al., 1996). MDCK cells derived from a clone expressing the RSV(A) receptor were plated at 105 cells/well of a 24-well tissue culture dish (Costar Corp., Cambridge, MA) and 12 h later were incubated with concentrated recombinant RCAS-BP(A) virus for 12 h at 37°C. Afterward, 1 ml of growth medium was added, and the cells were grown to confluency. Expression of mutant human TR was analyzed by immunofluorescence using B3/25, an mAb against the extracellular domain of the receptor, and a goat anti–mouse secondary antibody conjugated to FITC (Cooper Biomedical, Malvern, PA). Individual clones of MDCK cells expressing human TR were isolated by limited dilution.
Steady-State Cell Surface Distribution of Human TR
MDCK cells expressing human TR were plated at high density (1.5 × 106) onto 24-mm-diam Costar Transwell polycarbonate filters (0.4-μm pore size) and cultured for 3 d, with the media changed on the second day. 125Ilabeled diferric human transferrin (Tf) (ICN Biomedicals Inc., Costa Mesa, CA) was prepared by incubating 500 μg Tf with 40 μg chloramine T (Sigma Chemical Co., St. Louis, MO) and 0.5–1.0 mCi Na125I (Amersham Corp., Arlington Heights, IL) in a total volume of 150 μl of PBS. The reaction was stopped by adding 80 μg sodium metabisulfite (Fisher Scientific, Pittsburgh, PA) in 10 μl of PBS. 125I-labeled Tf was separated from free 125I on a Sephadex G-25 column equilibrated in PBS. To determine the steady-state cell surface distribution of human TR, filter-grown cells were incubated in DME for 1 h at 37°C and then shifted to 4°C and washed with PBS+ (PBS with 1 mM CaCl2 and 1 mM MgCl2) containing 0.5% BSA (BSA-PBS+). Cells were then incubated for 1 h at 4°C with 4 μg/ml 125I-labeled Tf in BSA-PBS+ added to either side of the monolayer (150 μl basolaterally or 350 μl apically). Under these conditions, less than 0.1% of the 125I-labeled Tf crossed the monolayers. Cells were then washed three times at 4°C with BSA-PBS+ (2 ml per monolayer surface), and the amount of radioactivity specifically bound to the cells was determined by excising the filters and counting them in a gamma counter.
Polarized Cell Surface Delivery of Newly Synthesized Human TR
Filter-grown MDCK monolayers expressing human TR were incubated for 30 min at 37°C in methionine- and cysteine-free DME (2 ml per monolayer surface) containing 0.5 mCi/ml 35S-labeled methionine-cysteine (ICN Biomedicals, Irvine, CA) and 1% dialyzed fetal bovine calf serum. Monolayers were then washed twice with DME and chased for 20 or 40 min at 37°C in DME containing a 10-fold excess of unlabeled methionine and cysteine. The cells were then chilled with two washes in ice-cold DME at 4°C. Monolayers were then incubated for 30 min in DME containing 100 μg/ml trypsin added to either the apical or the basolateral surface (Worthington Biochemical Corp., Freehold, NJ). DME containing 100 μg/ml trypsin inhibitor (Sigma Chemical Co.) was added to the opposite surface. Tryptic cleavage of surface receptors generates a soluble ∼70-kD TR extracellular domain fragment that was immunoprecipitated from the apical and basolateral media using B3/25 mAb and then analyzed on 10% SDS– polyacrylamide gels (Omary and Trowbridge, 1981). Dried gels were exposed to preflashed XAR film (Eastman Kodak, Rochester, NY), and quantitation of radioactivity was performed (model 425 PhosphorImager; Molecular Dynamics, Sunnyvale, CA). No TR tryptic fragments were detected in immunoprecipitates of media containing trypsin inhibitor.
Polarized Recycling of Internalized Human TR
Filter-grown MDCK monolayers expressing human TR were incubated for 1 h at 37°C in DME and then for 1 h at 37°C with 4 μg/ml 125I-labeled Tf in DME containing 0.5% BSA (BSA-DME) (150 μl basolaterally and 350 μl apically). Cells were then washed three times at 4°C with BSADME (2 ml per monolayer surface), and surface-bound Tf was removed with >95% efficiency using deferoxamine mesylate as previously described (Jing et al., 1990; Odorizzi et al., 1996). Cells were then washed three times with BSA-DME at 4°C and incubated at 37°C for 90 min in BSA-DME containing 100 μg/ml unlabeled Tf (1 ml per monolayer surface). Afterward, the radioactivity released into the apical and basolateral media, as well as the cell-associated radioactivity, was determined.
Internalization Efficiencies of Human TR
MDCK cells expressing human TR were plated at a density of 7.5 × 104 cells/cm2 in a 24-well tissue culture plate (Costar Corp.) and cultured at 37°C for 1 d. The cells were incubated for 1 h at 37°C in 1 ml of serum-free DME and then incubated with 150 μl of 4 μg/ml 125I-labeled Tf in BSADME for 1 h at 37°C. The media was removed, and the cells were washed three times at 4°C with 1 ml BSA-PBS+. The cells were incubated at 4°C twice for 3 min with 0.5 ml of 0.2 M acetic acid, 0.5 M NaCl, pH 2.4, to remove surface-bound 125I-labeled Tf (Hopkins and Trowbridge, 1983). Cells were removed from the wells with two washes in 0.5 ml of 1 M NaOH, and radioactivity in the acid wash and the cell lysate was determined. Prolonged incubation with the acid wash did not affect the radioactivity released (Jing et al., 1990). The internalization efficiencies of mutant human TR relative to the wild-type human TR were determined from the steady-state intracellular distribution of 125I-labeled Tf and calculated as previously described (Jing et al., 1990; Odorizzi et al., 1994).
Results
Residues 19–41 in the TR Cytoplasmic Tail Are Necessary and Sufficient for Basolateral Sorting
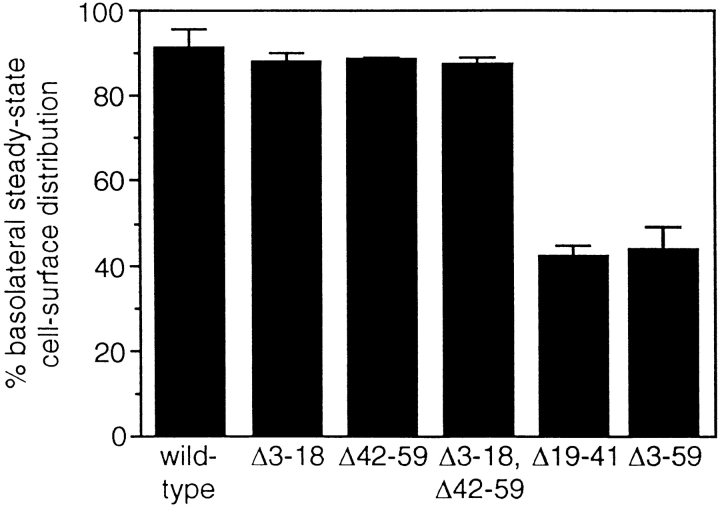
Using a retroviral vector derived from RSV(A) (Hughes et al., 1990), mutant human TR were stably expressed in MDCK cells in which the RSV(A) receptor (Bates et al., 1993) had been expressed, rendering them susceptible to infection (Odorizzi et al., 1996). To localize the basolateral sorting information within the cytoplasmic tail of TR, the steady-state cell surface distribution of mutant human TR was determined by measuring the binding of 125I-labeled human Tf to the apical and basolateral surfaces at 4°C. As shown in Fig. 1, deletion of residues 3–18 or residues 42–59 did not influence the polarized basolateral localization of TR, and ∼90% of the receptors were still expressed at the basolateral surface when both regions were deleted simultaneously. In contrast, removal of residues 19–41 from the TR cytoplasmic tail resulted in an essentially random distribution of receptors at both surfaces (Fig. 1), similar to the nonpolarized cell surface expression of a mutant tailless TR (Δ3–59) in MDCK cells (Odorizzi et al., 1996).
Figure 1.
Residues 19–41 of the TR cytoplasmic tail are necessary and sufficient to target TR to the basolateral surface of MDCK cells. Filter-grown MDCK cells expressing wild-type and mutant TR were incubated either apically or basolaterally for 1 h at 4°C with 125I-labeled Tf (4 μg/ml). After washing away unbound Tf, the amount of 125I-labeled Tf specifically bound on each surface was determined (mean ± standard error of three independent experiments).
The polarized basolateral expression of wild-type TR in MDCK cells results from the basolateral sorting of newly synthesized receptors as well as the selective basolateral recycling of internalized receptors (Odorizzi et al., 1996). Therefore, we investigated the biosynthetic cell surface delivery of mutant TR by pulse-labeling newly synthesized receptors with 35S-labeled methionine/cysteine and chasing them to the surface of filter-grown monolayers at 37°C for 20 or 40 min. Subsequently, the apical or basolateral surface was treated with trypsin at 4°C, which cleaves the TR external domain near the transmembrane region, resulting in the release of an ∼70-kD fragment, which was immunoprecipitated using B3/25 mAb (Omary and Trowbridge, 1981) and quantitated after SDS-PAGE and phosphorimage analysis. The data obtained using this protocol were the same regardless of whether a 20- or 40-min chase was used and strongly suggested that residues 19–41 are sufficient to promote the polarized transport of newly synthesized TR to the basolateral surface, whereas deletion of residues 19–41 resulted in the nonpolarized delivery of newly synthesized TR to both surfaces (Fig. 2 A). However, to exclude the possibility that the values for basolateral delivery of newly synthesized receptors obtained using relatively short chase periods were influenced by differences in either the rate of delivery of newly synthesized receptors to the apical or basolateral surface or their subsequent rates of internalization from each surface, more extensive kinetic studies were performed in which cells were pulse-labeled for 15 min and then chased for 30 min, 1 h, or 2 h. As shown in Fig. 2 B, using the tryptic cleavage protocol described above gave results similar to those shown in Fig. 2 A. The values for basolateral delivery of newly synthesized mutant TR with residues 19–41 deleted tend to decrease with time, suggesting that transport to the basolateral surface may be more rapid than to the apical surface. Further, experiments in which cleavage of surface TRs was performed at 37°C by continuous incubation with trypsin during the chase period also gave the same results (Fig. 2 B). We conclude, therefore, that residues 19–41 are sufficient to mediate polarized delivery of newly synthesized TR to the basolateral surface.
Figure 2.
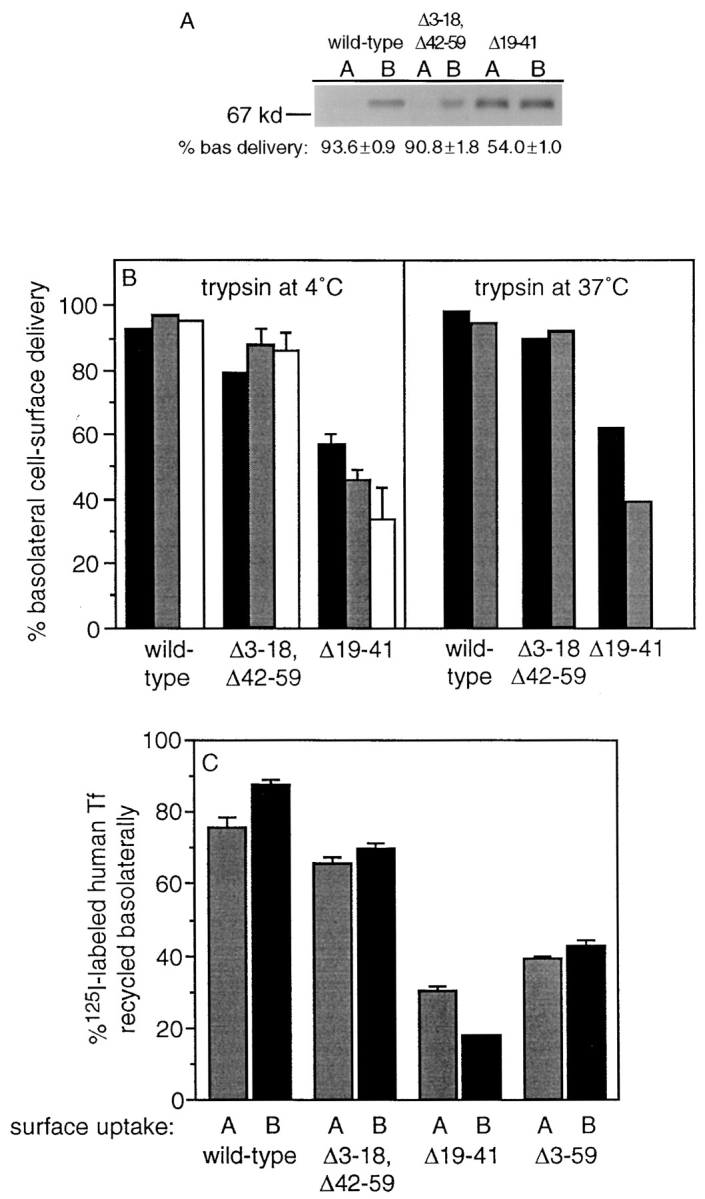
Residues 19–41 of the TR cytoplasmic tail are necessary and sufficient for basolateral sorting in both the biosynthetic and endocytic pathways. (A) Newly synthesized wild-type and mutant TR expressed in filter-grown MDCK cells were pulse- labeled with 35S-labeled methionine/cysteine for 30 min and then chased to the cell surface for 20 min at 37°C. Receptors at the apical or basolateral surface were then cleaved at 4°C for 30 min with trypsin (100 μg/ml). Trypsin inhibitor (100 μg/ml) was included in the opposite media. B3/25 mAb immunoprecipitates of the ∼70-kD TR extracellular fragment in the media collected from each surface were analyzed on SDS–polyacrylamide gels and quantitated by phosphorimage analysis (mean ± standard error of three independent experiments). No material could be detected in immunoprecipitates from the media containing trypsin inhibitor. (B) Newly synthesized receptors were pulse-labeled as described above and chased at 37°C for 30 min (black bars), 60 min (gray bars), or 120 min (open bars). Trypsin was added to the apical or basolateral surface of the monolayers at 4°C as described above or was included in the apical or basolateral media during the 37°C chase. TR external domain fragments were immunoprecipitated and analyzed by SDS-PAGE as described above. (C) Filter-grown MDCK cells expressing wild-type and mutant TR were incubated either apically or basolaterally at 37°C for 1 h with 125I-labeled Tf. Monolayers were then washed at 4°C, and surface-bound 125I-labeled Tf was removed with deferoxamine mesylate. Cells were then incubated at 37°C for 90 min, and the appearance of 125I-labeled Tf in the apical and basolateral media was determined (mean ± standard error of three independent experiments). More than 90% of the internalized 125I-labeled Tf recycled after 90 min at 37°C.
To measure the recycling of mutant TR in MDCK cells, monolayers were loaded for 1 h at 37°C with 125I-labeled Tf from either the apical or basolateral surface; then surface-bound Tf was removed at 4°C with deferoxamine mesylate, monolayers were re-incubated at 37°C for 90 min, and the release of radioactivity into the apical and basolateral media was monitored. Fig. 2 C shows that residues 19– 41 promote the basolateral sorting of TR internalized from either cell surface domain, whereas deletion of residues 19–41 results in the loss of polarized basolateral recycling. Thus, residues 19–41 in the TR cytoplasmic tail are sufficient for efficient basolateral sorting in both the biosynthetic and endocytic pathways.
The Basolateral Sorting Signal of TR Is Distinct from the Internalization Signal and Is Not Tyrosine Based
Basolateral sorting signals identified within the cytoplasmic tails of several membrane proteins have been demonstrated to be dependent upon tyrosine and colinear with the internalization signals within these proteins (Brewer and Roth, 1991; Matter et al., 1992; Geffen et al., 1993; Prill et al., 1993; Höning and Hunziker, 1995; Monlauzeur et al., 1995). The four-residue tyrosine-based internalization signal, Y20TRF23 is contained within residues 19–41 of the TR cytoplasmic tail (Collawn et al., 1990). To determine whether YTRF also contributes to the basolateral sorting signal of TR, we examined the biosynthetic and endocytic sorting of mutant receptors in which the activity of the internalization signal was severely reduced by substitution of alanine for either Tyr-20 or Phe-23 (Collawn et al., 1990). As shown in Table I, alanine substitution of either aromatic residue abrogated rapid endocytosis. In contrast, substitution of alanine for Tyr-20 had no effect on the polarized basolateral delivery of newly synthesized receptors and resulted in only a small decrease in polarized basolateral recycling, while alteration of Phe-23 did not affect the basolateral sorting of TR in either the biosynthetic or endocytic pathways. We conclude, therefore, that the basolateral sorting signal within region 19–41 of the TR cytoplasmic tail is distinct from the YTRF internalization signal and is not tyrosine based.
Table I.
The Basolateral Sorting Signal of TR Is Distinct from the Internalization Signal and Is Not Tyrosine Based
| TR construct | Internalization efficiency (%)* | Percentage of basolateral biosynthetic delivery | Percentage of basolateral recycling | |||||
|---|---|---|---|---|---|---|---|---|
| Basolateral uptake | Apical uptake | |||||||
| Wild-type TR | 100 | 93.6 ± 0.9‡ (11)§ | 87.5 ± 1.3 (6) | 75.7 ± 2.7 (6) | ||||
| Δ3-59 | 15.0 ± 2.9 (2) | 51.2 ± 2.3 (4) | 42.7 ± 1.7 (6) | 39.2 ± 0.7 (6) | ||||
| Y20→ A | 17.0 ± 2.1 (2) | 92.4 ± 2.0 (4) | 79.1 ± 0.7 (3) | 62.2 ± 1.9 (3) | ||||
| F23→ A | 10.0 ± 0.0 (2) | 94.0 ± 0.6 (3) | 83.2 ± 0.0 (2) | 75.3 ± 0.9 (2) | ||||
The internalization efficiencies expressed relative to wild-type TR were calculated from the steady-state intracellular distribution of internalized 125I-labeled Tf.
Mean ± standard error.
Number of independent experiments.
Residues 29–35 Are Important for Basolateral Sorting of Newly Synthesized TR
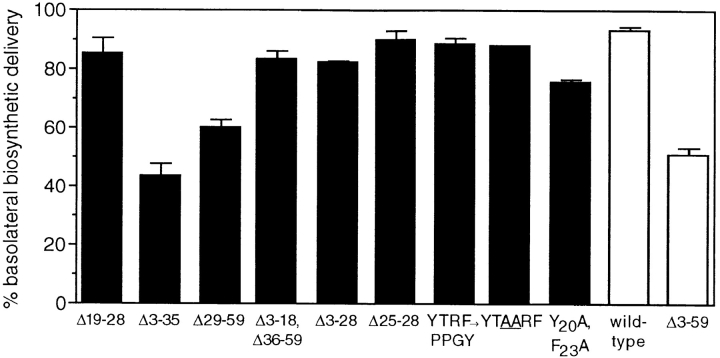
To further define the structural requirements for basolateral sorting in the biosynthetic pathway, we analyzed the polarity of cell surface delivery of newly synthesized TR containing various mutations encompassing residues 19– 41. As shown in Fig. 3, deletion of residues 19–28 resulted in only a small decrease in basolateral sorting of newly synthesized receptors, indicating that amino-terminal residues within region 19–41, including the YTRF internalization signal, are not required for basolateral sorting in the biosynthetic pathway. Deletion of residues 3–28 or residues 25–28 also had no effect on basolateral sorting of newly synthesized TR. In contrast, deletion of residues 3–35 abolished the polarized basolateral delivery of newly synthesized receptors, and mutant TR containing a membrane-proximal deletion of residues 29–59 were also delivered in an essentially nonpolarized manner to both the apical and basolateral surfaces, similar to the cell surface delivery of newly synthesized tailless TR (Fig. 3). Basolateral sorting was almost completely restored in mutant TR containing a truncated cytoplasmic tail in which residues 3–18 and residues 36–59 had been deleted (Fig. 3). Taken together, these results suggest that residues 29–35 are most important for basolateral sorting of TR in the biosynthetic pathway.
Figure 3.
Residues 29–41 of the TR cytoplasmic tail are the most important for basolateral sorting of newly synthesized TR. Newly synthesized wild-type and mutant TR expressed in filter-grown MDCK cells were pulse-labeled and chased to the cell surface, and the ∼70-kD TR extracellular fragment derived from receptors at either the apical or basolateral surface was immunoprecipitated and quantitated as described in the legend to Fig. 2 A.
To further demonstrate that the YTRF internalization signal is distinct from the basolateral sorting signal of TR, we examined the cell surface delivery of a mutant TR in which YTRF had been replaced with PPGY, a sequence from the cytoplasmic tail of lysosomal acid phosphatase that is predicted from two-dimensional NMR studies to form a β-turn (Eberle et al., 1991). The PPGY tetrapeptide sequence is inactive as an internalization signal in place of YTRF in the TR cytoplasmic tail (Collawn et al., 1993). Nevertheless, substitution of PPGY for YTRF did not affect the polarized basolateral delivery of newly synthesized TR (Fig. 3), indicating that the specific sequence of the TR internalization signal is not required for basolateral sorting in the biosynthetic pathway. We also examined the biosynthetic cell surface delivery of a mutant TR in which two alanine residues had been inserted between Thr-21 and Arg-22. This mutation (YTAARF) disrupts the relative position of the two hydrophobic residues within the putative tight turn and abolishes high-efficiency endocytosis (Collawn et al., 1993) but does not affect the polarized basolateral delivery of newly synthesized TR (Fig. 3). However, a small decrease in basolateral sorting resulted from the simultaneous substitution of alanine for both Tyr-20 and Phe-23, suggesting that complete removal of aromatic amino acids from this region results in a more substantial alteration of the structure of the cytoplasmic tail, which can modestly affect recognition of the basolateral sorting signal in the biosynthetic pathway.
Structural Requirements for Basolateral Sorting of TR in the Endocytic Pathway
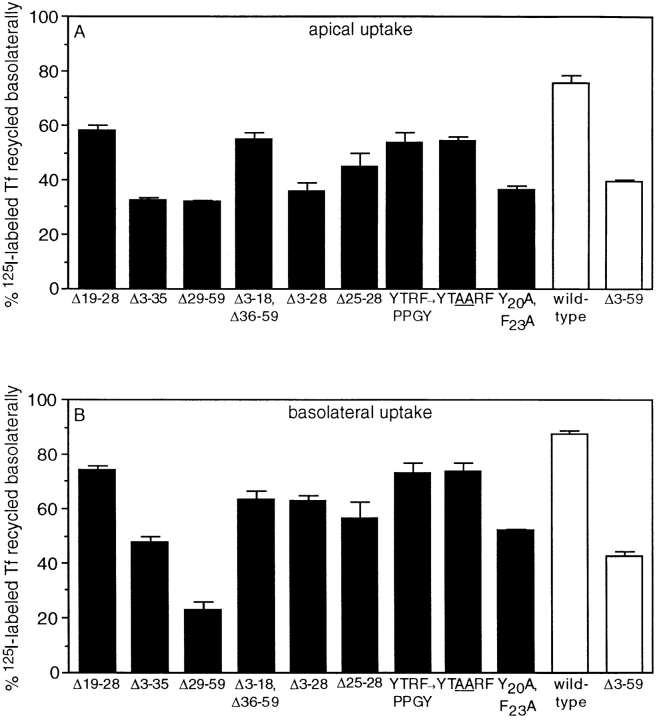
To determine whether residues 29–35 also mediate basolateral sorting of internalized TR from the endocytic pathway, we measured the polarity of recycling of the same set of mutant TR described above. The results of this analysis were more complex than for basolateral sorting of newly synthesized TR and revealed significant differences between the structural requirements for basolateral sorting of newly synthesized receptors and receptors internalized from either the basolateral or apical surface. As shown in Fig. 4, deletion of residues 19–28 resulted in a modest decrease in basolateral sorting of TR internalized from either the basolateral or apical surface, suggesting that this region is not very important for basolateral sorting of TR in the endocytic pathway. As in the case of basolateral sorting in the biosynthetic pathway, basolateral sorting of TR in the endocytic pathway was abolished either by deletion of residues 3–35 or by the internal deletion of residues 29–59 from the cytoplasmic tail. Mutant TR containing a truncated cytoplasmic tail in which residues 3–18 and 36– 59 had been deleted were selectively recycled to the basolateral surface with an efficiency intermediate between tailless and wild-type TR. Taken together, these results suggest that residues 29–36 are required for basolateral sorting of internalized TR from the endocytic pathway but are not sufficient for sorting with the same efficiency as wild-type TR, especially for receptors internalized from the apical surface.
Figure 4.
The structural requirements for basolateral sorting of TR in the endocytic pathway differ from the structural requirements for sorting in the biosynthetic pathway. The endocytic pathways of MDCK cells expressing wild-type or mutant TR were loaded with 125I-labeled Tf for 1 h at 37°C from either the apical (A) or basolateral (B) border. Monolayers were then washed at 4°C, and the amount of radiolabel recycled to the apical or basolateral surface after 90 min at 37°C was determined as described in the legend to Fig. 2 C.
Analysis of the remaining TR mutants gave significantly different results to those obtained for basolateral sorting of newly synthesized receptors. Most striking was that altering both Tyr-20 and Phe-23 to alanine residues essentially abolished selective basolateral delivery of receptors internalized from either the apical or basolateral surface. In addition, deletion of residues 25–28 had a more pronounced effect on basolateral sorting of TR from the endocytic pathway than from the biosynthetic pathway. These results indicate that, in contrast to basolateral sorting in the biosynthetic pathway, basolateral sorting of TR in the endocytic pathway is impaired by mutations located throughout the region spanning residues 19–41. One interpretation of these data is that residues located throughout this 23–amino acid region directly contribute to the basolateral sorting signal recognized in the endocytic pathway. An alternative explanation, suggested by the relatively efficient basolateral sorting of mutant TR with residues 19–28 deleted, is that other mutations within this region (i.e., alteration of Tyr-20 and Phe-23 to alanine residues) that abrogate basolateral sorting of TR from the endocytic pathway impair sorting by inducing a conformational change in the TR cytoplasmic domain.
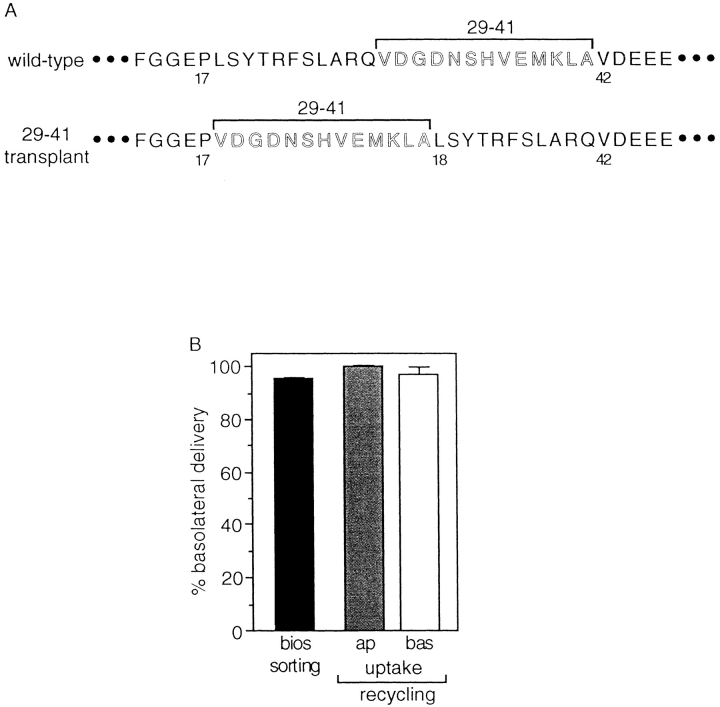
To attempt to distinguish between these possibilities, we constructed a mutant TR in which residues 29–41 were inserted between Pro-17 and Leu-18 (Fig. 5 A). The rationale for this construct was that if the basolateral sorting signal recognized in the endocytic pathway was comprised of residues throughout the 23–amino acid region spanning amino acids 19–41, then inversion of the carboxy- and amino-terminal sequences should abolish activity. As shown in Fig. 5 B, newly synthesized mutant TR containing the sequence inversion were efficiently sorted to the basolateral surface, which is consistent with previous data indicating that residues 19–28 do not contribute to the basolateral sorting signal recognized in the biosynthetic pathway. Importantly, these mutant TR were also efficiently targeted to the basolateral surface after internalization from either the apical or basolateral surface, providing strong evidence that residues within the region spanning amino acids 19–28 do not contribute directly to the basolateral sorting signal recognized in the endocytic pathway.
Figure 5.
TR is sorted efficiently to the basolateral surface upon transplantation of residues 29–41 to a more membrane-distal position in the cytoplasmic tail. (A) The amino acid sequence of the TR cytoplasmic tail depicting the transplantation of residues 29– 41 to a more membrane-distal position located between Pro-17 and Leu-18. (B) The fraction of newly synthesized and internalized mutant 29–41 transplant TR sorted basolaterally was determined as described in the legend to Fig. 2.
Further Analysis of the TR Basolateral Sorting Signal
The primary structure of residues 29–41 of the TR cytoplasmic domain reveals no similarities with previously identified basolateral sorting signals. However, within this region is the sequence R27QVD30. A similar sequence, RNVD, is found in the cytoplasmic domain of the pIgR, and the valine residue within this sequence has been demonstrated to be relatively important for basolateral sorting of newly synthesized receptors (Aroeti et al., 1993). However, alteration of either Val-29 or Val-36 in the TR cytoplasmic tail had only a small effect on the basolateral sorting of receptors in either the biosynthetic or endocytic pathways (Table II), demonstrating that, unlike the pIgR basolateral sorting signal (Aroeti et al., 1993), valine residues do not play an important role in the TR basolateral sorting signal.
Table II.
Valine Residues Are Not Important for Basolateral Sorting, Whereas G31 DNS34 Is Required for Basolateral Sorting in the Biosynthetic Pathway but Not the Endocytic Pathway
| TR construct | Percentage of basolateral biosynthetic delivery | Percentage of basolateral recycling | ||||
|---|---|---|---|---|---|---|
| Basolateral uptake | Apical uptake | |||||
| Wild-type TR | 93.6 ± 0.9* (11)‡ | 87.5 ± 1.3 (6) | 75.7 ± 2.7 (6) | |||
| V29→ A§ | 84.3 ± 3.6 (3) | 72.6 ± 2.0 (3) | 89.7 ± 4.2 (3) | |||
| V36→ A§ | 76.7 ± 6.9 (3) | 65.6 ± 1.1 (3) | 81.9 ± 4.5 (3) | |||
| G31DNS34 | 61.2 ± 3.2 (3) | 79.9 ± 1.5 (3) | 90.1 ± 1.8 (3) | |||
| → YTRF | ||||||
Mean ± standard error.
Number of independent experiments.
The V29→ A and V36→ A substitutions were constructed in the mutant TR Δ3–18, Δ42–59.
Also located within residues 29–41 of the TR cytoplasmic domain is the sequence G31DNS34, which as described earlier, along with the YTRF internalization signal, is predicted to adopt a tight turn conformation (Collawn et al., 1990). YTRF is efficiently recognized as an internalization signal when substituted for GDNS in this position (Collawn et al., 1993; see also Pytowski et al., 1995). As shown in Table II, substitution of YTRF for GDNS was found to severely impair basolateral sorting of newly synthesized TR, demonstrating that this specific sequence is required for recognition in the biosynthetic pathway. In contrast, substitution of YTRF for GDNS has no effect on basolateral sorting in the endocytic pathway (Table II), further demonstrating that the structural requirements for recognition of the TR basolateral sorting signal in the biosynthetic and endocytic pathways of MDCK cells are not identical.
Discussion
We have shown that residues 19–41 of the 61–amino acid cytoplasmic domain of human TR are sufficient for basolateral sorting of receptors in both the biosynthetic and endocytic pathways of MDCK cells. As deletion of these residues abrogates basolateral sorting of TR, we also conclude that there is not another basolateral sorting signal located outside this region of the TR cytoplasmic tail. The basolateral sorting signal located within region 19–41 is neither colinear with the TR internalization signal nor tyrosine based. Importantly, the structural requirements for basolateral sorting in the biosynthetic and endocytic pathways, although similar, are not identical. This observation has implications for the mechanism of basolateral sorting in MDCK cells.
TR Basolateral Sorting Signal
The results from our study of a series of TR deletion mutants are in contradiction with an earlier study that suggested the TR basolateral sorting signal is located within the membrane-proximal region of the cytoplasmic tail between residues 42–61 (Dargemont et al., 1993). This region was proposed to contain basolateral sorting information since ∼80% of a mutant TR containing an internal deletion of residues 6–41 was detected at the basolateral border at steady state, and newly synthesized mutant receptors were found to be delivered predominantly to the basolateral surface (Dargemont et al., 1993). Our more extensive studies, however, are internally consistent and show that residues 19–41 of the TR cytoplasmic tail are both necessary and sufficient for basolateral sorting in both the biosynthetic and endocytic pathways. In addition, >90% of mutant receptors in which residues 42–59 have been deleted are sorted basolaterally in both the biosynthetic and endocytic pathways (Odorizzi, G., and I.S. Trowbridge, unpublished observations). We have no obvious explanation for the results of Dargemont et al. (1993); however, the analysis of this single TR mutant leaves open the possibility that this deletion fortuitously creates a basolateral sorting signal that is not present in the wild-type receptor. It is noteworthy that the membrane-proximal region of the TR cytoplasmic tail is less conserved than the amino-terminal portion of the cytoplasmic tail spanning residues 1–41 (Collawn et al., 1993), arguing against an important functional role for this region. However, one conserved sequence motif within the membrane proximal region is a cluster of four acidic amino acids, DE/DEE, at positions 43–46. It has been suggested that a similar cluster of acidic residues is an important feature of the LDLR basolateral sorting signals (Matter et al., 1994). This requirement cannot be a general feature of basolateral sorting signals, however, as the cluster of acidic residues in the TR cytoplasmic domain can be deleted without loss of basolateral sorting activity.
We have clearly demonstrated that the basolateral sorting signal of TR is distinct from the internalization signal and is not tyrosine based. Substitution of alanine for either Tyr-20 or Phe-23 completely abrogates high-efficiency internalization but has little effect on basolateral sorting (Table I). Three other well-characterized basolateral sorting signals have been identified that do not share structural requirements with internalization signals. Two of these signals, the membrane-distal basolateral sorting signal of LDLR (Matter et al., 1992) and the basolateral sorting signal of VSV glycoprotein G (Thomas et al., 1993), are dependent upon tyrosine for activity. As in the case of the TR basolateral sorting signal, the 17–amino acid juxtamembrane region of the pIgR that is necessary and sufficient for basolateral sorting (Casanova et al., 1991) is independent of tyrosine (Okamoto et al., 1992). Nevertheless, there is no clear relationship between the basolateral sorting signals in TR and pIgR (see below), nor have any significant structural similarities between tyrosine-dependent basolateral sorting signals been found.
Structural Requirements for Basolateral Sorting in the Biosynthetic and Endocytic Pathways
The analysis of TR deletion mutants revealed that the structural requirements for basolateral sorting of TR in the biosynthetic and endocytic pathways are related, but not identical. The most important elements of the TR basolateral sorting signal recognized in the biosynthetic pathway have been further localized by deletion analysis to residues 29–35. Consistent with this conclusion, substitution of G31DNS34 with YTRF impairs basolateral sorting of newly synthesized TR. Substitution of G31DNS34 with YTRF had no effect on the efficiency of basolateral sorting of TRs in the biosynthetic pathway. This mutation provided the most clear-cut evidence that the structural requirements for basolateral sorting in the biosynthetic and endocytic pathways differ. The structure of the basolateral sorting signal recognized in the endocytic pathway remains enigmatic. The modest effect of deleting residues 19–28 on basolateral sorting of internalized TR and the fact that transplantation of residues 29–41 to a different position in the TR cytoplasmic tail did not affect basolateral sorting of internalized receptors suggests that the most important residues contributing to the TR basolateral sorting signal recognized in the endocytic pathway are located within the region spanning residues 29–41. However, whereas residues located on the amino-terminal side of region 29–41 could be removed without substantially affecting the basolateral sorting of newly synthesized receptors, deletion of residues 3–28 or 25–28 of the TR cytoplasmic tail significantly reduced basolateral sorting of internalized receptors in the endocytic pathway. Furthermore, simultaneous substitution of alanine residues for both Tyr-20 and Phe23 only modestly affected basolateral sorting in the biosynthetic pathway, whereas sorting in the endocytic pathway was completely abrogated. Our interpretation of these data is that these mutations have an indirect effect on the efficiency of basolateral sorting of internalized TR because deletion of residues 3–18 or 19–28 does not affect the efficiency of sorting.
Previous studies comparing the structural requirements for basolateral sorting of the LDLR and pIgR in the biosynthetic and endocytic pathways emphasized the similarities between the sorting signals recognized in each pathway, and significant differences in the structural requirements for sorting in the biosynthetic and endocytic pathways were not reported (Matter et al., 1993; Aroeti and Mostov, 1994). In these experimental systems, however, it is not possible to load the endocytic pathway from the apical or basolateral surface under conditions approaching steady state and then measure quantitatively the delivery of internalized receptors to each surface. Moreover, in recent studies we have shown that a TR chimera containing the major histocompatibility complexclass II invariant chain cytoplasmic tail is selectively expressed on the basolateral surface of MDCK cells as a result of basolateral sorting in both the biosynthetic and endocytic pathways (Odorizzi and Trowbridge, 1997). However, the precise structural requirements for basolateral sorting of the chimeric receptors in the biosynthetic and endocytic pathways were also different, indicating that this observation is not limited to basolateral sorting of TR.
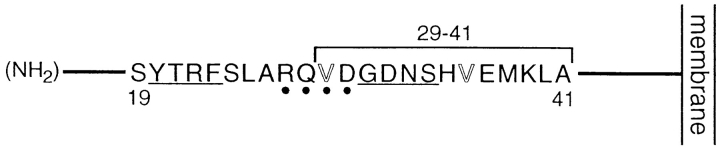
More detailed analysis of the region spanning residues 29–41 failed to clarify the structural basis for basolateral sorting of TR in the endocytic pathway. Included within this region are two valine residues, at positions 29 and 36. The 17-amino acid region of the pIgR that mediates basolateral sorting also contains two valine residues, one of which, Val-660, was found to be relatively important for activity (Aroeti et al., 1993). Two-dimensional NMR studies suggest that Val-660 in the pIgR is part of a β-turn comprised of the sequence RNVD (Aroeti et al., 1993). Although Val-29 in TR is found within a similar sequence, RQVD, this motif lies outside of the most important region of the TR cytoplasmic tail for basolateral sorting, and alteration of Val-29 to alanine also does not markedly affect the efficiency of basolateral sorting. Alteration of Val36 to alanine also had only a modest effect on basolateral sorting efficiency. As substitution of G31DNS34 with YTRF does not decrease the efficiency of basolateral sorting of TR in the endocytic pathway, these residues cannot be specifically required. However, since the GDNS motif, like the YTRF internalization signal, is predicted to adopt a β-turn conformation (Collawn et al., 1993), substitution of YTRF for GDNS may preserve secondary structure in this region, which may be the reason why activity of the basolateral sorting signal recognized in the endocytic pathway is unaffected. Fig. 6 summarizes the relevant features of the region of the cytoplasmic tail important for basolateral sorting of TR described in this study.
Figure 6.
Features of the TR basolateral sorting signal. Residues 19–41, which are necessary and sufficient for basolateral sorting of TR, are shown. Each of the two four-residue sequences within this region that are predicted to adopt a tight turn conformation, the YTRF internalization signal and the sequence GDNS (Collawn et al., 1990), are underlined. The region most important for basolateral sorting is within residues 29–41, and the TR basolateral sorting signal is neither tyrosine based nor colinear with the YTRF internalization signal. Also indicated is the four-residue sequence RQVD, which is similar to the sequence RNVD found to be important for basolateral sorting of pIgR (Aroeti et al., 1993). However, the sequence RQVD is not required for basolateral sorting of TR, and neither Val-29 nor Val-36 is important for activity of the TR basolateral sorting signal.
Implications for the Mechanism of Basolateral Sorting in MDCK Cells
Current models of polarized sorting suggest that either the same basolateral sorting signal is recognized at two different locations within MDCK cells, the TGN and the endosome (Matter and Mellman, 1994), or, alternatively, polarized sorting of newly synthesized membrane proteins and receptors internalized from the plasma membrane occurs within the same membrane compartment, recently proposed to be the endosome (Aroeti and Mostov, 1994; Matter and Mellman, 1994). Each of these models must be an oversimplification, however, since our results clearly show that the structural requirements for efficient basolateral sorting of newly synthesized TR and receptors internalized from the plasma membrane are not identical. One interpretation of these data is that polarized sorting of TR occurs at a site in the biosynthetic pathway that is distinct from basolateral sorting in the endocytic pathway and that the sorting machinery at these two locations are not identical and exhibit subtle differences in their recognition of basolateral sorting signals. For example, such a situation might occur if the recognition of basolateral sorting signals was mediated by different adaptor protein complexes. Alternatively, if basolateral sorting of newly synthesized and internalized TR is an iterative process similar to endosomal sorting in nonpolarized cells (Dunn et al., 1989) and newly synthesized TR were subject to more rounds of sorting than internalized receptors, then basolateral targeting of newly synthesized receptors could be more efficient than internalized receptors and, therefore, less sensitive to mutations that decrease sorting efficiency, despite the fact that both receptor populations are sorted by the same basolateral sorting machinery.
Our results further question the extent to which tyrosinebased internalization signals can be used as a paradigm for basolateral sorting signals. Several basolateral sorting signals are tyrosine dependent, and as for tyrosine-based internalization signals, a β-turn has been proposed to be a common feature of basolateral sorting signals (Aroeti et al., 1993). However, unlike tyrosine-based internalization signals, sequence patterns that might reflect a common structural motif have not been identified among basolateral sorting signals. Thus, basolateral sorting signals may involve a larger stretch of amino acids and may be more sensitive to changes in the surrounding secondary structure. As a consequence, the common structural features of basolateral sorting signals and their relationship to internalization signals may only be understood when the three-dimensional structures of both types of signal can be compared.
Acknowledgments
This work was supported by National Institutes of Health grant DK 50825 to I.S. Trowbridge. G. Odorizzi is a member of the University of California, San Diego, Department of Biology, Salk Institute Graduate Program and was supported in part by the Chapman Charitable Trust and National Cancer Institute training grant T32-CA64041.
Footnotes
1. Abbreviations used in this paper: LDLR, low-density lipoprotein receptor; MDCK, Madin-Darby canine kidney; pIgR, polymeric immunoglobulin receptor; RSV(A), Rous sarcoma virus subtype A; Tf, transferrin; TR, transferrin receptor.
Address all correspondence to I.S. Trowbridge, Department of Cancer Biology, The Salk Institute for Biological Studies, San Diego, CA 92186– 5800. Tel.: (619) 453-4100 ext. 1241. Fax: (619) 457-4765.
Greg Odorizzi's present address is Division of Cellular and Molecular Medicine, University of California, La Jolla, CA 92093-0668.
References
- Aroeti B, Mostov KE. Polarized sorting of the polymeric immunoglobulin receptor in the exocytic and endocytic pathways is controlled by the same amino acids. EMBO (Eur Mol Biol Organ) J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P, Young JAT, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science (Wash DC) 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov K. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–77. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing S, Trowbridge IS, Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Collawn JF, Lai A, Domingo D, Fitch M, Hatton S, Trowbridge IS. YTRF is the conserved internalization signal of the transferrin receptor, and a second YTRF signal at position 31-34 enhances endocytosis. J Biol Chem. 1993;268:21686–21692. [PubMed] [Google Scholar]
- Dargemont C, Le Bivic A, Rothenberger S, Iacopetta B, Kühn LC. The internalization signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO (Eur Mol Biol Organ) J. 1993;12:1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle W, Sander C, Klaus W, Schmidt B, von Figura K, Peters C. The essential tyrosine of the internalization signals in lysosomal acid phosphatase is part of a β turn. Cell. 1991;67:1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Geffen I, Fuhrer C, Leitinger B, Weiss M, Huggel K, Griffiths G, Spiess M. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993;268:20772–20777. [PubMed] [Google Scholar]
- Höning S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, S., C. Petropoulos, M. Federspiel, P. Sutrave, S. Forry-Schaudies, and J. Bradac. 1990. Vectors and genes for improvement of animal strains. J. Reprod. Fertil. 41(Suppl.):39–49. [PubMed]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO (Eur Mol Biol Organ) J. 1994;13:2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter W, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Jing S, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter KJ, Whitney AJ, Yamamoto EM, Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993;74:1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Monlauzeur L, Rajasekaran A, Chao M, Rodriguez-Boulan E, Le Bivic A. A cytoplasmic tyrosine is essential for the basolateral localization of mutants of the human nerve growth factor receptor in Madin-Darby canine kidney cells. J Biol Chem. 1995;270:12219–12225. doi: 10.1074/jbc.270.20.12219. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell surface polarity from bacteria to mammals. Science (Wash DC) 1992;258:948–955. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Recombinant Rous sarcoma virus vectors for avian cells. Methods Cell Biol. 1994;43:79–98. doi: 10.1016/s0091-679x(08)60599-3. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for MHC class II invariant chain trafficking in polarized MDCK cells. J Biol Chem. 1997;272:11757–11762. doi: 10.1074/jbc.272.18.11757. [DOI] [PubMed] [Google Scholar]
- Odorizzi CG, Trowbridge IS, Xue L, Hopkins CR, Davis CD, Collawn JF. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994;126:317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto CT, Shia S-P, Bird C, Mostov KE, Roth MG. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J Biol Chem. 1992;267:9925–9932. [PubMed] [Google Scholar]
- Omary MB, Trowbridge IS. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981;256:12888–12892. [PubMed] [Google Scholar]
- Prill V, Lehmann L, von Figura K, Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO (Eur Mol Biol Organ) J. 1993;12:2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytowski B, Judge TW, McGraw TE. An internalization motif is created in the cytoplasmic domain of the transferrin receptor by substitution of a tyrosine at the first position of a predicted tight turn. J Biol Chem. 1995;270:9067–9073. doi: 10.1074/jbc.270.16.9067. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chainterminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Fuller SD. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Tabor S, Richardson CC. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci USA. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;5:3313–3320. [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]