Abstract
Occludin is an integral membrane protein localizing at tight junctions in epithelial and endothelial cells. Occludin from confluent culture MDCK I cells resolved as several (>10) bands between 62 and 82 kD in SDS-PAGE, of which two or three bands of the lowest M r were predominant. Among these bands, the lower predominant bands were essentially extracted with 1% NP-40, whereas the other higher M r bands were selectively recovered in the NP-40–insoluble fraction. Alkaline phosphatase treatment converged these bands of occludin both in NP-40–soluble and -insoluble fractions into the lowest M r band, and phosphoamino acid analyses identified phosphoserine (and phosphothreonine weakly) in the higher M r bands of occludin. These findings indicated that phosphorylation causes an upward shift of occludin bands and that highly phosphorylated occludin resists NP-40 extraction. When cells were grown in low Ca medium, almost all occludin was NP-40 soluble. Switching from low to normal Ca medium increased the amount of NP-40–insoluble occludin within 10 min, followed by gradual upward shift of bands. This insolubilization and the band shift correlated temporally with tight junction formation detected by immunofluorescence microscopy. Furthermore, we found that the anti–chicken occludin mAb, Oc-3, did not recognize the predominant lower M r bands of occludin (non- or less phosphorylated form) but was specific to the higher M r bands (phosphorylated form) on immunoblotting. Immunofluorescence microscopy revealed that this mAb mainly stained the tight junction proper of intestinal epithelial cells, whereas other anti-occludin mAbs, which can recognize the predominant lower M r bands, labeled their basolateral membranes (and the cytoplasm) as well as tight junctions. Therefore, we conclude that non- or less phosphorylated occludin is distributed on the basolateral membranes and that highly phosphorylated occludin is selectively concentrated at tight juctions as the NP-40–insoluble form. These findings suggest that the phosphorylation of occludin is a key step in tight junction assembly.
Occludin is an integral membrane protein localizing at tight junctions in epithelial and endothelial cells. It was first identified in the chicken using monoclonal antibodies (Furuse et al., 1993) and most recently in mammalian species (Ando-Akatsuka et al., 1996; Saitou et al., 1997). Occludin consists of four transmembrane domains, three cytoplasmic domains (long COOHterminal and short NH2-terminal domains, and one short intracellular turn), and two extracellular loops. Among these domains, the first extracellular loop is characterized by an unusually high content of tyrosine and glycine residues (∼60%). The sequence of the COOH-terminal, ∼150 amino acids, is relatively conserved among species, and it is reportedly bound to ZO-1, a 220-kD tight junction-associated, lethal(1)discs large-1 (dlg)-like peripheral membrane protein (Stevenson et al., 1986; Anderson et al., 1988; Itoh et al., 1993; Willott et al., 1993; Furuse et al., 1994). Together with other tight junction undercoat-constitutive proteins such as ZO-2 (Gumbiner et al., 1991; Jesaitis and Goodenough, 1994), cingulin (Citi et al., 1988), 7H6 antigen (Zhong et al., 1993), and symplekin (Keon et al., 1996), ZO-1 appears to link occludin to the actin-based cytoskeleton (Madara, 1987; Citi, 1993; Gumbiner, 1993).
Tight junctions play the dual roles of barrier and fence in epithelial and endothelial cells. They create the primary barrier to the diffusion of solutes through the paracellular pathway and maintain cell polarity as a boundary between the apical and basolateral plasma membrane domains (for reviews see Schneeberger and Lynch, 1992; Gumbiner, 1987, 1993). As the morphological counterpart of the barrier function, in thin section electron microscopy, tight junctions appear as a series of discrete sites of apparent fusion, involving the outer leaflet of the plasma membrane of adjacent cells (Farquhar and Palade, 1963). Occludin is localized at these apparent fusion points (Furuse et al., 1993), and its overexpression results in transepithelial electric resistance (TER)1 elevation (Balda et al., 1996; McCarthy et al., 1996). Furthermore, most recently a synthetic peptide corresponding to the second extracellular loop of chicken occludin was reported to perturb the tight junction barrier in a very specific manner (Wong and Gumbiner, 1997). In freeze fracture electron microscopy, tight junctions appear as a set of continuous, anastomosing intramembranous particle strands, which are thought to work as a fence in the plasma membranes. Immunoelectron microscopic studies of freeze fracture replicas revealed that occludin is at least one of the major components of the tight junction strand itself (Fujimoto, 1995; Furuse et al., 1996), and a truncated occludin lacking its COOH-terminal cytoplasmic domain works in a dominant–negative manner, resulting in the destruction of a fence between apical and basolateral membrane domains (Balda et al., 1996). These findings indicate that occludin is a key component of tight junctions structurally as well as functionally.
The functions of tight junctions are dynamically regulated, but the molecular mechanism remains elusive. For example, knowledge of how the permeability of endothelial cells is elevated during an inflammatory reaction (Lum and Malik, 1994) and how the permeability of intestinal epithelial cells is controlled during absorption (Madara and Pappenheimer, 1987) is still fragmentary. Occludin is possibly involved in this regulation mechanism of tight junctions at two distinct levels: transcriptional and posttranslational. Indeed, occludin expression appears to be tightly regulated at the transcriptional level. Occludin mRNA is detected in epithelial and endothelial cells by Northern blotting but not in fibroblastic cells (Saitou et al., 1997).
In connection with posttranslational modification, it is noteworthy that chicken, as well as mammalian occludin, resolves on SDS-PAGE as several closely migrating bands, the smallest of which is the most intense (Furuse et al., 1993; Saitou et al., 1997). In this study we show that serine/ threonine-phosphorylation shifted the occludin band upward as multiple bands and that highly phosphorylated occludin resists detergent extraction. Calcium switch experiments revealed that tight junction formation is accompanied by the insolubilization and phosphorylation of occludin. Furthermore, we found that one of our mAbs raised against chicken occludin is specific for phosphorylated occludin and that this mAb mainly stained the tight junction proper. These findings suggest that phosphorylation is essential for occludin to form functional tight junctions.
Materials and Methods
Antibodies and Cells
Rat anti–mouse occludin mAb (MOC37) and rabbit anti–mouse occludin pAbs (F4 and F5) were raised against the cytoplasmic domain of mouse occludin produced in Escherchia coli (Saitou et al., 1997). Rat anti– chicken occludin mAbs (Oc-1, Oc-2, Oc-3) and a mouse anti–rat ZO-1 mAb (T8-754) were raised and characterized as described (Itoh et al., 1991; Furuse et al., 1993, 1996). Anti–chicken occludin pAb (F44) was raised in rabbits against the COOH-terminal cytoplasmic domain of chicken occludin that was produced in E. coli.
MDCK I cells were grown in minimal essential medium (MEM) supplemented with 5% FCS. Mouse epithelial cells, MTD-1A, were cultured in DMEM containing 10% FCS, and T84 human epithelial cells were grown in a 1:1 mixture of DMEM and Ham's F-12 medium containing 5% FCS.
Immunoprecipitation
MDCK I cells were cultured on two 24-mm filters (Transwell; Corning Costar Corp., Cambridge, MA), washed three times with ice-cold PBS, and then lysed in 500 μl of ice-cold NP-40–IP buffer (25 mM Hepes/ NaOH, pH 7.4, 150 mM NaCl, 4 mM EDTA, 25 mM NaF, 1% NP-40, 1 mM Na3VO4, 1 mM APMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin). Cells were scraped into a 1.5-ml microcentrifuge tube and gently rotated for 30 min at 4°C. After centrifugation (10,000 g for 30 min) the supernatant was collected as the NP-40–soluble fraction. The pellet was resuspended in 100 μl of SDS-IP buffer (25 mM Hepes, pH 7.5, 4 mM EDTA, 25 mM NaF, 1% SDS, 1 mM Na3VO4) using Kontes homogenizers (Kontes, Vineland, NJ), and the homogenate was combined with 900 μl of NP-40–IP buffer, which was used to wash the homogenizer. The lysate was passed 10 times through a 27G needle and then gently rotated again for 30 min at 4°C. After centrifugation (10,000 g for 30 min), the supernatant was used as the NP-40–insoluble fraction. The mixture of equal volume of NP-40–soluble and -insoluble fractions is called “total fraction.”
For immunoprecipitation, 4 μl of anti-occludin pAb (F5 or the mixture of F4 and F5) and a 15 μl bed vol of rec-protein G–Sepharose 4B (Zymed Labs., Inc., South San Francisco, CA) were added to each fraction and rotated for 3 h at 4°C. Beads were washed five times with 1 ml of NP-40–IP buffer, from which immunoprecipitates were eluted by boiling in the SDSPAGE sample buffer for 10 min. Samples were then separated by gel electrophoresis followed by immunoblotting or autoradiography.
Alkaline Phosphatase Treatment
After immunoprecipitation, beads were washed three times with 1 ml of NP-40–IP buffer and three times with 1 ml of AP buffer (50 mM Tris-HCl, pH 8.2, 50 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM APMSF). They were then resuspended in 200 μl of AP buffer containing 20 U of calf intestine alkaline phosphatase (Takara Shuzo Co., Ltd., Ohtsu, Japan). To check the specificity of the phosphatase, a phosphatase inhibitor (100 mM β-glycerophosphate, 25 mM NaF, 4 mM EDTA, 1 mM Na3VO4) was used. After a 1 h incubation at 30°C with occasional mixing, beads were washed three times with 1 ml of NP-40–IP buffer and boiled with SDS-PAGE sample buffer to elute the immunoprecipitates.
Gel Electrophoresis and Immunoblotting
Samples were resolved by one-dimensional SDS-PAGE as described by Laemmli (1970) and electrophoretically transferred to a nitrocellulose membrane (Protran, 0.45 μm pore size; Schleicher & Schuell, Dassel, Germany). This membrane was incubated with primary antibodies, which were visualized using a blotting detection kit (Amersham Intl., Buckinghamshire, UK).
Metabolic Labeling and Phosphoamino Acid Analysis
Confluent monolayers of MDCK I cells were grown on filters, washed three times with phosphate-free MEM, and then incubated for 30 min in phosphate-free MEM containing 1% FCS dialyzed against 0.9% NaCl, 10 mM Hepes buffer (pH 7.4). Thereafter, [32P]orthophosphate (Phosphorous-32; NEN Life Science Products, Boston, MA) was added at a concentration of 0.2 mCi/ml, cultured for 24 h, and then processed for immunoprecipitation.
Immunoprecipitates were resolved by gel electrophoresis and transferred to PVDF membranes (Immobilon; Millipore Corp., Bedford, MA), and signals were analyzed using a Fujix Bioimage Analyzer system (Bas 2000; Fuji Film Co. Ltd., Tokyo, Japan). Phosphoamino acids were analyzed based on the method of Boyle et al. (1991) with minor modifications. 32P-labeled phosphorylated occludin bands in the NP-40–insoluble fraction were excised from membranes and hydrolyzed in 200 μl of 6 M HCl at 110°C for 60 min. The hydrolysate was lyophilized using a speed vac concentrater (Savant Instruments Inc., Holbrook, NY) and resuspended in 10 μl of pH 3.5 buffer (5% glacial acetic acid, 0.5% pyridine) containing cold phosphoamino acid standards. The sample was then spotted onto thin-layer cellulose plates (EM Science, Gibstown, NJ), and two-dimensional electrophoresis was proceeded on a thin-layer system (NA-4000; Nippon Eido Co., Tokyo, Japan), using pH 3.5 buffer for the first dimension and pH 1.9 buffer (2.2% formic acid, 7.8% glacial acetic acid) for the second. The positions of 32P-labeled phosphoamino acids were determined by autoradiography, and cold phosphoamino acid standards were visualized by ninhydrin staining.
Low Ca Medium Culture and Ca Switch
Confluent monolayers of MDCK I cells were grown in normal calcium (NC) medium (MEM with 5% FCS and 1.8 mM CaCl2), washed three times with PBS, and then transferred to the low calcium (LC) medium (SMEM [calcium-free MEM] supplemented with 5 μM CaCl2 and 5% FCS that had been pretreated with Chelex resin [BioRad Labs., Inc., Hercules, CA]; Gumbiner et al., 1988). For the Ca switch, MDCK I cells in NC medium were trypsinized in PBS containing 1 mM EDTA, washed with PBS, and then plated on filters or cover slips in LC medium at a density of 2 × 105 cells/cm2. After a 36-h incubation in LC, cells were transferred to NC medium.
Immunofluorescence Microscopy
For indirect immunofluorescence microscopy, cells were cultured on cover slips and fixed in 1% formaldehyde in PBS for 15 min. After three washes with PBS they were permeabilized with 0.2% Triton X-100 in PBS for 15 min, soaked in blocking solution (PBS containing 1% BSA) for 15 min, and then incubated with first antibodies for 1 h in a moist chamber. The samples were washed three times with the blocking solution and then incubated for 30 min with the secondary antibodies, FITC-conjugated goat anti–rat IgG (Tago, Inc., Burlingame, CA) or rhodamine-conjugated goat anti–mouse IgG (Chemicon International, Inc., Temecula, CA). Samples were then washed with PBS three times, mounted in PBS containing 1% p-phenylenediamine and 90% glycerol, and examined using a fluorescence microscope (Zeiss Axiophot photomicroscope; Carl Zeiss, Inc., Thornwood, NY).
Chicken intestine was frozen in liquid nitrogen. About 7 μm-thick sections were cut in a cryostat, mounted on cover slips, and air dried. They were fixed in 95% ethanol at 4°C for 30 min and then in 100% acetone at room temperature for 1 min. After three washes with PBS, the sections were soaked in blocking solution for 15 min, incubated with primary antibodies for 60 min, washed three times with blocking solution containing 0.1% Triton X-100, and then incubated with secondary antibodies for 30 min. The samples were washed with PBS three times, mounted in PBS containing 1% p-phenylenediamine and 90% glycerol, and examined using a fluorescence microscope (Zeiss Axiophot photomicroscope; Carl Zeiss, Inc.) or a confocal fluorescence microscope (MRC 1024; BioRad Labs., Inc.) equipped with the photomicroscope.
Isolation of Junctional Fraction from Chick Liver
The junctional fraction was prepared from the liver of newly hatched or 1-d-old chicks through the crude membrane and the bile canaliculi fractions according to the method described previously (Tsukita and Tsukita, 1989; Furuse et al., 1993). The isolated junctional fraction was treated with SDS-IP buffer and processed for immunoprecipitation as described above.
Results
Multiple Banding and Detergent Solubility of Occludin
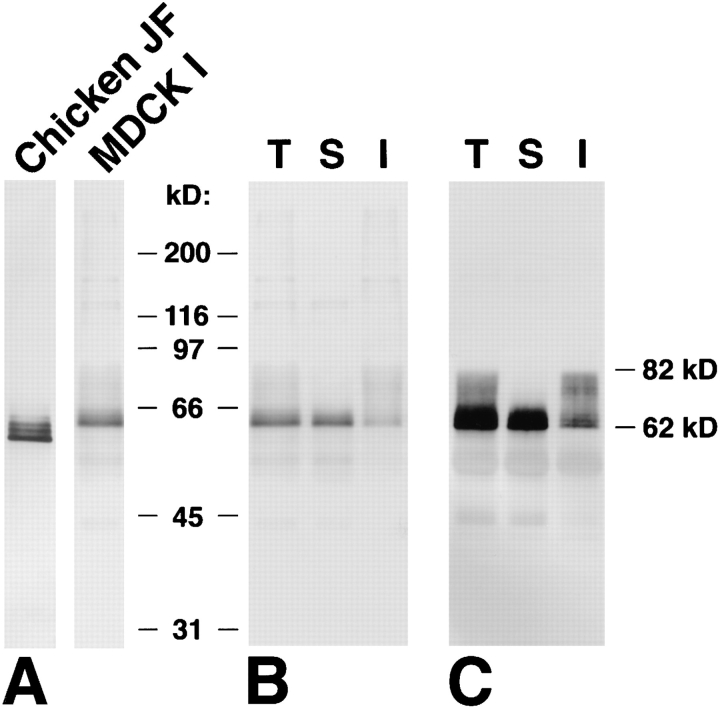
When the chicken junctional fraction isolated from liver and the whole cell lysate from cultured MDCK I cells were resolved by electrophoresis and immunoblotted with the respective anti-occludin Abs, both chicken and dog occludin migrated as multiple bands (Fig. 1 A). The apparent M r of occludin in the confluent culture of MDCK I cells was distributed from 62 and 82 kD, and two or three bands of the lowest M r were predominant. This multiple banding of occludin was found in all species so far examined.
Figure 1.
Multiple banding pattern and detergent solubility of occludin. (A) Immunoblots of the isolated junctional fraction from the chick liver (Chicken JF) and the whole cell lysate of MDCK I cells (MDCK I) with anti–chicken occludin mAb (Oc-2) and anti–mouse occludin pAb (F4), respectively. The apparent molecular masses of chicken and dog occludin were distributed between 58 and 66 kD and 62 and 82 kD, respectively. (B) Anti-occludin pAb (F4) immunoblots of the total (T), NP-40–soluble (S), and NP-40–insoluble (I) fractions of confluent MDCK I cells (see Materials and Methods). (C) Anti-occludin mAb (MOC37) immunoblots of the anti-occludin pAb (F4 + F5) immunoprecipitates from the total (T), NP-40–soluble (S), and NP-40–insoluble (I) fractions of confluent MDCK I cells. Since the amount of occludin in each fraction was fairly small (B), both NP-40–soluble and -insoluble occludins were recovered by immunoprecipitation, electrophoresed, and immunoblotted (C). Comparison between B and C revealed that the efficiency of immunoprecipitation from NP-40–soluble fraction is almost the same as that from NP40–insoluble fraction. Higher M r bands of occludin were selectively recovered in the NP-40–insoluble fraction.
As a first step to physiologically explain the multiple banding of occludin, we divided occludin from cultured MDCK I cells into NP-40–soluble and -insoluble fractions. Confluent cultures of MDCK I cells, which were grown on filters and showed >2,000 Ωcm2 of TER, were solubilized with 1% NP-40, and the supernatant was used as the NP40–soluble fraction. The pellet was further solubilized with lysis buffer containing 1% SDS, which was then used as the NP-40–insoluble fraction. Occludin was almost undetectable in the SDS-insoluble sediment by immunoblotting. Since the amount of occludin in each fraction was fairly small (Fig. 1 B), both NP-40–soluble and -insoluble occludins were recovered by immunoprecipitation with antioccludin pAb, electrophoresed, and immunoblotted with anti-occludin mAb. Fig. 1 C shows that under the NP-40 extraction conditions used in this study, the predominant lower M r two or three bands of occludin were mostly recovered in the NP-40–soluble fraction, and the higher M r bands were partitioned into the NP-40–insoluble fraction. The same result was obtained when mouse and human cultured epithelial cells, MTD-1A and T84 cells, respectively, were used (data not shown).
Electrophoretic Mobility and Phosphorylation Level of Occludin
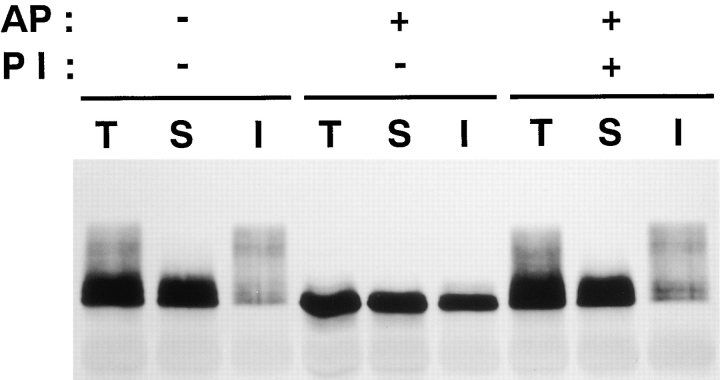
It is likely that some posttranslational modification causes the multiple banding of occludin. We first determined whether or not phosphorylation increases apparent M r of occludin in SDS-PAGE. The immunoprecipitated occludin from NP-40–soluble and -insoluble fractions were then treated with alkaline phosphatase (Fig. 2). This procedure significantly decreased the apparent M r of NP-40–soluble and -insoluble occludin bands to the level of the lowest M r band. This activity of alkaline phosphatase was completely suppressed by its inhibitor, indicating that phosphorylation causes the upward shift of occludin band in SDS-PAGE.
Figure 2.
Alkaline phosphatase treatment. Anti-occludin pAb (F4 + F5) immunoprecipitates from the total (T), NP-40–soluble (S), and NP-40–insoluble (I) fractions of confluent MDCK I cells were incubated in the presence (+) or absence (−) of alkaline phosphatase (AP) and its specific inhibitor (PI) and then immunoblotted with anti-occludin mAb (MOC37). Alkaline phosphatase significantly decreased the apparent molecular masses of NP-40–soluble and -insoluble occludin bands to the level of the lowest M r band, and its inhibitor completely suppressed this effect.
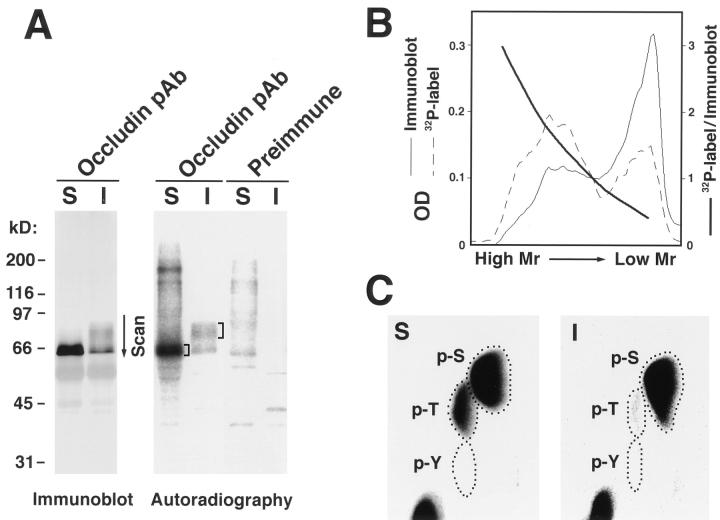
To determine which types of amino acid residues are phosphorylated, we analyzed phosphoamino acids using cultured MDCK I cells that were metabolically labeled with [32P] orthophosphate. Both NP-40–soluble and -insoluble occludins were labeled, although the latter were phosphorylated more heavily in terms of specific activity than the former (Fig. 3 A). Within the insoluble occludin, the specific activity was considerably higher in the higher M r bands than the lower M r bands (Fig. 3 B). The [32P]phosphoamino acids were then released by partial acid hydrolysis of the 32P-labeled higher M r bands of NP40–insoluble occludin as well as the 32P-labeled NP-40–soluble occludin, and identified by thin-layer electrophoresis. As shown in Fig. 3 C, in NP-40–soluble occludin, both serine and threonine residues were phosphorylated whereas in higher M r bands of NP-40–insoluble occludin, serine residues were predominantly phosphorylated with slight threonine phosphorylation. Phospho-tyrosine was undetectable either in NP-40–soluble or -insoluble occludin.
Figure 3.
Phosphoamino acid analysis of the NP-40–soluble and -insoluble occludin. (A) Anti-occludin mAb (MOC37) immunoblots (Immunoblot) and accompanying autoradiograms (Autoradiography) of anti-occludin pAb (F5) immunoprecipitates from the NP-40–soluble (S) and NP40–insoluble (I) fractions of confluent MDCK I cells metabolically labeled with [32P]orthophosphate. Control experiments were performed using preimmune serum (Preimmune). (B) Relative specific activity of occludin bands. The region marked by an arrow in the immunoblot and autoradiogram lanes of NP40–insoluble occludin was scanned by densitometry (A, Scan). Relative specific activity of each occludin band was calculated as autoradiogram density/immunoblot density. (C) The marked region in the autoradiogram lane of 32P-labeled NP-40–soluble and -insoluble occludins was excised and processed for phosphoamino acid analysis. The positions of phosphoserine (p-S), phosphothreonine (p-T), and phosphotyrosine (p-Y) were determined by autoradiography through comparison with the ninhydrin staining profiles of unlabeled phosphoamino acid standards. In NP-40–soluble occludin, both serine and threonine residues were phosphorylated (S), whereas in higher M r bands of NP-40–insoluble occludin, serine residues were predominantly phosphorylated with slight phosphorylation of threonine residues (I).
Behavior of Occludin during Destruction and Formation of Tight Junctions
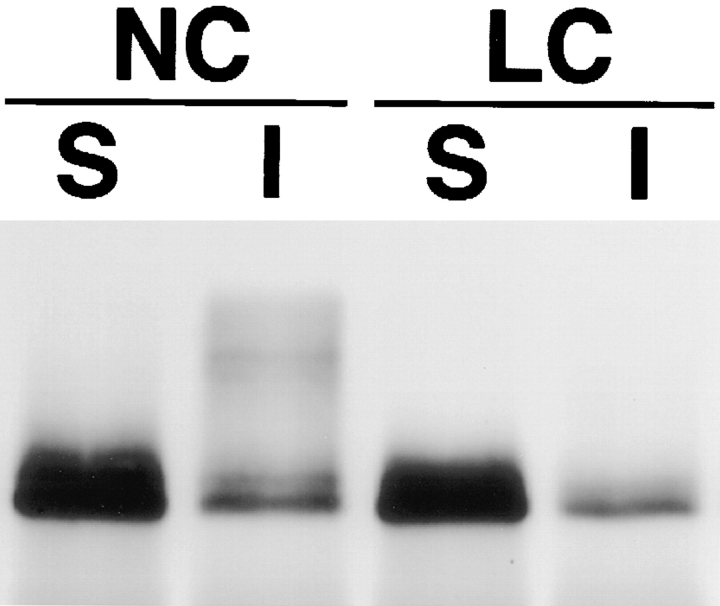
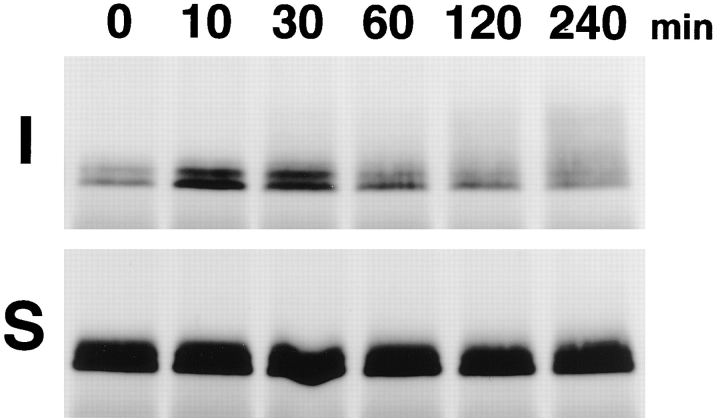
We examined the relationship between NP-40–insoluble occludin and tight junction formation. First, the banding patterns of NP-40–soluble and -insoluble occludin of MDCK I cells were compared under conditions of normal (1.8 mM) and low (5 μM) calcium medium. Fig. 4 shows that under low calcium (LC) conditions, almost all occludin was solubilized by 1% NP-40 and migrated as two or three lower M r bands in SDS-PAGE, whereas the insoluble fraction was fairly small. Furthermore, the total amount of occludin was significantly decreased under LC conditions. We then transferred cells from the LC to the normal calcium (NC) medium (Fig. 5). Within 10 min the amount of occludin recovered as the NP-40–insoluble fraction was significantly increased, followed by a gradual increase of their apparent M r. No significant change of either the banding pattern or the amount of the NP-40–soluble occludin was detected after the Ca switch. The same results were obtained also from confluent cultures of mouse (MTD-1A) and human (T84) epithelial cells (data not shown).
Figure 4.
Multiple banding pattern and detergent solubility of occludin in MDCK I cells grown in normal calcium (1.8 mM; NC) or low calcium (5 μM; LC) medium for 24 h. Anti-occludin pAb (F4 + F5) immunoprecipitates from the NP-40–soluble (S) and NP-40–insoluble (I) fractions of confluent MDCK I cells were immunoblotted with anti-occludin mAb (MOC37). Under low calcium conditions, the amount of NP-40–insoluble occludin was fairly small.
Figure 5.
Insolubilization and upward band shift of occludin in MDCK I cells after switching from low (5 μM) to normal (1.8 mM) calcium medium. 0, 10, 30, 60, 120, and 240 min after the Ca switch, the anti-occludin pAb (F4 + F5) immunoprecipitates from the NP-40–insoluble (I) or NP-40–soluble (S) fractions of confluent MDCK I cells were immunoblotted with anti-occludin mAb (MOC37). Within 10 min after switching, the amount of occludin recovered as the NP-40–insoluble portion was significantly increased, followed by a gradual increase of their apparent molecular masses.
The behavior of occludin as well as ZO-1 during the formation of tight junctions initiated by the Ca switch was then examined by immunofluorescence microscopy using confluent MDCK I cells (Fig. 6). In the LC medium, occludin signal was detected mainly from small granular structures scattered in the cytoplasm, and ZO-1 was found on ring-like structures that may consist of actin filament bundles. Some large granular structures and some ring-like structures were occasionally occludin/ZO-1 double positive. Within 10 min after the cells were transferred to the NC medium, both occludin and ZO-1 started to accumulate and colocalize at the cell–cell borders as discontinuous lines. Around 60 min after the Ca switch, this accumulation process appeared to reach a plateau, resulting in the continuous linear concentration of occludin and ZO-1 at junctional regions.
Figure 6.
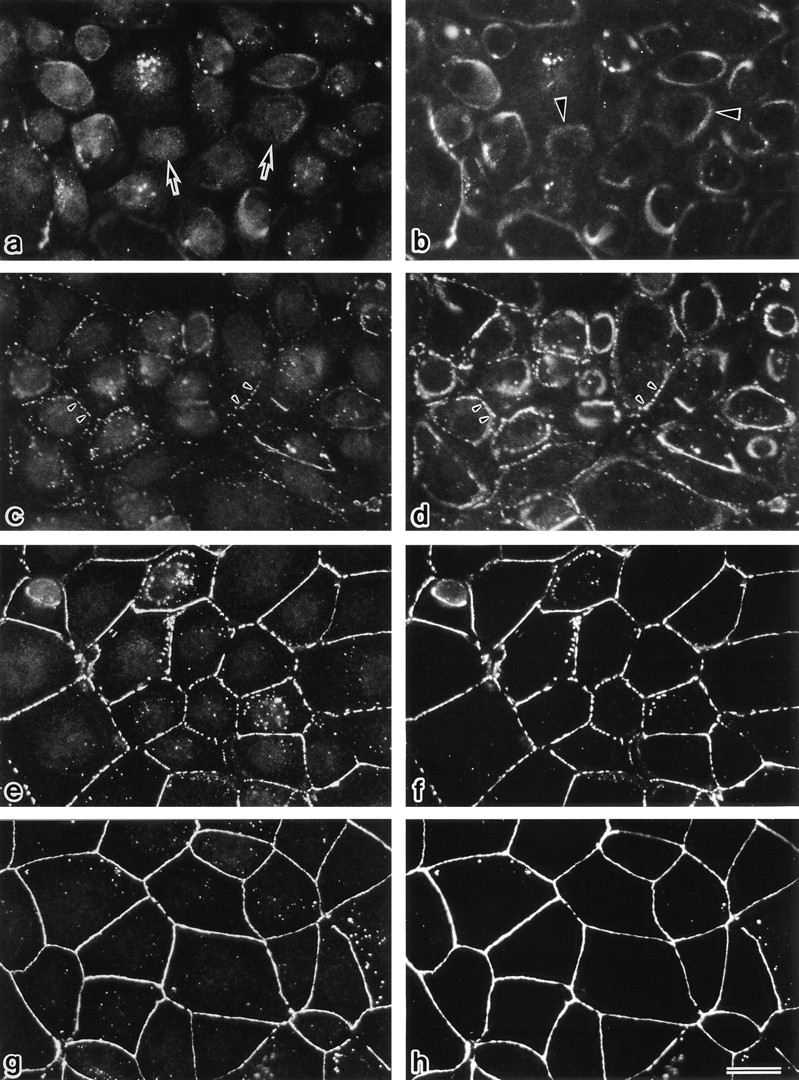
Formation of tight junctions in MDCK I cells after a Ca switch from low (5 μM) to normal (1.8 mM) calcium medium. 0 (a and b), 10 (c and d), 30 (e and f), and 60 (g and h) min after the Ca switch, confluent MDCK I cells were fixed and immunofluorescently stained with rat anti-occludin mAb (a, c, e, and g) and mouse anti–ZO-1 mAb (b, d, f, and h). In the low calcium medium (a and b), occludin signal was detected mainly from small granular structures in the cytoplasm (arrows), and ZO-1 signal was from ring-like structures (arrowheads). Within 10 min after the Ca switch (c and d), both occludin and ZO-1 gradually began to accumulate and colocalize at cell–cell borders (arrowheads). 30–60 min after the Ca switch (e–h), both occludin and ZO-1 were colocalized in a linear fashion at junctional regions. Even 60 min after the Ca switch, the epithelial sheet was still leaky in terms of TER. Bar, 20 μm.
A Monoclonal Antibody Specific for Phosphorylated Type of Occludin
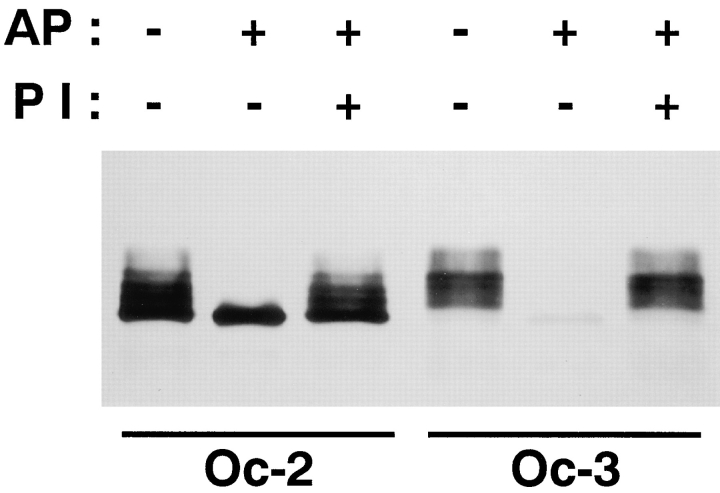
In the previous study we obtained three mAbs that recognized distinct epitopes of chicken occludin: Oc-1, Oc-2, and Oc-3 (Furuse et al., 1993). When the isolated junctional fraction from chick liver was immunoblotted with these mAbs, the banding pattern obtained from Oc-3 differed from those with Oc-1 and Oc-2 (Fig. 7; see Fig. 1 in Furuse et al., 1993). Oc-3 did not recognize the predominant lower M r bands of occludin, which were clearly detected by Oc-1 and Oc-2. This suggests that Oc-3 is specific for phosphorylated occludin. To evaluate this notion, chicken occludin in the isolated junctional fraction was solubilized using 1% SDS, immunoprecipitated with anti–chicken occludin pAb, treated with alkaline phosphatase in the absence or presence of its specific inhibitor, and then immunoblotted with Oc-2 or Oc-3 (Fig. 7). Immunoblots of chicken occludin with Oc-2 showed that alkaline phosphatase converged the multiple bands into the lowest M r of 58 kD, which was suppressed by the phosphatase inhibitor. Oc-2 detected this dephosphorylated occludin, whereas Oc-3 hardly recognized it. We concluded that Oc-3 is specific for the phosphorylated type.
Figure 7.
Two distinct anti–chicken occludin mAbs, Oc-2 and Oc-3. Chicken occludin in the isolated junctional fraction was solubilized using 1% SDS, immunoprecipitated with anti–chicken occludin pAb (F44), incubated in the presence (+) or absence (−) of alkaline phosphatase (AP) and its inhibitor (PI), and then immunoblotted with Oc-2 or -3. Oc-3 could not detect dephosphorylated occludin, whereas Oc-2 recognized both phosphorylated and dephosphorylated occludin.
Since Oc-2 or Oc-3 does not recognize mammalian occludin (Furuse et al., 1993), we examined the subcellular distribution of the phosphorylated occludin in chicken tissues using Oc-3 and compared it with the Oc-2 staining, which represents the distribution of total occludin. As shown in Fig. 8, Oc-2 stained the junctional complex regions of intestinal epithelial cells in a linear fashion. In addition, the basolateral membrane domains and the cytoplasm of these cells was also stained in a dotted manner. By contrast, Oc-3 mainly stained tight junction propers, showing a very weak signal only from the basolateral membrane domains. These observations indicate that the highly phosphorylated form of occludin is selectively concentrated at the tight junction proper.
Figure 8.
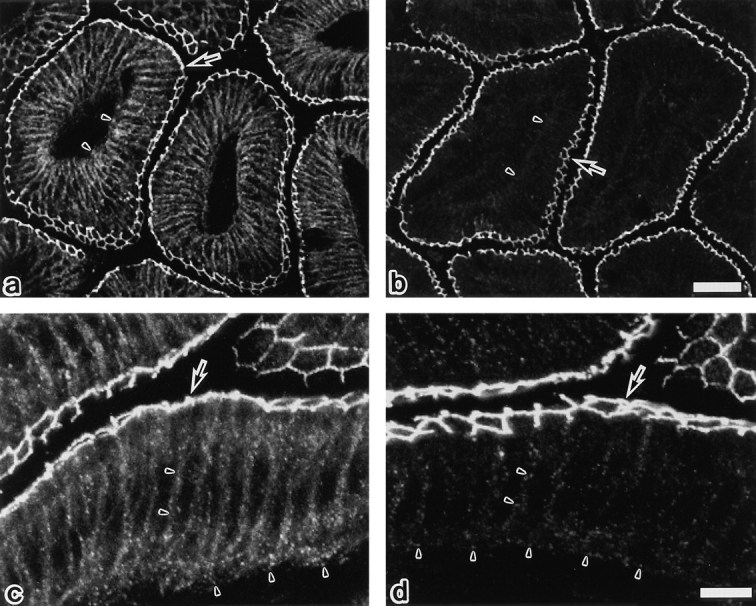
Confocal immunofluorescence microscopy of frozen sections of chick intestinal epithelial cells with anti–chicken occludin mAb, Oc-2 (a and c), or Oc-3 (b and d). Oc-2 stained both the junctional complex regions (arrows) and the basolateral membrane domains (arrowheads) in linear and dotted manners, respectively. By contrast, Oc-3 mainly stained the tight junction region (arrows), showing a very weak signal only from the basolateral membranes (arrowheads). In our previous study (Furuse et al., 1993) it was emphasized that Oc-2 is specific for tight junctions without paying special attention to its staining at the basolateral membrane domains, but as shown here, the difference in the staining pattern is significant between Oc-2 and -3. Bars: (a and b) 30 μm; (c and d) 10 μm.
Discussion
Occludin has been characterized in various species by its multiple bands on SDS-PAGE (Furuse et al., 1993; Saitou et al., 1997). The present study showed that occludin is phosphorylated at serine and threonine residues, which shifts the occludin band to assume the multiple profile in SDS-PAGE. Canine occludin is detected as at least 10 closely migrating bands between 62 and 82 kD. This suggests that several serine and threonine residues can be phosphorylated per occludin molecule and that the degree of the upward band shift of occludin roughly parallels its phosphorylation level. Phosphoamino acid analyses of higher and lower M r bands of occludin suggested that threonine residues are phosphorylated followed by heavy serine phosphorylation during the upward shift of occludin bands. However, it remains elusive whether these serine/ threonine residues are phosphorylated sequentially or randomly in each molecule and whether or not the phosphorylation of some residues is functionally more important than that of others.
Highly phosphorylated occludin from confluent cultures of MDCK I cells resisted extraction with 1% NP-40. Judging from the alkaline phosphatase treatment, not only the nonphosphorylated but also the less phosphorylated form of occludin was solubilized with 1% NP-40. Although the optimal concentration of NP-40 used here was empirically determined as 1%, it can be concluded that the highly phosphorylated type of occludin is more resistant to detergent extraction than the less phosphorylated type. When MDCK I cells were cultured in the LC medium, tight junctions disappeared, most occludin became soluble with 1% NP-40, and highly phosphorylated occludin was hardly detected. The total amount of occludin appeared to be decreased, suggesting the up-regulated degradation and/or down-regulated de novo synthesis of occludin under LC conditions. When these cells were transferred to the NC medium, tight junctions began to be assembled. With a similar time course, the amount of NP-40–insoluble occludin markedly increased, followed by a gradual upward shift of bands. Considering that tight junction strands are resistant to detergent extraction (Stevenson and Goodenough, 1984; Stevenson et al., 1988), these findings suggested that highly phosphorylated occludin predominantly composes the tight junction proper. This conclusion was confirmed by the immunofluorescence analyses of chicken epithelial cells using the mAb Oc-3, which is specific for phosphorylated occludin.
Occludin is at least one of the major components of tight junction strands in situ (Furuse et al., 1993; Fujimoto et al., 1995; Saitou et al., 1997). Furthermore, judging from occludin overexpression studies using insect Sf9 cells, occludin by itself may form tight junction strand-like structures, probably through oligomerization (Furuse et al., 1996). On the other hand, occludin binds to ZO-1 (then to cytoskeletons) at its COOH-terminal ∼150 amino acid region. Therefore, at present there are at least two possible molecular mechanisms by which occludin can become resistant to NP-40 extraction: oligomerization and cytoskeleton association. Further analyses are required to evaluate these two possibilities.
Understanding of the behavior of occludin during tight junction formation is still limited compared to that of adhesion molecules in other intercellular junctions. For example, in epithelial cells, cadherins are reportedly associated with β catenin in the endoplasmic reticulum, appear on the basolateral membrane surface as cadherin–αβ catenin complexes, and laterally aggregate (or oligomerize) at the most apical part of the lateral membrane to form adherens junctions (Hinck et al., 1994; Näthke et al., 1994). As for tight junction formation, the following questions should be addressed. Where does occludin associate with ZO-1 and other tight junction-associated peripheral membrane proteins? Where are occludin molecules oligomerized? Is occludin (or occludin–peripheral membrane proteins complex) targeted to the basolateral membranes or directly to the junctional complex area? Introduced exogenous chicken occludin reportedly concentrates in cytoplasmic vesicular structures in MDCK I cells, when the transfectants are cultured in LC medium (McCarthy et al., 1996). As shown here, endogenous occludin is also distributed in cytoplasmic granular structures under LC conditions, and most of them do not colocalize with ZO-1. Although the Ca switch appears to recruit these cytoplasmic occludins, together with ZO-1, to the cell–cell contact regions, at present it is difficult to exclude the possibility that some or all of the cytoplasmic occludin-positive granular structures occur as a result of endocytosis under LC conditions. The difference between the Oc-2 and -3 staining patterns in chicken epithelial cells suggests that non- or less phosphorylated occludin is first targeted to the basolateral membranes from the cytoplasm and that further phosphorylation induces occludin to concentrate into the junctional complex region.
Another issue that should be discussed is the similarity between occludin and connexin in terms of phosphorylation as well as structure. Connexin is an integral channel protein functioning at gap junctions (Bruzzone et al., 1996; Kumar and Gilula, 1996). Connexin also bears four transmembrane domains, although there is no sequence similarity between connexin and occludin (Furuse et al., 1993; Ando-Akatsuka et al., 1996). Connexin43 is reportedly serine phosphorylated in gap junction communicationcompetent cells (Musil et al., 1990). This phosphorylation mostly occurs after the arrival of non- or slightly phosphorylated connexin43 at the plasma membrane (Musil and Goodenough, 1991; Laird et al., 1995). The strong correlation between the formation of functional gap junctions and the phosphorylation of connexin is very similar to that between the formation of tight junctions and the phosphorylation of occludin.
Studies using various protein kinase activators and inhibitors have revealed that protein phosphorylation, especially the protein kinase C–dependent type, plays an important role in tight junction assembly and functions (Balda et al., 1991, 1993; Citi, 1992; Denisenko et al., 1994; Citi and Denisenko, 1995; Stuart and Nigam, 1995). However, a positive correlation has not been obtained between the tight junction assembly and the phosphorylation level of tight junction proteins such as ZO-1 and -2, p130, and cingulin (Balda et al., 1993; Citi and Denisenko, 1995). We found here, however, that occludin is heavily serine/threonine phosphorylated in a similar time course as that of tight junction formation after a Ca switch, and that the highly phosphorylated occludin is selectively concentrated at tight junctions. This suggests that protein phosphorylation is directly involved in tight junction assembly and provides a new experimental approach to studying the molecular mechanism of the regulation of tight junction assembly. In our next step we should determine which serine/threonine residues of occludin are phosphorylated in vivo, which kinases phosphorylate these residues, whether mutations at these residues affect the tight junction assembly and function, and which signaling up or down regulates occludin phosphorylation. Studies along these lines will clarify in molecular terms, how the barrier and fence functions of tight junctions are regulated in vivo.
Acknowledgments
We would like to thank all the members of our laboratory (Department of Cell Biology, Kyoto University Faculty of Medicine) for helpful discussions throughout this study. Our thanks are also due to Drs. T. Moriguchi and E. Nishida (Department of Genetics and Molecular Biology, Institute for Virus Research, Kyoto University) for technical help with the phosphoamino acid analysis.
This work was supported in part by a Grant-in-Aid for Cancer Research and a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science and Culture of Japan to S. Tsukita.
Footnotes
1. Abbreviations used in this paper: LC and NC, low and normal calcium; TER, transepithelial electric resistance.
Please address all correspondence to Dr. Shoichiro Tsukita, Department of Cell Biology, Kyoto University, Faculty of Medicine, Konoe-Yoshida, Sakyo-ku, Kyoto 606, Japan. Tel.: (81) 75-753-4372; Fax: (81) 75-753-4660; E-mail: htsukita@mfour.med.kyoto-u.ac.jp
References
- Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita Sh. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat–kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thinlayer cellulose plates. Methods Enzymol. 1991;201:110–148. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Goodenough DA. The cellular internet: on-line with connexins. Bioessays. 1996;18:709–718. doi: 10.1002/bies.950180906. [DOI] [PubMed] [Google Scholar]
- Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol. 1992;117:169–178. doi: 10.1083/jcb.117.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Denisenko N. Phosphorylation of the tight junction protein cingulin and the effects of protein kinase inhibitors and activators in MDCK epithelial cells. J Cell Sci. 1995;108:2917–2926. doi: 10.1242/jcs.108.8.2917. [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature (Lond) 1988;33:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Denisenko N, Burighel P, Citi S. Different effects of protein kinase inhibitors on the localization of junctional proteins at cell-cell contact sites. J Cell Sci. 1994;107:969–981. doi: 10.1242/jcs.107.4.969. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–409. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., K. Fujimoto, N. Sato, T. Hirase, Sa. Tsukita, and Sh. Tsukita. 1996. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. 109:429–435. [DOI] [PubMed]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorlin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Yonemura S, Nagafuchi A, Tsukita Sa, Tsukita Sh. A220-kD undercoat-constitutive protein: Its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol. 1991;115:1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita Sa, Tsukita Sh. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1 , a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophiladiscs-large tumor suppresser protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird DW, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol. 1987;253:C171–C175. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita Sh, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distribution of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, M., Y. Ando-Akatsuka, M. Itoh, M. Furuse, J. Inazawa, K. Fujumoto, and Sh. Tsukita. 1997. Mammalian occludin in epithelial cells: Its expression and subcellular distribution. Eur. J. Cell Biol. In press. [PubMed]
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Goodenough D. Zonula occludentes in junctional complex-enriched fractions from mouse liver: preliminary morphological and biochemical characterization. J Cell Biol. 1984;98:1209–1221. doi: 10.1083/jcb.98.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Bullivant S. The epithelial tight junction: Structure, function and preliminary biochemical characterization. Mol Cell Biochem. 1988;83:129–145. doi: 10.1007/BF00226141. [DOI] [PubMed] [Google Scholar]
- Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita, Sh., and Sa. Tsukita. 1989. Isolation of cell-to-cell adherens junctions from rat liver. J. Cell Biol. 108:31–41. [DOI] [PMC free article] [PubMed]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-I is homologous to the Drosophila discs-large tumor suppresser protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin, and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]